Abstract
Background & Aims
There is a pressing need to develop effective preventative therapies for post–endoscopic retrograde cholangiopancreatography pancreatitis (PEP). We showed that early PEP events are induced through the calcium-activated phosphatase calcineurin and that global calcineurin deletion abolishes PEP in mice. A crucial question is whether acinar cell calcineurin controls the initiation of PEP in vivo.
Methods
We used a mouse model of PEP and examined the effects of in vivo acinar cell-specific calcineurin deletion by either generating a conditional knockout line or infusing a novel adeno-associated virus–pancreatic elastase improved Cre (I–iCre) into the pancreatic duct of a calcineurin floxed line.
Results
We found that PEP is dependent on acinar cell calcineurin in vivo, and this led us to determine that calcineurin inhibitors, infused within the radiocontrast, largely can prevent PEP.
Conclusions
These results provide the impetus for launching clinical trials to test the efficacy of intraductal calcineurin inhibitors to prevent PEP.
Keywords: Adeno-Associated Virus, Calcineurin B1, FK506, Cyclosporine A, Intraductal Delivery
Abbreviations used in this paper: AAV, adeno-associated virus; Cn, calcineurin; CreERT2, Cre recombinase estrogen receptor T2; CsA, cyclosporine A; Ela, pancreatic elastase I; ERCP, endoscopic retrograde cholangiopancreatography; IL, interleukin; LSL, Lox-Stop-Lox; LVNS, low volume normal saline; MPO, myeloperoxidase; PEG, polyethylene glycol; PEP, post-ERCP pancreatitis; Tm, tdTomato Red reporter
See editorial on page 6.
Summary.
This work establishes that pancreatic acinar cell calcineurin is a critical mediator of post–endoscopic retrograde cholangiopancreatography pancreatitis, using a mouse model. Importantly, the work led us to discover that calcineurin inhibitors, infused along with the radiocontrast, largely can prevent the procedural complication.
Endoscopic retrograde cholangiopancreatography (ERCP) is a common gastrointestinal procedure that confers a risk of acute pancreatitis ranging between 1% and 15%.1 The efficacy of widely accepted strategies to prevent post-ERCP pancreatitis (PEP) such as pretreatment with rectal indomethacin2 recently have been challenged.3, 4 The search for PEP prevention requires uncovering central mechanisms that initiate PEP. By using an ex vivo surrogate model of PEP, derived by isolating primary mouse and human pancreatic acinar cells, we recently showed that common radiocontrast agents used during ERCP induce acinar cell inflammatory signaling and injury through the activation of the calcium-activated phosphatase calcineurin (Cn).5 In an in vivo model of PEP in mice, we found that global Cn knockout mice (deficient in CnAβ) or systemic inhibition of Cn with frequent dosing of the Cn inhibitors FK506 or cyclosporine A (CsA) prevented PEP. Because Cn is expressed ubiquitously, a crucial unanswered question is whether acinar cell Cn blockade by itself is sufficient to prevent PEP in vivo.
Materials and Methods
Reagents and Animals
All reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless specified otherwise. Mice carrying loxP-flanked (floxed) alleles of CnB1 (CnB1f/f; backcrossed to a C57BL/6 strain) were a kind gift from Dr Gerald Crabtree.6 The Cre recombinase estrogen receptor T2 (Ela-CreERT2) mutant line was a kind gift from Dr Craig Logsdon, and it contains a transgenic insertion of a full-length acinar cell-specific mouse pancreatic elastase I (Ela) promoter that drives a tamoxifen-inducible CreERT2.7 This line also was backcrossed to a C57BL/6 strain. Lox-Stop-Lox (LSL)-tdTomato Red reporter (Tm) mice were obtained from the Jackson Lab (Farmington, CT).8 Both male and female genetically engineered mice were used for the in vivo studies. Eight- to 10-week-old wild-type male and female Swiss Webster mice weighing 25 g were used to assess the efficacy of intraductal administration of FK506 and CsA. All mice were housed at 22ºC with a 12-hour light-dark cycle and maintained on standard laboratory chow with free access to food and water. All animal experiments were performed using a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Generation of Conditional Pancreatic Acinar Cell-Specific CnB1 Knockouts
CnB1f/f mice were crossed with Ela-CreERT2 mice to generate homozygous Ela-CreERT2/CnB1f/f strains. To delete CnB1 in pancreatic acinar cells (CnB1Δ/Δ), CreERT2/CnB1f/f mice received a cumulative dose of 5–6 mg tamoxifen given intraperitoneally either daily or every other day for a total duration of 5–6 days. PEP was induced 1 week after the last tamoxifen injection. CnB1f/f lines lacking the Ela-CreERT2 insertion served as controls, and they also received tamoxifen.
