Abstract
Objective
Genome-wide association study (GWAS) has been successful in identifying obesity risk genes by single-variant association analysis. For this study, we designed steps of analysis strategy and aimed to identify multi-variant effects on obesity risk among candidate genes.
Methods
Our analyses were focused on 2,137 African American participants with body mass index measured in the Jackson Heart Study and 657 common single nucleotide polymorphisms (SNPs) genotyped at 8 GWAS-identified obesity risk genes.
Results
Single-variant association test showed that no SNPs reached significance after multiple testing adjustment. The following gene-gene interaction analysis, which was focused on SNPs with unadjusted p-value < 0.10, identified 6 significant multi-variant associations. Logistic regression showed that SNPs in these associations did not have significant linear interactions; examination of genetic risk score evidenced that 4 multi-variant associations had significant additive effects of risk SNPs; and haplotype association test presented that all multi-variant associations contained one or several combinations of particular alleles or haplotypes, associated with increased obesity risk.
Conclusions
Our study evidenced that obesity risk genes generated multi-variant effects, which can be additive or non-linear interactions, and multi-variant study is an important supplement to existing GWAS for understanding genetic effects of obesity risk genes.
Keywords: gene-gene interaction, SNP association, genetic risk score, haplotype, obesity
Introduction
Obesity is a risk factor of many common diseases, such as hypertension, type 2 diabetes mellitus, cardiovascular disease and sleep apnea. Over the past decades, obesity prevalence continues to increase, and more than one-third of adults and 47.8% of non-Hispanic African American adults are obese in the USA (Ogden et al., 2014). High prevalence of obesity is becoming a major public health issue and the impact is substantial. The cause of obesity is partly attributed to unhealthy life styles including excessive calorie intake and lower physical activity (Catenacci et al., 2009). However, not everyone who leads a sedentary life-style or over consumes food becomes obese, and the predisposition to obesity depends on genetic components.
Twin and family heritability studies have indicated that up to 80% of the variance in obesity, as measured by BMI, can be explained by genetic factors (Silventoinen and Kaprio, 2009; Hasselbalch, 2010; Iranzo-Tatay et al., 2015). Genome-wide association studies (GWAS) by single-variant analysis have succeeded in identifying obesity risk variants from different genes, including fat mass and obesity associated (FTO), neuronal growth regulator 1 (NEGR1), neurexin 3 (NRXN3), transmembrane protein 18 (TMEM18), transcription factor AP-2 beta (TFAP2B) and melanocortin 4 receptor (MC4R) (Welter et al., 2014a). However, risk variants identified so far have tiny effects, widely known as the missing heritability (Manolio et al., 2009), and it is often a challenge to detect these variants following GWAS. In contrast to a single-variant analysis, study of multi-variant effects among GWAS-identified obesity genes can help to improve test of risk variants while individual variants may not have detectable effects in a sample.
Multi-variant effects, especially gene-gene interactions, are considered as important components of the genetic architecture influencing susceptibility to common human diseases (Culverhouse et al., 2002). Investigation of obesity risk genes reported by GWAS evidenced that SNPs with effects that are weak or absent alone can jointly exert a large longitudinal effect on body mass index (BMI) from childhood to adulthood (Mei et al., 2012). Multi-variant effects are complicated, and gene-gene interaction study by multifactor dimensionality reduction (MDR) showed that genetic risk variants can jointly present additive effects, linear or nonlinear interactions in different forms (Hahn et al., 2003; Greene et al., 2010). Study of obesity risk genes and examination of their multi-variant effects will be an important supplement to existing GWAS and help to improve understanding of genetic influences on obesity development.
In the USA, African-Americans are more obese than European-American (Lewis et al., 2000). For this study, we investigated African-American sample of the Jackson Heart Study (JHS) cohort (Fuqua et al., 2005), and designed steps of analysis strategy that emphasized on test of multi-variant associations among obesity risk genes reported by previous GWAS. Our purpose is to examine if these genes generate multi-variant effects on obesity risk and measure their sizes by deliberately designed analysis strategy.
Materials and methods
Study Participants
The JHS is a large community-based observational study of African Americans, and participants were recruited from urban and rural areas of the three counties of Hinds, Madison and Rankin in the Jackson, MS (Fuqua et al., 2005). Weight of participant was measured on a balance scale, in light clothing and without shoes, and recorded to the nearest 0.5 kg. The body mass index (BMI), as a measure of obesity, was calculated as weight in kilograms divided by height in meters squared. Obesity status was defined according to BMI (Bidulescu et al., 2011). Individuals with BMI < 30 kg/m2 were considered as control, and those with BMI ≥ 30 kg/m2 were considered as obese cases. The study protocol was approved by the Institutional Review Boards at the University of Mississippi Medical Center.
SNP Genotyping
The Affymetrix Human SNP Array 6.0 (Affymetrix® Inc., Santa Clara, CA) was used for genome-wide SNP genotyping. Genomic DNA was quantitated and DNA quality was evaluated via gel electrophoresis. The genomic DNA samples were processed according to standard Affymetrix procedures for processing of the assay. The data were processed for genotype calling using the Affymetrix Genotypic Console software using the Birdseed calling algorithm version2.0 (Affymetrix®, Inc., Santa Clara, CA)(Korn et al., 2008).
Obesity Risk Genes and Quality Control
Obesity risk genes were identified by searching against the Catalog of Published Genome-Wide Association Studies (www.genome.gov/gwastudies/) (Welter et al., 2014b), limited to diseases/trait categories of obesity and related traits. Top reported genes that had SNPs with association p-value ≤ 5 × 10-8 were selected as candidate genes for multi-variant study. Genomic map positions of candidate genes were identified based on Human NCBI36/hg18 Assembly. SNPs within upstream 5kb to downstream 5kb of candidate genes were extracted from JHS GWAS data. PLINK 1.90 (Purcell et al., 2007) was applied to calculate individual missing rate, genotype missing rate and minor allele frequency (MAF), to test Hardy-Weinberg equilibrium (HWE), and to measure pairwise linkage disequilibrium (LD). The criteria for SNP quality control included the genotype missing rate <5%, minor allele frequency (MAF) ≥5% and p-value of Hardy-Weinberg equilibrium (HWE) > 0.001.
Statistical Analysis
Demographic variables were analyzed with age, gender and BMI. The t and Chi-square test were respectively applied to examine distribution difference of continuous and category variables. The analysis was performed using Stata software (version 13.0; College Station, TX, USA), and p-values were obtained based on two-sided test.
