Abstract
Background
Rates of young adult cannabis use are rising, perceived harm is at its historical nadir, and most users do not want to quit. Most studies evaluating effects of cannabis use in young adults are cross-sectional, limiting causal inference. A method to reliably induce abstinence periods in cannabis users would allow assessment of the effects of abstinence and resumption of use on a variety of outcomes in a within subjects, repeated measure design.
Methods
We examined the efficacy and feasibility of a voucher-based contingency management procedure for incentivizing one month of continuous cannabis abstinence among young adults who reported at least weekly cannabis use, volunteered to participate in a laboratory study, and did not express desire to discontinue cannabis use long-term. Continuous cannabis abstinence was reinforced with an escalating incentive schedule, and self-report of abstinence was confirmed by frequent quantitative assays of urine cannabis metabolite (THCCOOH) concentration. New cannabis use during the abstinence period was determined using an established algorithm of change in creatinine-adjusted cannabis metabolite concentration between study visits.
Results
Thirty-eight young adults, aged 18–25, enrolled and 34 (89.5%) attained biochemically confirmed 30-day abstinence. Among those who attained abstinence, 93.9% resumed regular use within two-weeks of incentive discontinuation.
Conclusion
Findings support the feasibility and efficacy of contingency management to elicit short-term, continuous cannabis abstinence among young adult, non-treatment-seeking, regular cannabis users. Further work should test the effectiveness of this CM procedure for cannabis abstinence periods longer than one month, which may be required to evaluate some effects of abstinence.
Keywords: Contingency management, cannabis, marijuana, young adults, abstinence, methodology
1. INTRODUCTION
Cannabis is the most commonly used substance other than alcohol among young adults in the United States (Johnston et al., 2015; SAMHSA, 2014), with nearly 20% of young adults report using cannabis currently. Widespread policy changes regarding legal access to medical and non-medical cannabis are expected to increase rates of use further. This is concerning given that ongoing brain maturation occurring well into the third decade of life (Giedd et al., 1999) may increase vulnerability to negative consequences associated with regular cannabis exposure. Indeed, frequent use during this developmental period is the best predictor of persistent use in adulthood (Chen and Kandel, 1995; SAMHSA, 2014) and adverse cognitive (Gruber et al., 2012; Jacobus and Tapert, 2014; Lisdahl and Price, 2012; Lisdahl et al., 2014; Solowij et al., 2011), psychosocial (Chadwick et al., 2013; Degenhardt et al., 2013; Hall, 2014; Marmorstein and Iacono, 2011; Palamar et al., 2014), psychiatric (Di Forti et al., 2015; Volkow et al., 2016), substance use (Agrawal et al., 2004; Fergusson et al., 2006; Hurd et al., 2014; Lynskey et al., 2003; Swift et al., 2012), and academic outcomes (Ellickson et al., 2004; Fergusson and Boden, 2008; Maggs et al., 2015; Meier et al., 2015).
Though strong associations have been reported between frequent young adult cannabis exposure and negative outcomes, most studies of the effects of cannabis use are cross sectional, which impedes the ability to draw conclusions about causal effects of cannabis use. Prospective longitudinal trials and experimental manipulations are the gold-standard for determining causality. The former is challenging due to cost and time requirements if initial assessments are to occur before exposure and retains biases associated with the decision to initiate cannabis use prior to adulthood or not, and the latter poses ethical concerns for cannabis-naïve participants. A reliable method that would allow examination of change in clinical, cognitive or other measures in response to cannabis abstinence and resumption of use among young adults who regularly use cannabis may represent a viable strategy to study reversible effects of cannabis on outcomes of interest in this important population.
Contingency management (CM) may be an ideally suited approach for studying the potential consequences of cannabis use. Abstinence-based CM was developed according to basic tenets of behavior analysis and operant conditioning (Budney et al., 2001; Higgins and Petry, 1999; Meredith et al., 2014; Petry, 2000; Stanger and Budney, 2010): reinforcing behaviors increases the likelihood that they will recur (Skinner, 1969). From this perspective, addictive substance use is a learned behavior that is reinforced by desirable drug effects. Abstinence-based CM seeks to alter learned substance use behavior with provision of consistent, competing positive reinforcement (e.g., monetary rewards) for verified abstinence (Bigelow et al., 1981).
CM has well-established efficacy for changing substance use behavior (Higgins et al., 1991; Krishnan-Sarin et al., 2013; Petry et al., 2000; Reynolds et al., 2008; Stitzer et al., 1986), specifically cannabis use among treatment-seeking adults for total days abstinent (Kadden et al., 2007; Litt et al., 2013) and longer duration of continuous abstinence (Budney et al., 2000, 2006; Cooper et al., 2015; Copeland and Swift, 2009; Litt et al., 2013). CM is also efficacious for treatment-seeking adolescent (Kamon et al., 2005; Stanger et al., 2009, 2015; Stewart et al., 2015) and young adult cannabis users (Carroll et al., 2006; Montgomery et al., 2012), particularly when coupled with other psychosocial interventions such as cognitive behavioral therapy (CBT), motivational enhancement therapy (MET), and family therapy (Carroll et al., 2006; Kamon et al., 2005; Stanger et al., 2009, 2015; Stewart et al., 2015). However, participants in previous studies were seeking treatment (Kamon et al., 2005; Stanger et al., 2009) and/or met criteria for a cannabis use disorder (Carroll et al., 2006; Stanger et al., 2015). Although participants in prior trials were not necessarily motivated to stop using cannabis (e.g., Stewart et al., 2015), past findings may still only generalize to the most severely impacted young adult cannabis users given that most individuals do no seek treatment for cannabis use until after age 25 (SAMHSA, 2015), It is not known whether CM can be used to effectively induce a period of continuous cannabis abstinence in young adults who do not use cannabis daily, are not seeking treatment, but who nonetheless may be experiencing reversible effects of cannabis. The purpose of this study was to develop a method to reliably attain abstinence in young adult regular cannabis users for one month so that future studies can assess brain, cognitive, behavioral, and mood changes during four consecutive weeks of abstinence and after resumption of use, using a prospective, within-subject design that would allow more definitive conclusions regarding potential effects of abstinence in this important population.