CnB1Δ/Δ Genotyping
Genomic DNA was prepared from freshly isolated mouse pancreas and liver tissue, as described.9 Briefly, the tissue was minced on ice and homogenized in sodium chloride Tris-EDTA buffer containing proteinase K. The homogenates were incubated at 55ºC for 3 hours with intermittent vortexing. After inactivation of proteinase K, the homogenates were centrifuged at 4ºC, and the supernatants containing genomic DNA were precipitated with isopropanol. The precipitated genomic DNA was pelleted at 4ºC, washed with 70% ethanol, air-dried, and dissolved in 200 μL of 1 × Tris-EDTA buffer for polymerase chain reaction. A schematic of the location and size of the expected amplicons are provided in Figure 1. Primer sequences are shown in Table 1.
Figure 1.
Generation of acinar-specific Cn deletion using the Ela-CreERT2/CnB1f/f line. (A) Acinar cell Cn conditional knockout line (CnB1Δ/Δ) induced by crossing Ela-CreERT2 mice with CnB1f/f mice, followed by tamoxifen administration. (B) Schema of the CnB1 knockin allele containing loxP sites and schema of the Ela-CreERT2 transgene. Arrows denote forward and reverse primers designed to generate polymerase chain reaction products for the 5’ and 3’ loxP sites, Cre, and ERT2. Agarose gels showing the polymerase chain reaction products of expected size (C) in the non–tamoxifen-injected mice for the presence of the floxed alleles and the Ela-CreERT2 transgene or (D) confirming the floxed out state in the induced mouse. In the presence of acinar-specific Cre expression, the resulting amplicon is expected to be 168 bp in length, as shown in the gel from the pancreas, but not the liver of the CnB1Δ/Δ line. (E) Nuclear factor of activated T cells (NFAT) luciferase activity is diminished markedly in acinar cells from CnB1Δ/Δ but not from CnB1f/f controls, in response to radiocontrast (RC). *,#P < .05 relative to negative and positive controls, respectively. RLU, relative luminescence unit.
Table 1.
List of Primers Used in the Study
| Target amplicon | Forward primer | Reverse primer | Expected size, bp |
|---|---|---|---|
| 5’loxp site | TCTAGGTAATTAGGGCAGGTGC | GCTTCTTGAATCTCTTTCCTAG | 575 |
| 3’loxp site | GACAGCTATACAGAGAAACCCTG | AGCCTCCACATACACAGATAC | 286 |
| Cre | GCCTGCATTACCGGTCGA | TATCCTGGCAGCGATCGC | 440 |
| ERT2 | GCGATCCACGAAATGAAATG | GCAGGTTCATCATGCGGAAC | 501 |
| CnB1 (floxed out) | CAATGCAGTCCGCTGTAGTTC | AGCCTCCACATACACAGATAC | 168 |
The polymerase chain reaction products were separated on a 2% agarose gel and imaged. They were cut out, purified, and sequenced. All sequences were aligned to the National Center for Biotechnology Information database and manually verified to confirm CnB1 deletion and that each component (eg, Ela, Cre, and ERT2) was in frame.
Nuclear Factor of Activated T Cells–Luciferase Activity Assay
Isolated pancreatic acinar cells were infected with nuclear factor of activated T cells–luciferase adenovirus as previously described.10 Briefly, cells were incubated with adenovirus (titer 2 × 109 infectious units) for 30 minutes, and then were exposed to radiocontrast for approximately 6 hours. After stimulation, cells were collected, washed with phosphate-buffered saline once, lysed with 1× lysis buffer (E397A; Promega, Madison, WI), and centrifuged at 12,000 × g for 5 minutes at 4ºC. Luminescence was measured from the supernatant using the Luciferase Assay System (E1483; Promega) in a Synergy H1 plate reader (BioTek, Winooski, VT), and total protein, determined by the BCA kit (Thermo Scientific, Rockford, IL), was used to normalize the data.