We analyzed SNPs of JHS GWAS data, and the first 10 principal components (PCs) were calculated from the pruned genome-wide autosomal SNPs with MAF ≥ 0.05 and pair-wise r2 ≤ 0.1 by the EIGENSTRAT method (Price et al., 2006), which are used as covariates to adjust for potential population structure. For every genotyped SNP of candidate genes, additive genetic model was adopted to test single-variant association with obesity by logistic regression, adjusting for age, gender and PCs. The t-statistic and odds ratio were computed with p-values obtained through a two-sided test, and potential risk SNPs were identified if they had unadjusted p-value < 0.10. The analysis was performed using PLINK software.
Potential risk SNPs were further examined and those with pairwise r2 ≤ 0.2 based on HapMap reference data (Yoruba in Ibadan, Nigeria) (2003) were tested for gene-gene interactions by method of multifactor dimensionality reduction, MDR-Phenomics (Ritchie et al., 2001; Mei et al., 2007). MDR is a model-free approach that tests high-order gene x gene interactions without assuming any particular genetic model and estimating parameters. The method reduces dimensionality of multi-variant genotype information to a one-dimensional factor with two levels—“high-risk” and “low-risk” genotypes. The MDR analysis aimed to identify multi-variant effects and the test was based on the hypothesis that individual SNPs with weak effects, which may not be detectable in a single-variant study, can jointly exert a large effect on obesity risk. We applied the MDR method to test all 2- to 5-variant associations among the candidate SNPs that were trained and tested by 10-1 cross-validation, and measured by prediction error (PE). Association p-values, adjusted for multiple testing, were obtained by 1,000 permutation tests, and the significance was defined as adjusted p -value ≤ 0.05.
For significant multi-variant associations, linear interactions between SNPs were examined by logistic regression, and additive effects of SNPs were investigated through test of genetic risk score (GRS) association. A SNP risk allele was the one that had increased odds of obesity at the single-variant logistic regression analysis, and GRS was computed as the total number of risk alleles in the multi-variant association (Cornelis et al., 2009). Association of GRS with obesity was tested by logistic regression among men, women and total sample separately.
Significant multi-variant associations were lastly explored to investigate effects for combinations of particular alleles or haplotypes across SNPs by haplotype omnibus, conditional and specific analysis using PLINK: logistic regression with omnibus test was applied to examine overall haplotype association with obesity; independent SNP effects in a haplotype association were evaluated by haplotype conditional test and effect of a particular haplotype or combination of SNP alleles was measured by the specific analysis. For both the GRS and haplotype association tests, the Bonferroni correction was applied to adjust for multiple testing, and the threshold of significant p-value was defined as 0.05 divided by the number of significant multivariant associations identified by the MDR analysis.
Results
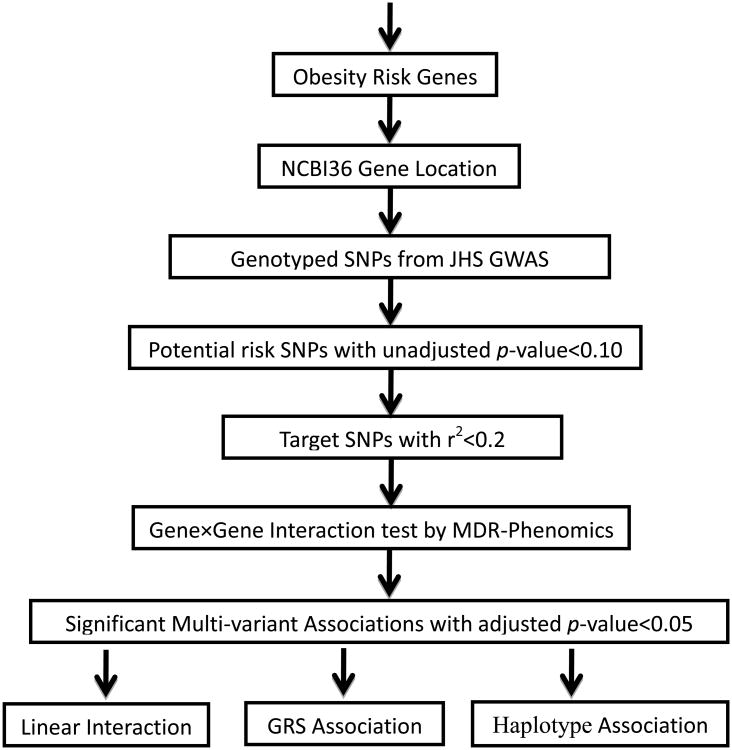
Steps of analysis strategy for present study were summarized in Figure 1. The study consists of 2,145 participants of African American with GWAS data. Eight participants with missed gender were removed, and the final sample size is 2,137, including 1,188 obese cases and 949 non-obese controls. The male participants account for 30.64% of cases and 50.26% of controls, and the difference was significant (p < 0.001). The male BMI was 35.64 ± 6.08 in case and 26.13 ± 2.67 in control, and the p-value of difference was p < 0.001. It was also significantly different for female BMI between case (37.98 ± 7.08) and control (26.13 ± 2.71) (p<0.001).
Figure 1.
Multi-variant analysis of obesity risk genes.
GWAS Candidate Genes and SNPs
We identified top 10 obesity candidate genes with SNP association p-values ≤ 5.0 × 10-8 from the GWAS catalog (Cotsapas et al., 2009; Meyre et al., 2009; Jiao et al., 2011; Berndt et al., 2013; Wheeler et al., 2013), and 8 genes were found to have genotyped SNPs in the JHS GWAS, whose characteristics were described in Supplementary Table 1. We retrieved gene map positions of transcription start and termination sites from the UCSC genome browser (Karolchik et al., 2014) based on the reference genome build of NCBI 36 assembly, and extracted 675 genotyped SNPs of the 8 candidate genes from the JHS GWAS. The SNP QC removed 1 variant with MAF<1% and 3 variants with HWE p<0.001, and the final 671 SNPs were used for the follow-up analyses.
Single-variant Association Analysis
We conducted test of single-variant association with obesity by logistic regression with age, gender and PCs as covariates. As a result, 57 SNPs had unadjusted p-value < 0.10, but no one is significant after Bonferroni correction. Of them, 30 SNPs that have mutual r2 <0.2 with the smallest p-values were identified. MC4R was widely reported as obesity risk gene. Although the gene did not have genotyped SNPs with unadjusted p-value < 0.10, we manually selected rs2229616 that had the smallest p-value with the 30 SNPs as potentially risk SNPs. These SNPs were examined for multi-variant and gene-gene interaction effects, and their MAFs, HWE p-values, association t-statistics, p-values and ORs were summarized in Table 1.