2. METHODS
This study was conducted between July and November, 2015. All enrolled participants gave written informed consent to a protocol approved by the Partners’ Human Subjects Review Committee.
2.1 Participants
Eligible participants were young adults, aged 18–25, who reported using cannabis at least weekly. They were recruited via peer referral and advertisements in the community that sought potential participants ‘who use marijuana and are between age 18 and 25′. Inclusion criteria included cannabis use in the week prior to the phone screen, English fluency, and willingness to stop using cannabis for 30 days and attend eight study visits over six weeks at the Massachusetts General Hospital (MGH).
2.2 Assessments of Participant Mood and Substance Use
At baseline, current and lifetime diagnoses of Axis I disorders were assessed with the Structured Clinical Interview for DSM-IV (SCID-IV), mood was assessed with the Mood and Anxiety Symptoms Questionnaire (MASQ; Watson et al., 1995), and current and lifetime symptoms of Attention Deficit/Hyperactivity Disorder (ADHD) were assessed with a DSM-IV symptom checklist. At baseline, substance use disorders were assessed using the SCID-IV, the Cannabis Use Disorder Identification Test – Revised (CUDIT-R; Adamson and Sellman, 2003) and Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993). Amount and frequency of substance use was assessed at every study visit using a modified Timeline Follow-Back method (Robinson et al., 2014). Cannabis withdrawal was assessed at every visit using the Cannabis Withdrawal Scale (CWS; Allsop et al., 2011).
2.3 Assessment of Cannabis Metabolites
Participants provided urine specimens at each session. Urine samples were shipped overnight to Dominion Diagnostics (Kingstown, RI, USA) to quantify 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) levels, a non-psychoactive metabolite of Δ9-tetrahydrocannabinol (THC) and the standard urine biomarker for cannabis use, using liquid chromatography/tandem mass spectrometry (LC-MS/MS). The lower limit of quantification (LOQ) was 5ng/mL and the maximum value quantified by the LC-MS/MS method was 500ng/mL. Samples with THCCOOH values ≥500ng/mL were further analyzed for THC using enzyme immunoassay (EIA), which had an upper LOQ of ≥900ng/mL.
Urine creatinine concentration was quantified and used to correct THCCOOH concentration for individual differences in hydration (Lafolie et al., 1991). The THCCOOH to creatinine concentrations ratio (CN-THCCOOH) was calculated by dividing the urinary THCCOOH concentration (ng/mL) by urine creatinine concentration (mg/mL), yielding ng THCCOOH/mg creatinine.
2.4 Contingency Management (CM) Procedure
The CM procedure consisted of a four-week abstinence-based incentive program consisting of seven study visits over 4 weeks, and a two-week follow-up visit. The first four visits occurred in the first week of the study, followed by three weekly visits. See Figure 1 for detailed visit schedule. In order to better ensure initial abstinence requirements were being met, more frequent visits were conducted during Week 1. This allowed for more frequent quantitative assessment of cannabinoid metabolites during early abstinence when CN-THCCOOH reductions are greatest (Budney et al., 2003). More frequent testing initially helps differentiate abstinence from reduction in severity of use and also provides better reinforcement of initial abstinence behavior (Schwilke et al., 2011; Stitzer and Vandrey, 2008). At the baseline visit, all participants completed an abstinence contract with a study staff member that clearly delineated the behavior to be monitored, schedule of monitoring, and contingencies (Budney and Higgins, 1998; Petry, 2000). Participants were then instructed to refrain from using cannabis for the next month (Visits 2–7). After Visit 7, abstinence was no longer reinforced, and participants were assessed two weeks later (Visit 8). Participants were asked to refrain from using illicit drugs and alcohol on the day of study visits.
Figure 1.