Adeno-Associated Virus 6 Constructs
Adeno-associated virus (AAV)6 plasmids were generated by cloning a pEla-iCre or pCMV-ZsGreen control vector into a pAAV-multiple cloning site plasmid (VPK-410; Cell Biolabs, San Diego, CA), as previously described.11, 12 Once cloned, the AAV6 plasmid was transfected into HEK293 cells along with 2 helper plasmids: (1) pAAV-RepCap (0912-06; Applied Viromics, Fremont, CA), which is a packaging plasmid that carries the serotype 6 rep and cap genes; and (2) pHelper (0913; Applied Viromics), which is a plasmid that carries the helper genes. Cells were collected 72 hours after transfection and suspended in lysis buffer containing 50 mmol/L Tris, 150 mmol/L NaCl, and 2 mmol/L MgCl2.
Purification of AAV6 for In Vivo Administration
AAV6 was purified as previously described.12, 13 Briefly, transfected HEK293 cells were freeze-thawed 3 times to release the AAV6 virus. Cell lysates were treated with benzonase (0.05 U) at 37ºC for 30 minutes, followed by 1% sodium deoxycholate at 37ºC for 30 minutes. Lysates were spun at 2500 × g for 10 minutes, and the supernatant was collected. AAV6 was precipitated using a 1:4 mixture of 40% polyethylene glycol (PEG-800) and 2.5 mol/L sodium chloride for 2 hours at 0ºC. The solution was spun at 2500 × g for 30 minutes to collect the PEG pellet. The pellet was resuspended in HEPES buffer (50 mmol/L), treated with an equal volume of 100% chloroform, spun at 2500 × g for 10 minutes, and air-dried for 30 minutes. Two-phase partitioning was performed using 50% ammonium sulfate and 40% PEG-800 and spun at 2500 × g for 15 minutes. The ammonium sulfate phase was collected and dialyzed using a 10-kilodalton molecular weight cut-off Slide-A-Lyser Dialysis Cassette (66810; Thermo Scientific) for 4 hours. Dialysis was repeated a second time for 16 hours. The AAVs were concentrated using a 50-kilodalton centrifugal filter unit (UFC905024; Millipore, Billerica, MA) and stored at -80ºC. The QuickTiter AAV Quantitation Kit (VPK-145; Cell Biolabs) was used to measure viral concentrations.
Pancreatic Ductal Infusion of AAV6 Into CnB1f/f Mice and Immunofluorescence
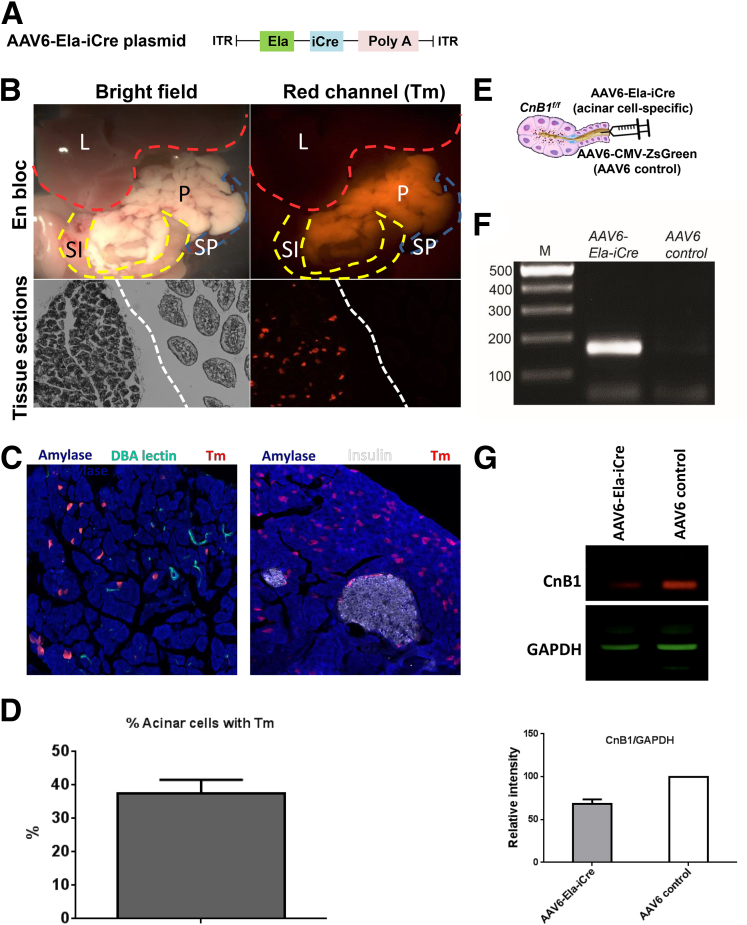
The surgical procedure for retrograde pancreatic ductal infusion of the AAV6 was as previously described.5 Briefly, 100 μL of purified AAV6 (titer 2 × 1012 plaque-forming units) was infused into the biliopancreatic duct at a rate of 10 μL/min for 10 minutes using a P33 peristaltic syringe pump (Harvard Apparatus, Holliston, MA). Surgical anesthesia was achieved by inhaling isoflurane and oxygen. A single injection of the analgesic buprenorphine (0.075 mg/kg) was given immediately after the surgery. Mice recovered on a heating pad for 30 minutes and