Table 1. Characteristics and single-variant associations.
| SNP Index | Gene | SNPs | Position | MAa | MAFb | Phwec | t | P | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NEGR1 | rs1334000 | 71806840 | A | 0.42 | 0.32 | -2.04 | 4.17E-02 | 0.88(0.06,0.78) |
| 2 | NEGR1 | rs3795698 | 71831344 | T | 0.1 | 0.7 | 2.5 | 1.26E-02 | 1.31(0.11,1.06) |
| 3 | NEGR1 | rs10493485 | 71843717 | T | 0.06 | 1 | -2.01 | 4.49E-02 | 0.75(0.14,0.57) |
| 4 | NEGR1 | rs11209823 | 71924195 | G | 0.02 | 1 | 1.73 | 8.43E-02 | 1.44(0.21,0.95) |
| 5 | NEGR1 | rs11802650 | 72227919 | A | 0.23 | 0.83 | -1.98 | 4.74E-02 | 0.86(0.08,0.74) |
| 6 | NEGR1 | rs2046503 | 72304061 | G | 0.29 | 0.52 | -2.03 | 4.19E-02 | 0.87(0.07,0.76) |
| 7 | NEGR1 | rs12132044 | 72306264 | A | 0.22 | 0.31 | -1.79 | 7.32E-02 | 0.87(0.08,0.75) |
| 8 | NEGR1 | rs12128707 | 72360707 | G | 0.05 | 1 | -2.31 | 2.10E-02 | 0.72(0.14,0.55) |
| 9 | NEGR1 | rs11209935 | 72422842 | A | 0.45 | 0.88 | 2.27 | 2.32E-02 | 1.15(0.06,1.02) |
| 10 | NEGR1 | rs12073950 | 72498028 | C | 0.16 | 0.2 | 1.75 | 7.98E-02 | 1.17(0.09,0.98) |
| 11 | TMEM18 | rs3187671 | 659023 | T | 0.24 | 0.21 | 2.84 | 4.51E-03 | 1.23(0.07,1.07) |
| 12 | ADCY3 | rs6545758 | 24928378 | C | 0.45 | 0.56 | 2.47 | 1.35E-02 | 1.17(0.06,1.03) |
| 13 | TFAP2B | rs987237 | 50911009 | G | 0.1 | 0.42 | 2.55 | 1.08E-02 | 1.30(0.10,1.06) |
| 14 | BDNF | rs11030119 | 27684678 | A | 0.3 | 0.86 | -2.7 | 6.87E-03 | 0.83(0.07,0.73) |
| 15 | NRNX3 | rs10498537 | 78908298 | T | 0.39 | 0.22 | 2.45 | 1.45E-02 | 1.17(0.06,1.03) |
| 16 | NRNX3 | rs4903847 | 78924585 | T | 0.46 | 0.6 | -2.53 | 1.13E-02 | 0.85(0.06,0.76) |
| 17 | NRNX3 | rs11159408 | 78944111 | A | 0.16 | 0.61 | 2.59 | 9.61E-03 | 1.25(0.09,1.06) |
| 18 | NRNX3 | rs7145924 | 79035334 | A | 0.14 | 0.41 | 2.69 | 7.08E-03 | 1.28(0.09,1.07) |
| 19 | NRNX3 | rs7153625 | 79119015 | A | 0.13 | 0.44 | 1.89 | 5.93E-02 | 1.20(0.10,0.99) |
| 20 | NRNX3 | rs8016688 | 79293258 | G | 0.24 | 0.66 | -3.61 | 3.04E-04 | 0.76(0.07,0.66) |
| 21 | NRNX3 | rs766958 | 79321265 | T | 0.05 | 0.67 | -2.06 | 3.97E-02 | 0.76(0.14,0.58) |
| 22 | NRNX3 | rs8021394 | 79336276 | C | 0.26 | 0.71 | 3.2 | 1.37E-03 | 1.26(0.07,1.09) |
| 23 | NRNX3 | rs2293836 | 79377043 | C | 0.13 | 0.34 | 2.11 | 3.45E-02 | 1.22(0.09,1.01) |
| 24 | NRNX3 | rs2293839 | 79387213 | G | 0.45 | 0.82 | 2.53 | 1.14E-02 | 1.17(0.06,1.04) |
| 25 | FTO | rs1861868 | 52347903 | T | 0.27 | 0.12 | 1.93 | 5.37E-02 | 1.15(0.07,1.00) |
| 26 | FTO | rs1421085 | 52358455 | C | 0.1 | 0.69 | 2.23 | 2.61E-02 | 1.27(0.11,1.03) |
| 27 | FTO | rs10521303 | 52466686 | G | 0.47 | 0.94 | 2.61 | 9.10E-03 | 1.18(0.06,1.04) |
| 28 | FTO | rs13335646 | 52556188 | G | 0.1 | 0.7 | 2.29 | 2.18E-02 | 1.28(0.11,1.04) |
| 29 | FTO | rs7202920 | 52558749 | A | 0.1 | 0.5 | -2.56 | 1.06E-02 | 0.77(0.10,0.63) |
| 30 | FTO | rs860713 | 52626966 | A | 0.26 | 0.39 | 2.68 | 7.27E-03 | 1.22(0.07,1.05) |
| 31 | MC4R | rs2229616 | 56190256 | T | 0.01 | 1 | 0.21 | 8.32E-01 | 1.06(0.28,0.61) |
MA: Minor allele.
MAF: Minor allele frequency.
PHWE: p-value of Hardy-Weinberg equilibrium test.
Multi-variant Association Analysis
We tested gene-gene interactions among the 31 candidate risk SNPs by the method of multifactor dimensionality reduction, MDR-Phenomics (Mei; Mei et al., 2007), and identified 6 significant multi-variant associations with adjusted p-value ≤ 0.05. However, logistic regression test showed that no linear interaction terms of SNPs in the associations reached significance. Results of the 6 multi-variant associations were summarized at Table 2, which included involved SNPs, corresponding genes, PE and permutation p-value (PPE) by MDR, Z statistic, standard error (SE) and interaction p-value (PGL) by logistic regression.