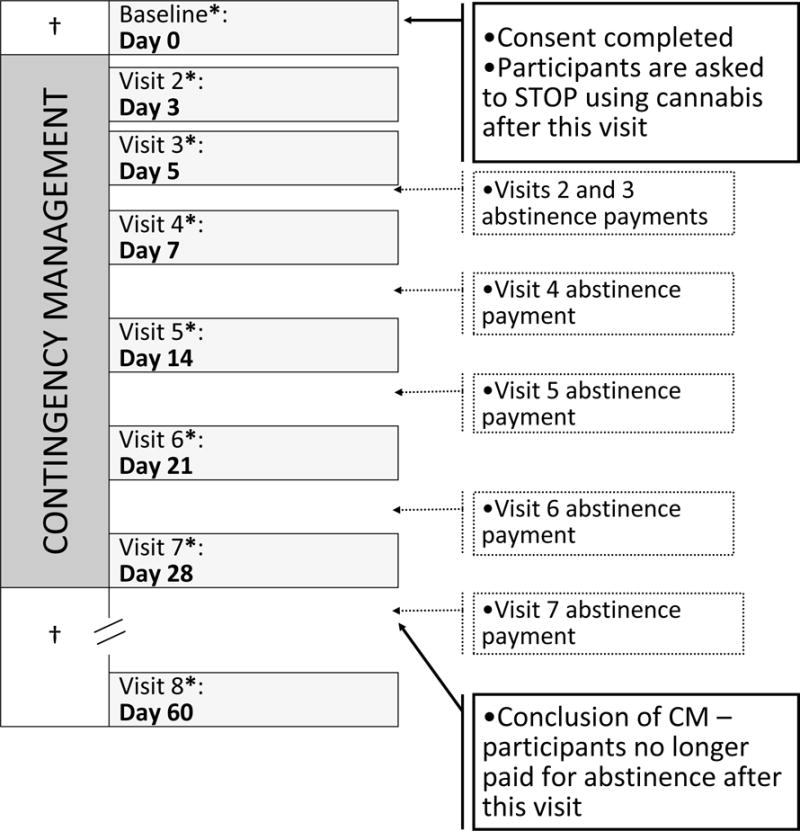
Participants were assessed 8 times over the course of approximately six weeks. Abstinence was reinforced between Visits 2 and 7. Visits 4, 5, 6, 7, and 8 had a scheduling window of ±2 days
†Participants using cannabis as usual
* Delivery of attendance payments
Participants earned incentives based on a two-track system for attendance and abstinence. Please see Table 1 for incentive schedule. The value of incentives for both attendance and abstinence escalated incrementally to encourage both study retention and achievement of longer periods of continuous abstinence (Roll and Howard, 2008; Roll and Shoptaw, 2006). Participants were told that they would revert to the initial attendance payment level ($5) following a missed study visit and that they would be discontinued from the study if abstinence was not confirmed. At the two-week follow-up visit (Visit 8), participants were compensated for attendance only. Participants abstinent for 30 days with full attendance earned $585 ($405 for continuous abstinence and $180 for full attendance). Incentives were provided on reloadable credit cards through Clinical Trials Payer (CT Payer), a secure web-based platform that facilitates HIPAA and HITECH safe clinical trial and study-related payments. Incentives for attendance were made available for use 15 minutes after each study visit. Incentives for abstinence were provided upon receipt of the quantitative urinalysis results (described below), typically about four days after specimen collection. Incentive payments for verified abstinence were added to the credit cards remotely between study visits (see Figure 1). Study staff called participants as soon as lab results were received to inform them of the results and the value added to their cards.
Table 1.
Contingency Management Payment Schedule
| Visit Number | Attendance | Abstinence | |
|---|---|---|---|
| 1 | Baseline | $5 | – |
| 2 | 3 days | $10 | $30 |
| 3 | 4 days | $15 | $45 |
| 4 | 1 week | $20 | $60 |
| 5 | 2 weeks | $25 | $75 |
| 6 | 3 weeks | $30 | $90 |
| 7 | 4 weeks | $35 | $105 |
| 8 | 2 week follow-up | $40 | – |
| Max Subtotal | $180 | $405 | |
| Max Total | $585 | ||
2.5 Determination of Cannabis Abstinence at Each Study Visit
Residual cannabinoid excretion was differentiated from new cannabis exposure using a statistical model developed by Schwilke and colleagues (2011). This model was empirically derived from urine CN-THCCOOH concentration ratios of consecutively collected specimen pairs (current specimen/prior specimen). This model takes into account the time between collection of specimens, which enhances the accuracy of prediction of new cannabis use (Smith et al., 2009). This formula yields an expected CN-THCCOOH ratio associated with specimen pairs during abstinence, and observed ratios that exceed this expected value are interpreted as new cannabis use.
Model parameters are specific to the CN-THCCOOH concentration of the first specimen collected in each pair as well as the desired level of specificity. For this study, we used a 95% level of certainty, which allows for a 2.5% probability that an observed CN-THCCOOH concentration ratio of specimen pairs would exceed the expected ratio and be falsely interpreted as new cannabis use (see supplementary information1 for additional model details and an example application of this formula in the current study). As sensitivity analyses, new use determinations with 80, 90, and 99% specificity are reported. New use determinations are also reported with the cutoff of 0.5 and 1.5 ratio in specimen pairs, widely used methods for determining new cannabis use in non-daily cannabis users originally recommended by Huestis and colleagues (1998). The former cutoff for new use (0.5) yields very low false positive rates, and the latter (1.5) allows some increase in metabolite concentration that could be consistent with no new use.
2.6 Data Analysis
All statistical analyses were performed using Stata 13.1. The ratio of CN-THCCOOH concentrations of each consecutive specimen pair was the primary outcome measure, and was used to assess cannabis abstinence for each participant at each time point. Specimens in which the THCCOOH concentration exceeded the upper LOQ could not be adjusted for creatinine and were excluded from analyses. When possible, the THCCOOH concentration from Visit 2 or Visit 3 was substituted for baseline THCCOOH concentrations that exceeded the upper LOQ. To determine change in CN-THCCOOH across 30 days of abstinence, a non-parametric Friedman test of differences among repeated measures was conducted, with pairwise comparisons evaluated using Wilcoxon Signed Ranks Test. Alpha was set at 0.05 for all statistical tests.
3. RESULTS
3.1 Participants
Fifty-six people were screened for participation and 38 were enrolled. No subject failed the screen due to unwillingness to stop using cannabis for 30 days. Seven declined to enroll due to not wanting to commit to eight study visits; four did not meet current substance use criteria; three no longer lived in the greater Boston area; and four were older than 25 years of age. Participant age range was 18 to 25, a majority was in or had completed some college, and nearly half had a lifetime psychiatric diagnosis. See Table 2. Participants reported an average age of onset of cannabis use at least once per week on most weeks of 17.7 years, and an average use of cannabis on 19.7 of the last 30 days. Over 58% met criteria for possible Cannabis Use Disorder on the CUDIT-R. See Table 3 for participant substance use characteristics.
Table 2.