were housed for 4–6 weeks with free access to food and water before induction of PEP. To verify the efficacy of the AAV6 infusion, LSL-Tm mice were used. One hundred microliters of purified AAV6Ela-iCre (titer 2 × 1012 plaque-forming units) was infused into the pancreatic duct as described earlier. Five weeks after the surgery, pancreas tissue, along with the abdominal organs en bloc, was imaged using a fluorescence dissecting microscope, then sectioned and immunostained. Polyclonal rabbit anti–α-amylase (Sigma) antibody was used for labeling acini, and polyclonal guinea pig anti-insulin (Dako, Carpinteria, CA) antibody was used for islets. Secondary antibodies were purchased from Jackson ImmunoResearch Labs (West Grove, PA). Ducts were labeled with biotinylated Dolichos biflorus agglutinin lectin (Vector Labs, Burlingame, CA). Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (Sigma). Images were acquired using a LSM710 confocal laser scanning microscope (Carl Zeiss, Jena, Germany). The percentage of acinar cells showing red fluorescence was quantified using the image analysis software Volocity (PerkinElmer Inc, Santa Clara, CA), for a total of 18 fields at 200× magnification from 2 independent mouse infusions. Acinar cells were identified by co-staining for amylase and 4′,6-diamidino-2-phenylindole.
Induction of PEP
PEP was induced as previously described.5 Briefly, 100 μL iohexol (Omnipaque 300; GE Healthcare, Princeton, NJ) was infused retrograde into the biliopancreatic duct at a rate of 20 μL/min for 5 minutes. Mice from the low-volume normal saline (LVNS) intraductal infusion group received retrograde infusion of 50 μL normal saline into pancreatic duct at a lower rate of 10 μL/min for 5 minutes. Mice were euthanized 24 hours after PEP induction by CO2 inhalation and cervical dislocation. Mice from the sham group received a laparotomy only. The experiments with the intraductal administration of Cn inhibitors initially were performed in females and then were replicated in both sexes. The 2 complementary conditional knockout experiments each were performed in a single batch, based on the availability of littermates as well as the relatively large volume of AAV required for each mouse infusion. The experiments with the intraductal administration of Cn inhibitors were performed in 3 separate batches of mice.
Serum Amylase and Interleukin 6 Measurements
Blood was collected by retro-orbital bleed 6 hours after PEP induction. Serum was prepared by centrifuging at 1500 × g for 10 minutes at 4ºC. Serum amylase was measured using a Phadebas Kit (Amersham Pharmacia, Rochester, NY), and interleukin (IL)6 was measured using a standard enzyme-linked immunosorbent assay (Biolegend, San Diego, CA).