Table 2. Multi-variant associations with obesity.
| Gene | SNP Index | PEa | PPEb | Z | SE | PGLc |
|---|---|---|---|---|---|---|
| NEGR1 | 3+7 | 0.441 | 0.013 | -1.216 | 0.209 | 0.224 |
| ADCY3+NRXN3 | 12+15 | 0.443 | 0.043 | -2.352 | 0.086 | 0.019 |
| BDNF+NRXN3 | 14+20 | 0.444 | 0.007 | -1.443 | 0.114 | 0.149 |
| NEGR1+NRXN3 | 1+9+22 | 0.427 | 0.047 | 0.320 | 0.148 | 0.749 |
| NEGR1+NRXN3 | 6+15+20 | 0.422 | 0.004 | -1.131 | 0.167 | 0.258 |
| NEGR1+NRXN3+FTO | 1+9+16+27 | 0.441 | 0.042 | 1.397 | 0.195 | 0.162 |
PE: Prediction error.
PPE: p-value of prediction error adjusted for multiple test by permutation.
PGL: p-value of linear interaction by logistic regression.
Of the 6 significant findings, there are three 2-variant associations including rs10493485 and rs12132044 (SNP 3 + 7) from NEGR1 (PPE = 0.013, PGL = 0.224), rs6545758 and rs10498537 (SNP 12 + 15) from ADCY3+NRXN3 (PPE = 0.043, PGL = 0.019), and rs11030119 and rs8016688 (SNP 14 + 20) from BDNF+NRXN3 (PPE = 0.007, PGL = 0.149); there are two 3-variant associations observed from NRGR1+NRXN3 including rs1334000, rs11209935 and rs8021394 (SNP 1 + 9 + 22) with PPE = 0.047 and PGL = 0.749, and rs2046503, rs10498537 and rs8016688 (SNP 6 +15 + 20) with PPE = 0.004 and PGL = 0.258; and the only 4-variant association of rs1334000, rs11209935, rs4903847 and rs10521303 (SNP 1 + 9 +16 +27) was identified from genes of NRGR1+NRXN3+FTO with PPE = 0.042 and PGL = 0.162.
GRS Association Analysis
We calculated the GRS for the additive genetic effect of the 6 significant multi-variant associations. Association test of the GRS was shown at the Table 3 and the threshold of significant p-value is 8.33×10-3. Multi-variant associations of SNP 14+20 and 1+9+22 had significant GRS effects in nearly all groups: the OR (p-value) was 1.27 (2.00E-03), 1.19 (9.20E-03) and 1.23 (2.68E-05) for SNP 14+20, and 1.26 (3.65E-04), 1.15 (7.03E-03) and 1.19 (7.61E-06) for SNP 1+9+22 in Men, Women and total sample respectively. The GRS associations of SNP 3+7 and 6+15+20 were significant in Men and total sample, but not in Women: the OR (p-value) was 1.33 (5.83E-03), 1.13 (0.14) and 1.19 (4.17E-03) for SNP 3+7, and 1.16 (1.90E-02), 1.10 (0.08) and 1.13 (1.81E-03) for SNP 6+15+20 in Men, Women and total sample respectively.
Table 3. GRS Associations with obesity.
| Combination | Men | Women | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z | SE | p-valuea | OR (95% CI)b | Z | SE | p-valuea | OR (95% CI) b | Z | SE | p-valuea | OR (95% CI)b | |
| 3+7 | 2.76 | 0.14 | 5.83E-03 | 1.33(1.09,1.63) | 1.48 | 0.09 | 0.14 | 1.13(0.96,1.32) | 2.87 | 0.07 | 4.17E-03 | 1.19(1.06,1.35) |
| 12+15 | 0.75 | 0.07 | 0.45 | 1.05(0.92,1.20) | -0.36 | 0.06 | 0.72 | 0.98(0.88,1.10) | 0.06 | 0.04 | 0.95 | 1.00(0.92,1.09) |
| 14+20 | 3.09 | 0.10 | 2.00E-03 | 1.27(1.09,1.48) | 2.61 | 0.08 | 9.20E-03 | 1.19(1.04,1.35) | 4.20 | 0.06 | 2.68E-05 | 1.23(1.12,1.35) |
| 1+9+22 | 3.56 | 0.08 | 3.65E-04 | 1.26(1.11,1.43) | 2.70 | 0.06 | 7.03E-03 | 1.15(1.04,1.26) | 4.48 | 0.05 | 7.61E-06 | 1.19(1.10,1.28) |
| 6+15+20 | 2.35 | 0.07 | 1.90E-02 | 1.16(1.02,1.32) | 1.76 | 0.06 | 0.08 | 1.10(0.99,1.22) | 3.12 | 0.05 | 1.81E-03 | 1.13(1.05,1.23) |
| 1+9+16+27 | 0.58 | 0.05 | 0.56 | 1.03(0.94,1.13) | 1.08 | 0.04 | 0.28 | 1.04(0.97,1.13) | 1.28 | 0.03 | 0.20 | 1.04(0.98,1.10) |
p-value of GRS association.
Odds ratio for every additional risk allele.
Haplotype Association Analysis
We examined haplotype effects of the 6 significant multi-variant associations, and the results were summarized in Table 4, including multi-variant haplotype, frequency, OR and p-value of haplotype omnibus (Pomni), conditional (Pcond) and specific (Pspec) association tests. The threshold of significant p-value is 8.33×10-3, based on the Bonferroni correction. For the 2-variant association of SNP 3+7 (NEGR1 rs10493485 and NEGR1 rs12132044), haplotype omnibus analysis showed that 4 haplotypes had overall effect of Pomni =0.028; conditional analysis indicated that SNP 3 and 7 had independent marginal effects with Pcond = 0.068 and 0.048 respectively. Compared to the TA, haplotypes of AA, TG and AG had OR of 2.06, 2.39 and 2.23, and none was significant. However, the specific haplotype association test showed that TA versus the group of AA, TG and AG had significant p-value of Pspec =3.92E-03.