Baseline Participant Characteristics
| Baseline Participant Characteristics (N=38) | |
|---|---|
| Demographics | |
| Sex, % Male | 57.9% |
| Age | 21.1 (1.7) (range: 18–25) |
| Ethnicity, % Hispanic or Latino | 13.2% |
| Race | |
| % White | 68.4% |
| % Black | 13.2% |
| % Other | 18.4% |
| Highest Level of Education | |
| % High school diploma or less | 5.3% |
| % Some college/in college currently | 73.7% |
| % 4-year college diploma or more | 21.0% |
| Intellectual/Academic Functioning | |
| Predicted full scale IQ (WTAR) | 106.1 (9.2) |
| Grade point average (on a 4.0 scale) | 3.2 (0.5) |
| Psychopathology | |
| % Lifetime SCID-IV diagnosis (excluding substance use) | 48.6% |
| Major Depressive Disorder: | |
| % Lifetime | 45.9% |
| % Current | 5.4% |
| Anxiety disorder (GAD, PTSD, Panic, Phobia) | |
| % Lifetime | 21.6% |
| % Current | 16.2% |
| Bipolar Disorder | |
| % Lifetime | 2.7% |
| % Current | 2.7% |
| Eating Disorder | |
| % Lifetime | 2.7% |
| % Current | 2.7% |
| % ADHD (Self-Report) | 23.7% |
| Concomitant Psychotropic Medications | |
| % Currently taking any psychotropic medication | 26.3% |
| % currently taking antidepressants | 7.9% |
| % currently taking anxiolytics | 2.6% |
| % currently taking mood stabilizers | 5.3% |
| % currently taking stimulants | 10.5% |
| Psychiatric Symptoms | |
| Childhood ADHD symptoms (max=9)* | |
| Inattention | 3.3 (2.8) |
| Hyperactivity | 4.2 (2.9) |
| Current ADHD symptoms (max=9)* | |
| Inattention | 3.6 (2.8) |
| Hyperactivity | 3.7 (2.6) |
| MASQ | |
| General Distress Anxious Symptoms | 21.7 (7.2) |
| Anxious Arousal | 25.2 (7.7) |
| General Distress Depressive Symptoms | 25.3 (9.9) |
| Anhedonic Depression | 57.1 (13.5) |
A score of ≥5 indicates symptoms consistent with ADHD
Table 3.
Participant Substance Use Characteristics
| Participant Substance Use Characteristics (N=38) | |
|---|---|
| Marijuana (MJ) Use | |
| Age of first use | 15.9 (2) |
| Age of regular use* | 17.7 (2.1) |
| Recency of last MJ use (in days) | 1.9 (2.7) |
| Frequency of use | |
| Days used in past 90 days | 53.6 (22.0) |
| Days used in past 30 days | 19.7 (7.5) |
| Times used in past 90 days | 103 [50, 164] |
| Times used in past 30 days | 42.5 (32.6) |
| Amount of use | |
| Grams consumed in past 90 days | 28.3 [11.3, 78.7] |
| Grams consumed in past 30 days | 11.3 [2.6, 31.3] |
| Severity of use | |
| MJ Use Disorder (SCID-IV) | |
| % Lifetime Abuse | 45.9% |
| % Current Abuse | 29.7% |
| % Lifetime Dependence | 29.7% |
| % Current Dependence | 18.9% |
| Baseline CUDIT | 14.7 (6.2) |
| % ≥ 8 | 89.5% |
| % ≥ 12 | 58.3% |
| Withdrawal | |
| Baseline | |
| Intensity | 29.5 [15, 49.75] |
| Negative Impact | 33 [22, 48.5] |
| Change from Baseline to Week 1 | |
| Intensity | −1 [−11, 5] |
| Negative Impact | 0 [−9, 3] |
| Change from Baseline to Week 4 | |
| Intensity | −3 [−24.5, 6] |
| Negative Impact | 0 [−16.5, 4] |
| Alcohol Use | |
| % Ever used | 100% |
| Age of 1st use | 15.5 (2) |
| Recency of last use | |
| % drank in last week | 89.5% |
| % drank in last month | 94.7% |
| % drank in last year | 100% |
| Binge drinking episodes | 20 [2.0, 62.5] |
| Frequency of use | |
| Days drank in past 90 days | 25.1 (14.1) |
| Days drank in past 30 days | 8.4 (5.0) |
| Amount of use | |
| Standard drink equivalents in past 90 days | 81.8 [54, 152.5] |
| Standard drink equivalents in past 30 days | 30.0 [12.0, 60.5] |
| Severity of use | |
| Alcohol Use Disorder (SCID-IV) | 10.8% |
| % Lifetime Abuse | 0% |
| % Current Abuse | 10.8% |
| % Lifetime Dependence | 0% |
| % Current Dependence | 9.0 (6.3) |
| Baseline AUDIT | 36.8% |
| % ≥ 8 | 18.4% |
| % ≥ 13 (female) or ≥ 15 (male) | |
| Nicotine Use† | |
| % Ever used | 89.5% |
| Age of 1st use | 17 (2.5) |
| Recency of use | |
| % smoked in last week | 47.4% |
| % smoked in last month | 63.2% |
| % smoked in last year | 81.6% |
| Frequency of use | |
| Days smoked in past 90 days | 2.5 [0, 15.75] |
| Days smoked in past 30 days | 1 [0, 4.5] |
| Times smoked in past 90 days | 2.5 [0, 27.5] |
| Times smoked in past 30 days | 1 [0, 7.0] |
| Amount of use | |
| Cigarette equivalents in past 90 days | 2.5 [0, 70.5] |
| Cigarette equivalents in past 30 days | 1 [0, 18.0] |
| Other Drug Use | |
| % use of any drug >10 times | 31.6% |
| Recency of use | |
| % use of any drug in past 30 days | 26.3% |
| % use of any drug in past 90 days | 68.4% |
| Severity of use | |
| Other Drug Use Disorder (SCID-IV) | |
| % Lifetime Abuse | 5.4% |
| % Current Abuse | 0% |
| % Lifetime Dependence | 2.7% |
| % Current Dependence | 0% |
Data available only for 26 participants.