Pancreatic Histopathology and Image Analysis
The pancreas, duodenum, and spleen were placed en bloc in a cassette to maintain anatomic orientation. The tissues were fixed in 4% paraformaldehyde at room temperature for 24 hours. Paraffin-embedded sections were stained with H&E. Ten systematically selected fields at 200× magnification were graded in a blinded fashion from the head of the pancreas, which was identified by its juxtaposition to the duodenum. The grading score gave equal weight (from 0 to 3) for edema, inflammatory infiltration, and necrosis, as described.14, 15 Edema indices were delineated further objectively by performing intensity thresholding using ImageJ software (National Institutes of Health, Bethesda, MD). At least 5 images from each slide were selected for the analysis. Each image was set to the same color threshold. Labeled areas within the parenchyma were marked as edema, and their surface area was calculated as a percentage of the total parenchymal area.
Immunohistochemistry
Immunohistochemistry for myeloperoxidase (MPO) was performed from paraffin-embedded tissue sections using a Leica Bond-Max Fully Automated immunohistochemistry and in-situ hybridization (ISH) Staining System (Leica, Buffalo Grove, IL) in a semiautomated manner. All of the products for the immunohistochemistry for MPO were purchased from Leica, including the primary antibody. The slides were loaded on the Bond system, and the program was set as follows: deparaffinized using Bond Dewax Solution (#AR9222), dehydrated with alcohol, incubated with MPO (#PA0491; ready-to-use) primary antibody for 15 minutes and with a Bond polymer refine detection kit (#DS9800). The slides were washed automatically using either Bond Wash Solution (#AR9590) or distilled water between each steps. After systematic optimization of the antibody using positive and negative control tissues, the ideal conditions for MPO were with no pretreatment needed, a 15-minute antibody incubation time, followed by 8 minutes post-primary and 8 minutes diaminobenzidine tetrahydrochloride hydrate incubation. Five systematically selected fields at 50× magnification were graded in a blinded fashion from the head of the pancreas. A score from 0 to 3 was used to grade the extent of brown color in each field.
Statistical Analysis
Data were expressed as means ± SEM, unless otherwise specified. Statistical analysis was performed using GraphPad Prism 6 (La Jolla, CA). Comparisons were performed using an unpaired t test. A P value of .05 or less was considered significant.
Results
To delete Cn selectively in pancreatic acinar cells, we crossed a mouse line containing floxed alleles for the critical regulatory subunit B1 (CnB1) with a tamoxifen-inducible Cre line driven by a full-length acinar-specific mouse Ela promoter7 (Figure 1). A mild model of PEP was first induced by infusing LVNS into the biliopancreatic duct. In this model, we found that there was a near-complete reduction in histologic damage among the acinar cell-specific CnB1-deficient mice (CnB1Δ/Δ) (Figure 2). Furthermore, we developed a more severe model of injury that mimics PEP by infusing radiocontrast at double the volume and rate of infusion within the same time frame as the mild model. In this model, we observed that the CnB1Δ/Δ mice also had a marked reduction in histologic damage by 75%, down to the level of the sham-operated negative control arm. Each parameter of the overall histologic score was diminished, including edema, inflammatory infiltrate (additionally examined by MPO staining), and necrosis. Serum IL6 increase also was reduced markedly. These findings indicate that acinar cell Cn mediates PEP in vivo. In addition to the mild and moderate models of PEP, we observed that the acinar cell-specific Cn deletion also protected against a disparate model of acute pancreatitis induced by infusion of the bile acid taurocholate (Figure 3). The findings lend further support to the broad importance of acinar cell Cn in mediating pancreatic injury.
Figure 2.
Acinar cell-specific deletion of Cn in the tamoxifen-induced Ela-CreERT2/CnB1f/f mouse protects against PEP. (A) Timeline for tamoxifen injections, followed by PEP induction. (B) Representative H&E sections of the pancreatic head from sham-operated LVNS intraductal infusion, and PEP modeled conditions, along with overall histologic severity scoring. (C) Subscoring for edema, inflammation, and necrosis from the pancreatic head. (D) Edema, assessed by image thresholding. (E) Serum IL6 and (F) MPO immunohistochemistry staining (left), and quantification (right) (n = 3–4 animals per condition). *,#P < .05 relative to control sham and each positive control, respectively.