Table 4. Haplotype associations with obesity.
| SNP Indexa | CHRb | Position | SNP | A1 | A2 | Pcondc | Pomnid | Haplotype | Freqe | OR | Pspecf |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 1 | 71843717 | rs10493485 | T | A | 6.81E-2 | 2.78E-2 | TA | 0.03 | Reference | 3.92E-3 |
| 7 | 1 | 72306264 | rs12132044 | A | G | 4.80E-2 | AA | 0.19 | 2.06(1.13,3.74) | 3.78E-1 | |
| TG | 0.03 | 2.39(1.07,5.34) | 9.93E-1 | ||||||||
| AG | 0.76 | 2.23(1.28,3.89) | 7.60E-2 | ||||||||
|
| |||||||||||
| 12 | 2 | 24928378 | rs6545758 | C | T | 6.12E-2 | 4.57E-3 | CT | 0.17 | Reference | 1.90E-2 |
| 15 | 14 | 78908298 | rs10498537 | T | C | 6.03E-3 | TT | 0.22 | 0.93(0.68,1.27) | 5.26E-1 | |
| CC | 0.28 | 0.97(0.72,1.28) | 1.85E-1 | ||||||||
| TC | 0.33 | 0.71(0.56,0.90) | 3.45E-4 | ||||||||
|
| |||||||||||
| 14 | 11 | 27684678 | rs11030119 | A | G | 4.91E-2 | 1.33E-3 | AG | 0.08 | Reference | 2.08E-3 |
| 20 | 14 | 79293258 | rs8016688 | G | A | 9.02E-3 | GG | 0.16 | 1.19 (0.77,1.84) | 4.79E-2 | |
| AA | 0.22 | 1.31(0.88,1.97) | 3.56E-1 | ||||||||
| GA | 0.54 | 1.60(1.15,2.23) | 2.22E-4 | ||||||||
|
| |||||||||||
| 1 | 1 | 71806840 | rs1334000 | A | C | 4.01E-3 | 2.15E-4 | AAC | 0.04 | Reference | 1.35E-2 |
| 9 | 1 | 72422842 | rs11209935 | A | G | 3.12E-3 | CAC | 0.07 | 0.70 (0.31,1.59) | 4.68E-3 | |
| 22 | 14 | 79336276 | rs8021394 | C | T | 3.14E-3 | AGC | 0.06 | 0.61 (0.27,1.41) | 7.59E-1 | |
| CGC | 0.09 | 0.49(0.29,0.93) | 9.03E-1 | ||||||||
| AAT | 0.17 | 0.43(0.22,0.85) | 4.09E-2 | ||||||||
| CAT | 0.17 | 0.74(0.41,1.33) | 2.35E-3 | ||||||||
| AGT | 0.15 | 0.43(0.23,0.78) | 3.67E-4 | ||||||||
| CGT | 0.26 | 0.55(0.30,0.99) | 5.21E-1 | ||||||||
|
| |||||||||||
| 6 | 1 | 72304061 | rs2046503 | G | A | 2.54E-2 | 2.01E-2 | GTG | 0.02 | Reference | 8.19E-1 |
| 15 | 14 | 78908298 | rs10498537 | T | C | 6.01E-2 | ATG | 0.06 | 0.79 (0.26,2.39) | 3.25E-1 | |
| 20 | 14 | 79293258 | rs8016688 | G | A | 0.42 | GCG | 0.05 | 0.62(0.19,1.95) | 8.67E-3 | |
| ACG | 0.11 | 0.70(0.28,1.72) | 6.41E-3 | ||||||||
| GTA | 0.08 | 1.11(0.38,3.20) | 1.34E-1 | ||||||||
| ATA | 0.23 | 1.01(0.41,2.49) | 1.87E-2 | ||||||||
| GCA | 0.13 | 0.79(0.32,1.93) | 2.70E-1 | ||||||||
| ACA | 0.31 | 0.98 (0.40,2.46) | 2.79E-1 | ||||||||
|
| |||||||||||
| 1 | 1 | 71806840 | rs1334000 | A | C | 1.21E-2 | 1.55E-3 | AATG | 0.04 | Reference | 2.10E-1 |
| 9 | 1 | 72422842 | rs11209935 | A | G | 1.02E-2 | CATG | 0.05 | 1.72(0.68,4.37) | 3.97E-2 | |
| 16 | 14 | 78924585 | rs4903847 | T | C | 3.02E-3 | AGTG | 0.05 | 1.22(0.48,3.10) | 2.40E-1 | |
| 27 | 16 | 52466686 | rs10521303 | G | T | 3.13E-3 | CGTG | 0.07 | 1.09(0.53,2.25) | 3.44E-1 | |
| AACG | 0.06 | 0.93(0.39,2.23) | 8.39E-1 | ||||||||
| CACG | 0.06 | 2.96(1.33,6.56) | 3.66E-4 | ||||||||
| AGCG | 0.05 | 2.66(1.16,6.10) | 4.51E-1 | ||||||||
| CGCG | 0.10 | 1.39(0.68,2.84) | 1.65E-1 | ||||||||
| AATT | 0.06 | 1.22(0.51,2.94) | 6.59E-1 | ||||||||
| CATT | 0.06 | 2.29(1.07,4.90) | 2.72E-2 | ||||||||
| AGTT | 0.05 | 1.19(0.54,2.64) | 6.95E-3 | ||||||||
| CGTT | 0.09 | 1.15(0.56,2.37) | 2.37E-1 | ||||||||
| AACT | 0.05 | 2.34(1.05,5.21) | 8.46E-1 | ||||||||
| CACT | 0.07 | 1.17(0.59,2.46) | 1.73E-1 | ||||||||
| AGCT | 0.06 | 0.57(0.25,1.33) | 1.57E-3 | ||||||||
| CGCT | 0.10 | 1.47(0.75,2.91) | 5.44E-1 | ||||||||
SNP index of multi-variant association.
Chromosome
p-value of SNP independent effect controlling for other SNPs in haplotype association test.
p-value for omnibus test of haplotype associations.
Frequency of haplotype or combination of alleles.
p-value of specific haplotype effect versus all other haplotypes.
Two-variant associations of SNP 12+15 (rs6545758 and rs10498537) had significant omnibus haplotype effects with Pomni =4.57E-03, and SNP 15 (Pcond =0.006) had an independent marginal effect with much smaller p-value than SNP 12 (Pcond =0.061). Haplotypes of TT, CC and TC versus CT had OR of 0.93, 0.97 and 0.71 respectively, and the specific test of haplotype TC versus all other haplotypes had OR=0.71 with significant p-value of Pspec = 3.45E-04. Multi-variant associations of SNP 14+20 (rs11030119 and rs8016688) also had significant omnibus haplotype effects with association p-value of Pomni =1.33E-3, and SNP 14 and 20 respectively had conditional effects with p-values of Pcond =0.049 and 0.009. ORs of GG, AA and GA versus reference haplotype AG were 1.19, 1.31 and 1.60 respectively and AG versus all other 3 haplotypes had specific effect with significant p-value of Pspec = 2.08×10-3.