Includes hookah and mulling
3.2 Abstinence Outcomes
Of the total number of urine specimens collected (N = 293), 8.5% were above the upper LOQ of 500ng/mL (10 samples at Visit 1, five samples at Visit 2, four samples at Visit 3, two samples at Visit 4, and four samples at Visit 8). There was wide inter-subject variability in the CN-THCCOOH concentrations in the first urine specimen. After removal of the samples with the greatest THCCOOH concentrations (>500ng/mL), the 73% of participants with quantifiable baseline THCCOOH concentrations had a median CN-THCCOOH concentration of 29.4ng/mL (IQR= 17.6, 85.6).
Thirty-day abstinence was confirmed in 89.5% of the sample (n = 34 of 38 enrolled), with 95% certainty (Schwilke et al., 2011). See Table 4. These participants completed all seven visits of the active CM protocol. Table 4 also highlights the percent new-use determination in this sample according to alternative upper prediction limits stipulated by Schwilke and colleagues (2011) and Huestis and Cone (1998). Of the four participants who did not achieve 30 days of abstinence, two were lost to follow-up (one after Visit 2 and one after Visit 6, and thus presumed to be non-abstinent), and two reported using cannabis one time between Visits 5 and 6 and were accurately classified as non-abstinent by the Schwilke algorithm at 80%, 90% and 95% accuracy as well as by the Huestis 0.5 and 1.5 ratio guidelines.
Table 4.
Percent New-Use Determinations Exceeding the Upper Prediction Limits Proposed by Huestis et al., 1998 and Schwilke et al., 2011
| Used in Study | Sensitivity Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Specimen Pairs | N† | 95% PI | 80% PI | 90% PI | 99% PI | 0.5 Ratio | 1.5 Ratio |
| Wk 1/Baseline | 34 | 0 | 0 | 0 | 0 | 0 | 5.9 |
| Wk 2/Wk 1 | 35 | 0 | 0 | 0 | 0 | 2.9 | 22.9 |
| Wk 3/Wk 2†† | 37 | 5.4 | 8.1 | 5.4 | 2.8 | 5.4 | 35.1 |
| Wk 4/Wk 3 | 34 | 0 | 0 | 0 | 0 | 0 | 25.7 |
Note.
The number of excluded pairs was as follows: Week 1/Baseline (3 pairs excluded because at least one specimen exceeded the LOQ); Week 2/Week 1 (2 pairs excluded because at least one specimen exceeded the LOQ)
Two participants reported resuming cannabis use after the Week 2 visit, and were therefore not included in subsequent data points.
Thirty-day abstinence with CM was associated with reduction in consecutive CN-THCCOOH concentrations (X2 = 83.9, p<0.0001). All pairwise comparisons between CN-THCCOOH values were significant (p-values < 0.0007), with the exception of Visit 6 and Visit 7 (p = 0.12) which were both close to zero. See Figure 2.
Figure 2.
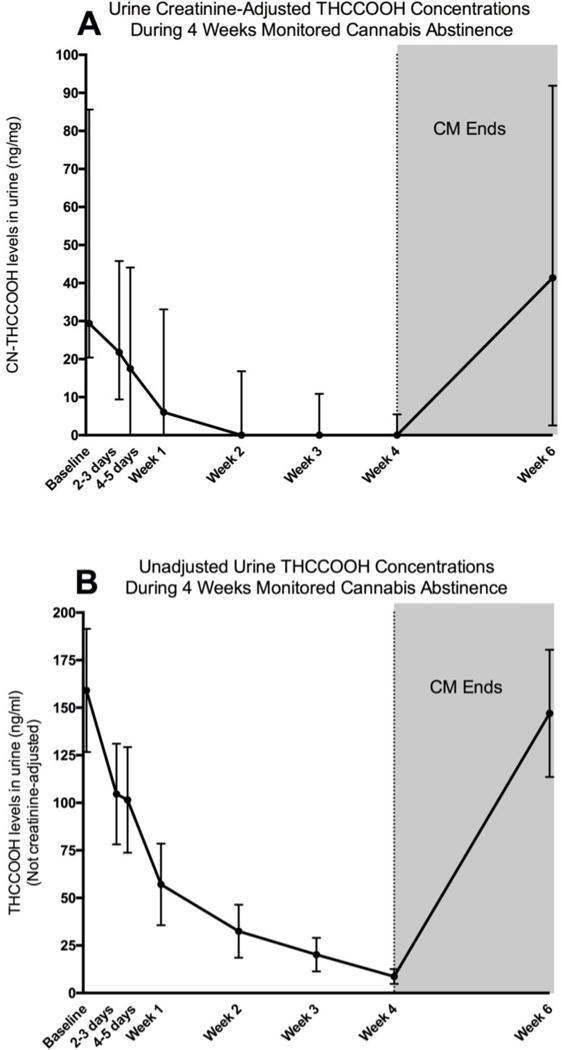
A. Urine creatinine-adjusted THCCOOH concentrations declined during four weeks of monitored cannabis abstinence. All values represent medians and interquartile ranges. Values are only presented for the participants who provided urine samples with THCCOOH values under the limit of detection (500ng/mL). B. Unadjusted THCCOOH concentrations declined during four weeks of monitored cannabis abstinence. All values represent means and standard errors. Values are coded as “501” for participants with urine THCCOOH values over the limit of detection (500ng/mL).
3.3 Resumption of Cannabis Use Two-Weeks Following End of CM
Among the 34 30-day abstinent participants, 97.1% (n=33) completed the two-week follow-up assessment (Visit 8), at which time 93.9% (n=31) had resumed using cannabis. CN-THCCOOH values at this visit were comparable to baseline levels among those who resumed use and whose THCCOOH levels were quantifiable (Baseline: Med=44.2, IQR= 20.4, 85.6; 2-Week Follow-Up: Med=43.9, IQR= 3.4, 99.9). See Figure 2.