Figure 3.
Acinar cell-specific deletion of Cn protects against bile acid infusion pancreatitis in mice. (A) Left: representative H&E sections, right: overall histologic severity. Histologic subscoring for (B) edema, (C) inflammation, and (D) necrosis. (E) Serum IL6 level at 24 hours (n = 3–4 animals per condition). #P < .05 relative to each positive control.
We complemented the breeding strategy for acinar cell Cn knockouts by generating an AAV6 vector, which houses an enhanced version of Cre (iCre)16 that is driven by a shorter, independently constructed rat Ela promoter17 (Figure 4). Among serotypes, AAV6, along with AAV8, offer the highest infection efficiency into acinar cells.12, 18 As proof of principle for targeting acinar cells, the AAV6-Ela-iCre induced acinar cell fluorescence in LSL-Tm mice. Immunostaining confirmed that the activation of iCre is restricted largely to acinar cells, but not ducts and islets. The percentage of acinar cells showing red fluorescence was 37% ± 3.88% and Western blot from the head of the pancreas showed that CnB1 expression was reduced by 33% (Figure 4). Despite the modest reduction in Cn, these mice were protected against PEP (Figure 5).
Figure 4.
Intraductal infusion of a novel AAV6-Ela-iCre construct targets the pancreatic acinar cell. (A) Schema of the AAV6-Ela-iCre plasmid, (B) LSL-Tm mice were infused with AAV6-Ela-iCre to examine the depth of Cre expression in acinar cells and acinar cell specificity. Red fluorescence was observed in the pancreas and excluded from neighboring organs such as the small intestine (SI), liver (L), and spleen (Sp). In the tissue sections, the dashed white line separates pancreas (left) from duodenum (right). (C) Immunofluorescence from pancreatic sections for acinar cells (amylase; blue), duct cells (Dolichos biflorus agglutinin lectin; green), and islets (insulin; white) in LSL-Tm mice. (D) Quantification of the percentage of acinar cells showing red fluorescence. (E) Schema for intraductal infusion of AAV6-Ela-iCre into CnB1f/f mice to delete Cn. (F) Expected polymerase chain reaction product of 168 bp seen in the pancreas from AAV6-Ela-iCre–infused mice, but not with AAV6 control infusion. (G) Western blot for CnB1 on top and densitometry at bottom with error bar representing SD. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. ITR, inverted terminal repeat.
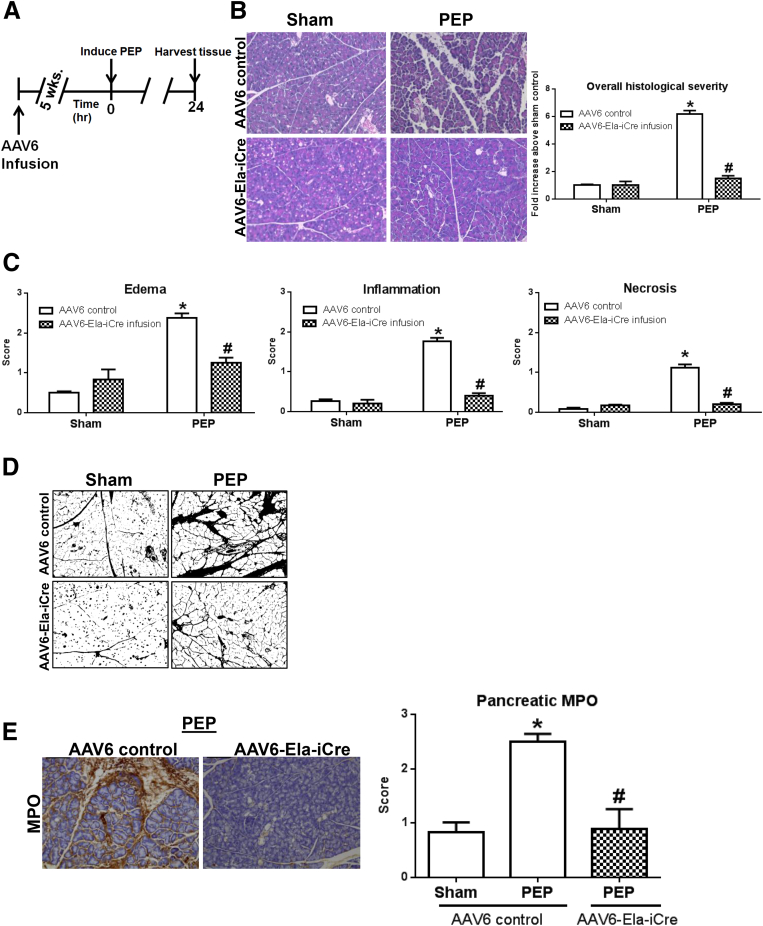
Figure 5.