For 3-variant association of SNP 1+9+22 (rs1334000, rs11209935 and rs8021394), omnibus test showed that haplotype effects had significant p-value of Pomni =2.15×10-4. The SNP 1, 9 and 22 had conditional effects with significant p-values of Pcond =0.004, 0.003 and 0.003 respectively. Compared to the AAC, all other haplotypes had specific effects with OR<1 and AGT had the smallest OR of 0.43 with significant Pspec = 3.67×10-4. For 3-variant association of SNP 6+15+20 (rs2046503, rs10498537 and rs8016688), omnibus test showed it had an overall effect with Pomni =0.02, which however did not reach significance. Conditional analysis presents that only SNP 6 had independent effect with p-value <0.05 (Pcond =0.025). Compared to reference haplotype GTG, haplotypes of GCG, ACG and ATA had specific effects with p-values of Pspec =0.009, 0.006 and 0.02 respectively.
For the 4-variant association of SNP 1+9+16+27 (rs1334000, rs11209935, rs4903847 and rs10521303), the p-value of the omnibus test was Pomni =1.55×10-3; the SNP 1,9,16 and 27 respectively had independent effects with p-values of Pcond = 0.012, 0.010, 0.003 and 0.003. Compared with reference haplotype AATG, all haplotypes except AACG showed OR >1 and CATG (Pspec = 0.04), CACG (Pspec = 3.66×10-4), CATT (Pspec = 0.03) and AGTT (Pspec = 6.95 ×10-3) had specific effects with p-value<0.05, of which the CACG presented the strongest risk effect (OR=2.96).
Discussion
GWAS to date have successfully identified many risk variants from different genes associated with obesity. These risk variants however typically present weak effect, and genetic effect from an obesity-risk gene is often hard to detect in an independent study following GWAS by single-variant association test. For this study, we examined 8 obesity-risk genes (NEGR1, ADCY3, TMEM18, TFAP2B, BDNF, NRXN3, FTO and MC4R) reported by previous GWAS in the African American sample of JHS. To improve detection of genetic effect, we focused on test of multi-variant associations among these candidate genes, of which individual variants may have undetectable modest or weak effects, following our steps of analysis strategy.
Our first step of analysis applied single-variant association test by logistic regression to identify potential risk variants of obesity with unadjusted p-value < 0.10 and pairwise r2<0.2. Investigation of these variants enables detection of joint effects from multiple variants, of which individual variants may have undetectable weak effects after adjusting for multiple testing. Of total 671 SNPs in the candidate genes, our test showed 57 SNPs were marginally significant, but none attained Bonferroni-adjusted significance (Table 1), and the final 31 candidate SNPs were selected to test for multi-variant effects.
Our second step of analysis applied the MDR method to test multi-variant associations among the candidate SNPs, and the significance was defined as adjusted p-value ≤ 0.05. The high-risk and low-risk genotypes of a multi-variant association is trained and validated by cross-validation, and the adjusted p-value is obtained using a permutation test. The characteristics of MDR make it an effective approach of testing multi-variant effect in any form, including additive and non-linear interaction, and it is especially powerful for testing interactions in the absence of any statistically significant independent main effects (Hahn et al., 2003).
Our MDR-based analysis identified 6 significant multi-variant associations with obesity after adjusting for multiple tests, including a 2-variant association (SNP 3+7) from NEGR1, two 2-variant associations (SNP 12+15 and 14+20) from ADCY3+NRXN3 and BDNF+NRXN3, two 3-variant associations (SNP 1+9+22 and 6+15+20) from NEGR1+NRXN3, and a 4-variant association (SNP 1+9+16+27) from NEGR1+NRXN3+FTO. Logistic regression test of linear interaction showed that only 2-variant association of SNP 12+15 had unadjusted p-value < 0.05 (Table 2, PGL=0.019). These results suggested that GWAS-identified risk genes contain complex multi-variant effects on obesity risk in African American, of which single variant may only yield weak effect and multiple variants do not have linear interaction.
Our third step of analysis examined if the 6 identified multi-variant associations contained additive effects of risk variants by GRS study. The GRS calculation and its association test were based on the hypothesis that the number of risk alleles in multi-variant association has linear correlation with log odds of obesity risk, and the significance level of p-value is 8.33×10-3 based on the Bonferroni correction. Previous studies (Elks et al., 2012; Rukh et al., 2013) have shown that the GRS test is effective in investigating additive genetic effects on obesity risk. Our analysis showed that GRS had significant linear association for multi-variant associations of SNP 3+7, 14+20, 1+9+22 and 6+15+20 that presented OR (p-value) as 1.19 (4.17E-03), 1.23 (2.68E-05), 1.19 (7.61E-06) and 1.13 (1.81E-03) respectively for every additional risk allele (Table 3). The significant GRS associations presented higher OR in the men than the women. However, for SNP 12+15 and 1+9+16+27, GRS did not show linear associations. Our study evidenced that multiple variants at GWAS-identified risk genes can jointly generate linear additive genetic effects on obesity risks.
We further examined effects for combinations of alleles over a multi-variant association by haplotype association tests. Combinations of alleles from different genes are considered as presence of potential inter-locus or gene-gene interactions (Akey et al., 2001). Our analysis is based on the hypothesis that a significant multi-variant association will present different distributions of haplotypes among candidate genes between case and control groups, e.g. higher probability of a particular combination of alleles among case individuals than control individuals. The haplotype omnibus test showed that the 6 identified multi-variant associations by MDR had p-values of overall haplotype effects from 2.78 ×10-2 to 2.15 ×10-4 and four of them (p-value < 8.33×10-3) were significant after Bonferroni correction of multiple testing (Table 4). The conditional analysis examined if SNPs involved in a multi-variant association independently contribute to haplotype association. Of the SNP combinations with significant omnibus effects, SNP 15 (Pcond = 0.006) and SNP 20 (Pcond = 0.009) were the major contributions to the haplotype associations of SNP 12+15 and SNP 14+20 respectively; all SNPs had strongly independent effects (Pcond ≤0.004) for haplotype associations of SNP 1+9+22; and SNP 16 and 27 had higher independent effects than SNP 1 and 9 in the haplotype association of SNP 1+9+16+27 (Table 4).
The specific haplotype test with OR calculation estimated effect for a combination of particular alleles from a multi-variant association. For SNP 12+15, the reference haplotype CT was high risk and all other haplotypes decreased obesity risk with OR <1.0; similarly, analysis showed that reference haplotypes of AG was low risk for SNP 14+20 and reference AAC was high risk for SNP 1+9+22. Specific haplotype analysis also indicated that allele combinations of TC and AGT had the lowest risk for SNPs 12+15 and 1+9+22, and GA and CACG had the highest risk for SNPs 14+20 and 1+9+16+27 respectively (Table 4).