4. DISCUSSION
This study aimed to examine the effectiveness of a modified CM approach for four weeks of continuous cannabis abstinence in non-treatment seeking young adults with a range of cannabis dependence scores. Here we show that CM can be used to induce short-term cannabis abstinence in non-treatment seeking young adults who report using cannabis weekly or more. This CM manipulation may serve as a useful methodological approach for examining the effects of cannabis abstinence on various brain, cognitive, behavior and psychiatric outcomes, which are more commonly investigated using cross sectional designs in this population. Policy changes have made investigation of the effect of cannabis use on these outcomes urgent, particularly among adolescents and young adults who may be differentially impacted by early cannabis exposure due to ongoing brain development.
With nearly 90% of the sample able to achieve 30-day cannabis abstinence, this study provides strong preliminary support for the feasibility of this CM protocol to study abstinence associated change in outcomes of interest. This high rate of abstinence is consistent with prior studies demonstrating the efficacy of CM in treatment-seeking and cannabis-dependent populations (Carroll et al., 2006; Kamon et al., 2005; Stanger et al., 2009, 2015). Young adulthood is a critical time to examine the effects of cannabis given that this is the peak developmental period for use (1 in 5 18 to 25 year olds are current cannabis users; SAMHSA, 2014) and use during this time is the best predictor of related problems (Chen and Kandel, 1995). Further, this study supports the efficacy of CM among cannabis users with psychiatric co-morbidities, as 48.6% of our sample met criteria for a lifetime Axis I psychiatric diagnosis, and 89.5% and 47.4% used alcohol and tobacco in the last week, respectively. While many studies restrict their samples to cannabis-dependent individuals and/or systematically exclude for co-morbid conditions, it has also become essential to elucidate cannabis’ residual effects among the “average” young adult cannabis user who may not use cannabis daily, is not seeking treatment, and presents with other drug use and psychiatric co-morbidities.
There are several aspects of the CM approach that merit discussion. Our protocol delivered CM alone, suggesting that CM without complimentary manualized treatments may be sufficient in incentivizing non-treatment seeking young adult cannabis users with a range of dependence scores to abstain from use for 30 days. Additionally, participants achieved nearly a 90% verified abstinence rate using delayed (rather than immediate) monetary rewards for abstinence. The quantitative urine drug testing utilized to monitor abstinence in the days immediately following the point of discontinued use took about four days for results, and therefore this approach required a delayed delivery of rewards for confirmed abstinence. Importantly, this approach eliminated the need for the “washout” period used in prior trials because most frequent cannabis users will test positive for cannabis several days to weeks after their last use (Goodwin et al., 2008; Hawks and Chiang, 1986) due to the storage and subsequent release of THC and THCCOOH in fat and relatively long half-life (Hunt and Jones, 1980; Lowe et al., 2009). The primary benefit of the present approach is that requiring a “washout” period may result in the systematic exclusion of individuals who are not able to achieve early continuous abstinence without more immediate contingent incentives to motivate abstinence efforts. Finally, despite the positive effect of CM in this sample, more than 90% of participants resumed use within two weeks of CM termination, consistent with other studies that show poor maintenance of gains post-treatment (Stanger et al., 2009, 2015). Future studies are needed that evaluate the benefit of alternative positive reinforcement strategies (e.g., increased magnitude of reinforcers, longer duration of intervention) as well as adding brief interventions (e.g., motivational interviewing) prior to the removal of the abstinence contingencies to promote continued abstinence.
Limitations of this study warrant consideration. A limitation includes the small sample size, precluding identification of predictors of abstinence. There is also no control group against which to evaluate the specificity of CM’s efficacy on cannabis abstinence. Additionally, a large proportion of this sample had a high level of education (>73% had completed at least some college coursework) and a major minority had a psychiatric co-morbidity, which may limit generalizability of findings to young adults with similar background characteristics. Finally, although the amount of compensation provided to participants was commensurate with other studies (e.g., Kamon et al., 2005; Stanger et al., 2015) and the use of contingencies for abstinence may actually increase the cost-effectiveness of clinical interventions (e.g., López-Núñez, et al., 2016), the high cost of conducting a protocol such of this may reduce its ability to be utilized when research budgets are modest.
Despite these limitations, this study provides important support for the use of CM as a tool to examine change in various outcomes of interest during four weeks of continuous cannabis abstinence. A checklist is provided as a resource for investigators interested to setting up a research study using a similar CM approach (Figure 3). From a clinical perspective, establishing the efficacy of CM in this population is critical given that current cannabis use is disproportionately represented among young adults yet this population is among the lowest to access treatment for use. Additionally, young adults are especially poised to experience greater consequences from early exposure due to ongoing brain development. CM may be a viable avenue for active secondary prevention that targets young people at an early stage of their cannabis-use. Early prevention among this vulnerable group, particularly during a time of rapid policy change surrounding cannabis’ legal status, may help to minimize problematic use, promote problem recognition, and facilitate informed choice regarding cannabis use and its potential consequences.
Figure 3.