Acinar cell-specific deletion of Cn by AAV6-Ela-iCre in CnB1f/f mouse protects against PEP. (A) Timeline for AAV6 infusion, followed by PEP induction. (B) Representative H&E sections from sham control and PEP conditions, along with overall histologic severity scoring. (C) Subscoring for edema, inflammation, and necrosis from the pancreatic head. (D) Edema, assessed by image thresholding. (E) MPO immunohistochemistry staining (left) and quantification (right) (n = 3–4 animals per condition). *,#P < .05 relative to control sham and each positive control, respectively.
Systemic inhibition of Cn with administration of multiple doses of Cn inhibitors before and after PEP induction was shown previously to protect against PEP.5 However, the current findings, with the 2 genetic Cn deletion models, that acinar cell Cn in vivo is necessary for PEP prompted us to question whether selectively targeting acinar cell Cn activity by administering a single, acute dose of Cn inhibitor, along with the radiocontrast infusion, could mitigate PEP. This unique compartmentalized method of delivery of a small amount of drug would additionally reduce the toxicity profile of the inhibitors. FK506 (1 μmol/L) and CsA (10 μmol/L) each were dissolved easily in the ready-to-use iohexol formulation, and the concentrations of each were chosen based on our previous ex vivo data.5 In contrast to the finding with the CnB1 conditional knockouts (ie, the CnB1Δ/Δ line), intraductal FK506 or CsA therapy did not affect the mild histologic damage seen with LVNS (Figure 6). This differential response likely represents incomplete Cn blockade with the current dosing schema. However, this pharmacologic intervention reduced the severity in the moderate model of PEP by 61% and 37% down to sham levels, respectively. Both serum IL6 and amylase levels also were reduced significantly.
Figure 6.
Intraductal (ID) administration of Cn inhibitors along with the radiocontrast infusion prevents PEP. (A) Representative H&E sections from sham-operated, LVNS, and PEP modeled conditions. The Cn inhibitors FK506 (1 μmol/L) or CsA (10 μmol/L) were delivered intraductally with the radiocontrast solution. (B) Overall histologic severity scoring. (C) Subscoring for edema, inflammation, and necrosis. (D) Serum IL6 level also at 24 hours and (E) serum amylase level at 6 hours (n = 3–10 animals per condition). *,#P < .05 relative to the control sham and each positive control, respectively.
Discussion
In summary, using 2 complementary genetic approaches to delete acinar cell Cn in vivo and in 2 severity models of PEP in mice, as well as a bile infusion model of pancreatitis, we show that PEP and pancreatitis can be largely prevented by acinar cell Cn deletion. Notably, even a partial deletion of acinar Cn (by approximately one third with the intraductal AAV strategy) led to marked protection against PEP. The translational corollary to these significant findings is that intraductal delivery of Cn inhibitors, to target acinar cell Cn in vivo, also was shown to reduce PEP. These novel findings reconcile the paradox that chronic and systemic administration of Cn inhibitors could predispose to pancreatitis and pancreatic fibrosis,19, 20 whereas acute and targeted delivery to the pancreas protects against pancreatitis. This work provides the impetus for launching clinical trials to test the efficacy of a novel ERCP infusion formulation containing Cn inhibitors to prevent PEP.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by National Institutes of Health grants DK093491 and DK103002 (S.Z.H.).