For this study, over 60% of the JHS sample were independent individuals, and the mixed family mainly had the size of 4 or less. Previous simulation studies have shown that ignoring family structure has little impact on power and the inflation of type I error is also not obvious for small pedigree size (McArdle et al., 2007). Considering the JHS sample characteristics, we therefore did not directly differentiate independent individuals from family member in the analysis models, and the first 10 principal components were instead integrated as covariates to adjust for both cryptic relatedness and population stratification in the sample (Wang et al., 2013).
For the significant multi-variant effects on obesity risk we have identified, they were all related to the gene of NRXN3 except the two-SNP effect from the NEGR1 (Table 2). The NRXN3 and the NEGR1 also presented a significant three-variant association. The NRXN3 encodes a family of proteins that actively function in the brain as cell adhesion molecules and receptors (Rowen et al., 2002). Genes of NEGR1, FTO, and BDNF observed to have significant interactions with the NRXN3 are all expressed particularly in the hypothalamus, which is crucial for energy balance and food intake (Bauer et al., 2009). The multi-variant results revealed potential mechanisms and pathways involving brain function genes that jointly regulate body adiposity and implied the role of central nervous system in the pathogenesis and development of obesity.
For this study, we detected multi-variant effects at GWAS-identified risk genes of obesity in African American, while individual variants did not have obvious effects. However, there were also a few limitations in the present study. Firstly, although our study was focused on examination of multi-variant effects among candidate genes, it lacks validation of the findings in an independent sample, which we will plan to confirm in the future study. Secondly, our GWAS candidate genes contained 671 SNPs, which will involve a large number of tests for high-dimensional interaction and multi-variant effects (e.g. up to 8.4 billion tests for 4-variant effects). To reduce the computational burden and perform a feasibility analysis, our study considered only those individual SNPs with potential weak effect or stronger and targeted those SNPs with unadjusted p-value < 0.10. However, this selection criteria may cause the missed detection of those multi-variant interactions while a SNP individually may not have effect or present the unadjusted p-value ≥ 0.10. Thirdly, our study is limited to 8 GWAS-identified risk genes and those genotyped common variants. Examinations of effects for rare variants and additional risk genes are also required in the follow-up studies.
Conclusions
In conclusion, we deliberately designed steps of analysis strategy for testing multi-variant effects on obesity risk among GWAS-identified genes in African Americans. The findings will help to understand complex genetic effects of obesity risk genes and elucidate biological mechanisms underlying obesity development. Specifically, our analysis showed that multiple SNPs can jointly produce a large effect on obesity risk, while individual variants did not have detectable effects in the sample. The results demonstrated that risk variants can generate additive and non-linear effects, and revealed that one or several combinations of alleles or haplotypes across SNPs led to increased obesity risk. Our study suggested that GWAS-identified risk genes affect obesity development more likely through a complex multi-variant interaction pattern than a single-variant effect, and multi-variant study can supplement existing GWAS for improving detection of obesity risk variants.
Supplementary Material
Highlights.
This study examined effects of 8 GWAS-identified genes on obesity risk in blacks
Steps of analysis strategy was carefully designed for testing multi-variant effects
Multi-variants without significant association individually exerted a large effect
Six multi-variant associations presenting complex effects were observed
Additive effects measured by genetic risk scores have been found
Haplotype omnibus, conditional and specific effects have been detected
Acknowledgments
The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The authors thank the participants of Jackson Heart Study for their contributions. We would also like to acknowledge the help of Alison Johnson from PROJECT Hope in the preparation of this manuscript.
Abbreviations
- GWAS
Genome-wide association study
- SNPs
Single nucleotide polymorphisms
- JHS
Jackson Heart Study
- BMI
body mass index
- MDR
multifactor dimensionality reduction
- SE
standard error
- MAF
minor allele frequency
- HWE
Hardy-Weinberg equilibrium
- LD
linkage disequilibrium
- PC
principal component
- PE
prediction error
- GRS
genetic risk score
- FTO
fat mass and obesity associated
- NEGR1
neuronal growth regulator 1
- NRXN3
neurexin 3
- TMEM18
transmembrane protein 18
- TFAP2B
transcription factor AP-2 beta
- MC4R
melanocortin 4 receptor
- ADCY3
adenylate cyclase 3
- BDNF
brain-derived neurotrophic factor
- CHRNA3
cholinergic receptor, nicotinic, alpha 3
- GIPR
gastric inhibitory polypeptide receptor
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shijian Liu, Shanghai Children's Medical Center, School of Public Health, Shanghai Jiaotong University School of Medicine, Shanghai, 200127, China.
Jim Wilson, Physiology & Biophysics, University of Mississippi Medical Center, Jackson, MS, 39216, USA.
Fan Jiang, Shanghai Children's Medical Center, School of Public Health, Shanghai Jiaotong University, School of Medicine, Shanghai, 200127, China.
Michael Griswold, Center of Biostatistics and Bioinformatics, University of Mississippi Medical Center, Jackson, MS, 39216, USA.
Adolfo Correa, Jackson Heart Study, University of Mississippi Medical Center, Jackson, MS, 39216, USA.