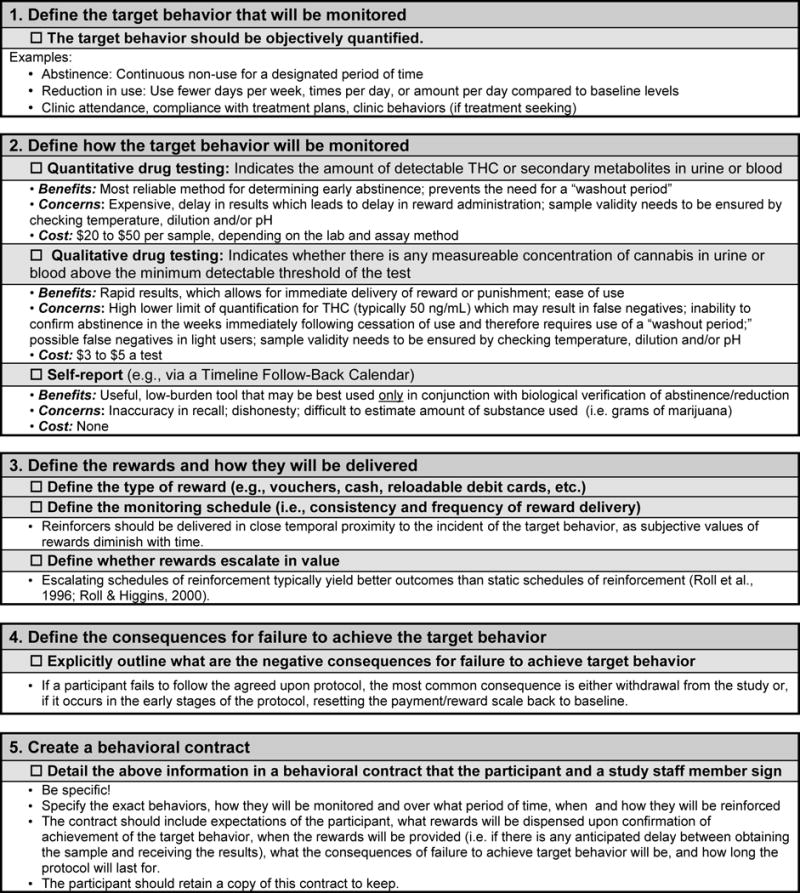
Key considerations for setting up a CM-based research protocol are delineated in checklist format.
Supplementary Material
Highlights.
Contingency management may be useful for studying the effects of cannabis in youth.
Nearly 90% of the sample attained biochemically confirmed 30-day abstinence.
Abstinence was associated with reduction in concentrations of cannabis metabolites.
Acknowledgments
Role of Funding Source
This publication was made possible by support from K24 DA030443 (Evins), K01DA034093 (Gilman), by the Harvard Medical School Norman E. Zinberg and Livingston Fellowships (Schuster), and by a Louis V. Gerstner III Research Scholar Award (Schuster).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Conflict of Interest Statement
Dr. Schuster, Ms. Hanly, and Dr. Gilman and Dr. Budney declare no conflicts of interest. Dr. Evins has received research grant support to her institution from Pfizer Inc, Forum Pharmaceuticals and GSK and honoraria for advisory board work from Pfizer and Reckitt Benckiser for work unrelated to this project. Dr. Vandrey is a consultant for Zynerba Pharmaceuticals and CW Botanicals, and has received honoraria for advisory board work from Insys Therapeutics for work unrelated to this project.
Contributors
Randi M. Schuster was primarily responsible for the design of the study, conducting the data analysis, and writing the first draft of the manuscript. Ailish Hanly assisted with data collection and analysis. Alan Budney provided assistance in study design and feedback on drafts of the manuscript. Ryan Vandrey provided assistance in study design, interpretation of findings, and feedback on drafts of the manuscript. A. Eden Evins provided assistance in study design, interpretation of findings, and feedback on drafts of the manuscript. All authors have read and approve the final version of the manuscript.
References
- Adamson SJ, Sellman JD. A prototype screening instrument for cannabis use disorder: the Cannabis Use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug Alcohol Rev. 2003;22:309–315. doi: 10.1080/0959523031000154454. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004;34:1227–1237. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Bigelow GE, Stitzer ML, Griffiths RR, Liebson IA. Contingency management approaches to drug self-administration and drug abuse: efficacy and limitations. Addict Behav. 1981;6:241–252. doi: 10.1016/0306-4603(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Budney A, Higgins ST. A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction. USDHHS; Rockville, MD: 1998. [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick B, Miller ML, Hurd YL. Cannabis use during adolescent development: susceptibility to psychiatric illness. Front Psychiatry. 2013;4:129. doi: 10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K, Chatters R, Kaltenthaler E, Wong R. Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report. Health Technol Assess. 2015;19:1–130. doi: 10.3310/hta19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Swift W. Cannabis use disorder: epidemiology and management. Int Rev Psychiatry. 2009;21:96–103. doi: 10.1080/09540260902782745. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, Patton GC. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. 2013;108:124–133. doi: 10.1111/j.1360-0443.2012.04015.x. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, Bianconi F, Gardner-Sood P, O’Connor J, Russo M, Stilo SA, Marques TR, Mondelli V, Dazzan P, Pariante C, David AS, Gaughran F, Atakan Z, Iyegbe C, Powell J, Morgan C, Lynskey M, Murray RM. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2:233–238. doi: 10.1016/S2215-0366(14)00117-5. [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ, Saner H. Antecedents and outcomes of marijuana use initiation during adolescence. Prev Med. 2004;39:976–984. doi: 10.1016/j.ypmed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103:969–976. doi: 10.1111/j.1360-0443.2008.02221.x. discussion 977–968. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction. 2006;101:556–569. doi: 10.1111/j.1360-0443.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zjdbendos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goodwin RS, Darwin WD, Chiang CN, Shih M, Li SH, Huestis MA. Urinary elimination of 11-nor-9-carboxy-delta9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol. 2008;32:562–569. doi: 10.1093/jat/32.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. Robin Room and cannabis policy: dangerous comparisons. Drug Alcohol Rev. 2014;33:612–616. doi: 10.1111/dar.12197. [DOI] [PubMed] [Google Scholar]
- Hawks RL, Chiang CN. Examples of specific drug assays. NIDA Res Monogr. 1986;73:84–112. [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Petry NM. Contingency management. Incentives for sobriety. Alcohol Res Health. 1999;23:122–127. [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt B):416–424. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20:2186–2193. doi: 10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring The Future National Survey Results On Drug Use: 1975–2014: Overview, Key Findings On Adolescent Drug Use. Institute For Social Research, The University Of Michigan; Ann Arbor: 2015. [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32:1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamon J, Budney A, Stanger C. A contingency management intervention for adolescent marijuana abuse and conduct problems. J Am Acad Child Adolesc Psychiatry. 2005;44:513–521. doi: 10.1097/01.chi.0000159949.82759.64. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Cavallo DA, Cooney JL, Schepis TS, Kong G, Liss TB, Liss AK, McMahon TJ, Nich C, Babuscio T, Rounsaville BJ, Carroll KM. An exploratory randomized controlled trial of a novel high-school-based smoking cessation intervention for adolescent smokers using abstinence-contingent incentives and cognitive behavioral therapy. Drug Alcohol Depend. 2013;132:346–351. doi: 10.1016/j.drugalcdep.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafolie P, Beck O, Blennow G, Boreus L, Borg S, Elwin CE, Karlsson L, Odelius G, Hjemdahl P. Importance of creatinine analyses of urine when screening for abused drugs. Clin Chem. 1991;37:1927–1931. [PubMed] [Google Scholar]
- Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc. 2012;18:678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Shollenbarger S. Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addict Rep. 2014;1:144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: randomized trial of contingency management and self-efficacy enhancement. Addict Behav. 2013;38:1764–1775. doi: 10.1016/j.addbeh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Núñez C, Alonso-Pérez F, Pedrosa I, Secades-Villa R. Cost-effectiveness of a voucher-based intervention for smoking cessation. Am J Drug Alcohol Abuse. 2016;42:296–305. doi: 10.3109/00952990.2015.1081913. [DOI] [PubMed] [Google Scholar]
- Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA. Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend. 2009;105:24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Maggs JL, Staff J, Kloska DD, Patrick ME, O’Malley PM, Schulenberg J. Predicting young adult degree attainment by late adolescent marijuana use. J Adolesc Health. 2015;57:205–211. doi: 10.1016/j.jadohealth.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein NR, Iacono WG. Explaining associations between cannabis use disorders in adolescence and later major depression: a test of the psychosocial failure model. Addict Behav. 2011;36:773–776. doi: 10.1016/j.addbeh.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Hill ML, Small PJ, Luthar SS. Associations of adolescent cannabis use with academic performance and mental health: a longitudinal study of upper middle class youth. Drug Alcohol Depend. 2015;156:207–212. doi: 10.1016/j.drugalcdep.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, Jarvis BP, Raiff BR, Rojewski AM, Kurti A, Cassidy RN, Erb P, Sy JR, Dallery J. The ABCs of incentive-based treatment in health care: a behavior analytic framework to inform research and practice. Psychol Res Behav Manag. 2014;7:103–114. doi: 10.2147/PRBM.S59792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery L, Petry NM, Carroll KM. Moderating effects of race in clinical trial participation and outcomes among marijuana-dependent young adults. Drug Alcohol Depend. 2012;126:333–339. doi: 10.1016/j.drugalcdep.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Fenstermaker M, Kamboukos D, Ompad DC, Cleland CM, Weitzman M. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438–446. doi: 10.3109/00952990.2014.943371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Dallery J, Shroff P, Patak M, Leraas K. A web-based contingency management program with adolescent smokers. J Appl Behav Anal. 2008;41:597–601. doi: 10.1901/jaba.2008.41-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28:154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Roll JM, Howard JT. The relative contribution of economic valence to contingency management efficacy: a pilot study. J Appl Behav Anal. 2008;41:629–633. doi: 10.1901/jaba.2008.41-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Shoptaw S. Contingency management: schedule effects. Psychiatry Res. 2006;144:91–93. doi: 10.1016/j.psychres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA, Pope HG, Huestis MA. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction. 2011;106:499–506. doi: 10.1111/j.1360-0443.2010.03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. Contingencies of Reinforcement: A Theoretical Analysis. Prentice Hall; New York: 1969. [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. J Anal Toxicol. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Lubman DI, Yucel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology. 2011;216:131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Stanger C, Budney AJ. Contingency management approaches for adolescent substance use disorders. Child Adolesc Psychiatr Clin N Am. 2010;19:547–562. doi: 10.1016/j.chc.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009;105:240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Scherer EA, Norton GE, Budney AJ. Clinic- and home-based contingency management plus parent training for adolescent cannabis use disorders. J Am Acad Child Adolesc Psychiatry. 2015;54:445–453 e442. doi: 10.1016/j.jaac.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DG, Felleman BI, Arger CA. Effectiveness of motivational incentives for adolescent marijuana users in a school-based intervention. J Subst Abuse Treat. 2015;58:43–50. doi: 10.1016/j.jsat.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bickel WK, Bigelow GE, Liebson IA. Effect of methadone dose contingencies on urinalysis test results of polydrug-abusing methadone-maintenance patients. Drug Alcohol Depend. 1986;18:341–348. doi: 10.1016/0376-8716(86)90097-9. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Vandrey R. Contingency management: utility in the treatment of drug abuse disorders. Clin Pharmacol Ther. 2008;83:644–647. doi: 10.1038/sj.clpt.6100508. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Behavioral Health Barometer: United States, 2015. Substance Abuse and Mental Health Services Administration; Rockville: 2015. (HHS Publication No. SMA–16–Baro–2015). [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Detailed tables. US Department of Health and Human Services, Office of Applied Studies; Rockville: 2014. [Google Scholar]
- Swift W, Coffey C, Degenhardt L, Carlin JB, Romaniuk H, Patton GC. Cannabis and progression to other substance use in young adults: findings from a 13-year prospective population-based study. J Epidemiol Community Health. 2012;66:e26. doi: 10.1136/jech.2010.129056. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, Bloomfield MA, Curran HV, Baler R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73:292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