References
- 1.Maranki J., Yeaton P. Prevention of post-ERCP pancreatitis. Curr Gastroenterol Rep. 2013;15:352. doi: 10.1007/s11894-013-0352-2. [DOI] [PubMed] [Google Scholar]
- 2.Elmunzer B.J., Scheiman J.M., Lehman G.A. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levenick J.M., Gordon S.R., Fadden L.L. Rectal indomethacin does not prevent post-ERCP pancreatitis in consecutive patients. Gastroenterology. 2016;150:911–917. doi: 10.1053/j.gastro.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman M.L., Kozarek R.A. Take 2 indomethacin (suppositories) and call me in the morning? The role of nonsteroidal anti-inflammatory drugs in protection against post-endoscopic retrograde cholangiopancreatography pancreatitis. Gastroenterology. 2016;150:805–808. doi: 10.1053/j.gastro.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 5.Jin S., Orabi A.I., Le T. Exposure to radiocontrast agents induces pancreatic inflammation by activation of nuclear factor-kappaB, calcium signaling, and calcineurin. Gastroenterology. 2015;149:753–764 e11. doi: 10.1053/j.gastro.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heit J.J., Apelqvist A.A., Gu X. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 7.Ji B., Song J., Tsou L. Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis. 2008;46:390–395. doi: 10.1002/dvg.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madisen L., Zwingman T.A., Sunkin S.M. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss W.M. Preparation of genomic DNA from mammalian tissue. Curr Protoc Mol Biol. 2001;2:2. doi: 10.1002/0471142727.mb0202s42. [DOI] [PubMed] [Google Scholar]
- 10.Muili K.A., Wang D., Orabi A.I. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem. 2013;288:570–580. doi: 10.1074/jbc.M112.428896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo P., Xiao X., El-Gohary Y. Specific transduction and labeling of pancreatic ducts by targeted recombinant viral infusion into mouse pancreatic ducts. Lab Invest. 2013;93:1241–1253. doi: 10.1038/labinvest.2013.113. [DOI] [PubMed] [Google Scholar]
- 12.Orabi A.I., Sah S., Javed T.A. Dynamic imaging of pancreatic nuclear factor kappaB (NF-kappaB) activation in live mice using adeno-associated virus (AAV) infusion and bioluminescence. J Biol Chem. 2015;290:11309–11320. doi: 10.1074/jbc.M115.647933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao X., Guo P., Prasadan K. Pancreatic cell tracing, lineage tagging and targeted genetic manipulations in multiple cell types using pancreatic ductal infusion of adeno-associated viral vectors and/or cell-tagging dyes. Nat Protoc. 2014;9:2719–2724. doi: 10.1038/nprot.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wildi S., Kleeff J., Mayerle J. Suppression of transforming growth factor beta signalling aborts caerulein induced pancreatitis and eliminates restricted stimulation at high caerulein concentrations. Gut. 2007;56:685–692. doi: 10.1136/gut.2006.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sendler M., Mayerle J., Lerch M.M. Necrosis, apoptosis, necroptosis, pyroptosis: it matters how acinar cells die during pancreatitis. Cell Mol Gastroenterol Hepatol. 2016;2:407. doi: 10.1016/j.jcmgh.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sztal T.E., Zhao M., Williams C. Zebrafish models for nemaline myopathy reveal a spectrum of nemaline bodies contributing to reduced muscle function. Acta Neuropathol. 2015;130:389–406. doi: 10.1007/s00401-015-1430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swift G.H., Craik C.S., Stary S.J. Structure of the two related elastase genes expressed in the rat pancreas. J Biol Chem. 1984;259:14271–14278. [PubMed] [Google Scholar]
- 18.Wang Z., Zhu T., Rehman K.K. Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes. 2006;55:875–884. doi: 10.2337/diabetes.55.04.06.db05-0927. [DOI] [PubMed] [Google Scholar]
- 19.Echigo Y., Inoue K., Kogire M. Effects of cyclosporine and tacrolimus (FK 506) on acute pancreatitis in mice. Arch Surg. 1995;130:64–68. doi: 10.1001/archsurg.1995.01430010066013. [DOI] [PubMed] [Google Scholar]
- 20.Vaquero E., Molero X., Tian X. Myofibroblast proliferation, fibrosis, and defective pancreatic repair induced by cyclosporin in rats. Gut. 1999;45:269–277. doi: 10.1136/gut.45.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]