References
- Akey J, Jin L, Xiong M. Haplotypes vs single marker linkage disequilibrium tests: what do we gain? Eur J Hum Genet. 2001;9:291–300. doi: 10.1038/sj.ejhg.5200619. [DOI] [PubMed] [Google Scholar]
- Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, van Vliet-Ostaptchouk JV, Wijmenga C, van der Schouw YT. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009;90:951–9. doi: 10.3945/ajcn.2009.27781. [DOI] [PubMed] [Google Scholar]
- Berndt SI, Gustafsson S, Magi R, Ganna A, Wheeler E, Feitosa MF, Justice AE, Monda KL, Croteau-Chonka DC, Day FR, Esko T, Fall T, Ferreira T, Gentilini D, Jackson AU, Luan J, Randall JC, Vedantam S, Willer CJ, Winkler TW, Wood AR, Workalemahu T, Hu YJ, Lee SH, Liang L, Lin DY, Min JL, Neale BM, Thorleifsson G, Yang J, Albrecht E, Amin N, Bragg-Gresham JL, Cadby G, den Heijer M, Eklund N, Fischer K, Goel A, Hottenga JJ, Huffman JE, Jarick I, Johansson A, Johnson T, Kanoni S, Kleber ME, Konig IR, Kristiansson K, Kutalik Z, Lamina C, Lecoeur C, Li G, Mangino M, McArdle WL, Medina-Gomez C, Muller-Nurasyid M, Ngwa JS, Nolte IM, Paternoster L, Pechlivanis S, Perola M, Peters MJ, Preuss M, Rose LM, Shi J, Shungin D, Smith AV, Strawbridge RJ, Surakka I, Teumer A, Trip MD, Tyrer J, Van Vliet-Ostaptchouk JV, Vandenput L, Waite LL, Zhao JH, Absher D, Asselbergs FW, Atalay M, Attwood AP, Balmforth AJ, Basart H, Beilby J, Bonnycastle LL, Brambilla P, Bruinenberg M, Campbell H, Chasman DI, Chines PS, Collins FS, Connell JM, Cookson WO, de Faire U, de Vegt F, Dei M, Dimitriou M, Edkins S, Estrada K, Evans DM, Farrall M, Ferrario MM, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45:501–12. doi: 10.1038/ng.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidulescu A, Liu J, Musani SK, Fox ER, Samdarshi TE, Sarpong DF, Vaccarino V, Wilson PW, Arnett DK, Din-Dzietham R, Taylor HA, Gibbons GH. Association of adiponectin with left ventricular mass in blacks: the Jackson Heart Study. Circ Heart Fail. 2011;4:747–53. doi: 10.1161/CIRCHEARTFAILURE.110.959742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenacci VA, Hill JO, Wyatt HR. The obesity epidemic. Clin Chest Med. 2009;30:415–44. vii. doi: 10.1016/j.ccm.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Qi L, Zhang C, Kraft P, Manson J, Cai T, Hunter DJ, Hu FB. Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Ann Intern Med. 2009;150:541–50. doi: 10.7326/0003-4819-150-8-200904210-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsapas C, Speliotes EK, Hatoum IJ, Greenawalt DM, Dobrin R, Lum PY, Suver C, Chudin E, Kemp D, Reitman M, Voight BF, Neale BM, Schadt EE, Hirschhorn JN, Kaplan LM, Daly MJ Consortium G. Common body mass index-associated variants confer risk of extreme obesity. Hum Mol Genet. 2009;18:3502–7. doi: 10.1093/hmg/ddp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culverhouse R, Suarez BK, Lin J, Reich T. A perspective on epistasis: limits of models displaying no main effect. Am J Hum Genet. 2002;70:461–71. doi: 10.1086/338759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks CE, Loos RJ, Hardy R, Wills AK, Wong A, Wareham NJ, Kuh D, Ong KK. Adult obesity susceptibility variants are associated with greater childhood weight gain and a faster tempo of growth: the 1946 British Birth Cohort Study. Am J Clin Nutr. 2012;95:1150–6. doi: 10.3945/ajcn.111.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6, 18–29. [PubMed] [Google Scholar]
- Greene CS, Himmelstein DS, Nelson HH, Kelsey KT, Williams SM, Andrew AS, Karagas MR, Moore JH. Enabling personal genomics with an explicit test of epistasis. Pac Symp Biocomput. 2010:327–36. doi: 10.1142/9789814295291_0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19:376–82. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- Hasselbalch AL. Genetics of dietary habits and obesity - a twin study. Dan Med Bull. 2010;57:B4182. [PubMed] [Google Scholar]
- Iranzo-Tatay C, Gimeno-Clemente N, Livianos-Aldana L, Rojo-Moreno L. Genetic and environmental contributions to body mass index in a Spanish adolescent twin sample. Med Clin (Barc) 2015;145:153–9. doi: 10.1016/j.medcli.2014.05.039. [DOI] [PubMed] [Google Scholar]
- Jiao H, Arner P, Hoffstedt J, Brodin D, Dubern B, Czernichow S, van't Hooft F, Axelsson T, Pedersen O, Hansen T, Sorensen TI, Hebebrand J, Kere J, Dahlman-Wright K, Hamsten A, Clement K, Dahlman I. Genome wide association study identifies KCNMA1 contributing to human obesity. BMC Med Genomics. 2011;4:51. doi: 10.1186/1755-8794-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolchik D, Barber GP, Casper J, Clawson H, Cline MS, Diekhans M, Dreszer TR, Fujita PA, Guruvadoo L, Haeussler M, Harte RA, Heitner S, Hinrichs AS, Learned K, Lee BT, Li CH, Raney BJ, Rhead B, Rosenbloom KR, Sloan CA, Speir ML, Zweig AS, Haussler D, Kuhn RM, Kent WJ. The UCSC Genome Browser database: 2014 update. Nucleic Acids Res. 2014;42:D764–70. doi: 10.1093/nar/gkt1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn JM, Kuruvilla FG, McCarroll SA, Wysoker A, Nemesh J, Cawley S, Hubbell E, Veitch J, Collins PJ, Darvishi K, Lee C, Nizzari MM, Gabriel SB, Purcell S, Daly MJ, Altshuler D. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet. 2008;40:1253–60. doi: 10.1038/ng.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Jacobs DR, Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, Williams OD. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–81. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle PF, O'Connell JR, Pollin TI, Baumgarten M, Shuldiner AR, Peyser PA, Mitchell BD. Accounting for relatedness in family based genetic association studies. Hum Hered. 2007;64:234–42. doi: 10.1159/000103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H. Multifactor dimensionality reduction-phenomics. http://hihg.med.miami.edu/software-download/mdr-phenomics.
- Mei H, Chen W, Jiang F, He J, Srinivasan S, Smith EN, Schork N, Murray S, Berenson GS. Longitudinal Replication Studies of GWAS Risk SNPs Influencing Body Mass Index over the Course of Childhood and Adulthood. PLoS One. 2012;7:e31470. doi: 10.1371/journal.pone.0031470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Cuccaro ML, Martin ER. Multifactor dimensionality reduction-phenomics: a novel method to capture genetic heterogeneity with use of phenotypic variables. Am J Hum Genet. 2007;81:1251–61. doi: 10.1086/522307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proenca C, Gaget S, Korner A, Kovacs P, Kiess W, Tichet J, Marre M, Hartikainen AL, Horber F, Potoczna N, Hercberg S, Levy-Marchal C, Pattou F, Heude B, Tauber M, McCarthy MI, Blakemore AI, Montpetit A, Polychronakos C, Weill J, Coin LJ, Asher J, Elliott P, Jarvelin MR, Visvikis-Siest S, Balkau B, Sladek R, Balding D, Walley A, Dina C, Froguel P. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41:157–9. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138–47. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen L, Young J, Birditt B, Kaur A, Madan A, Philipps DL, Qin S, Minx P, Wilson RK, Hood L, Graveley BR. Analysis of the human neurexin genes: alternative splicing and the generation of protein diversity. Genomics. 2002;79:587–97. doi: 10.1006/geno.2002.6734. [DOI] [PubMed] [Google Scholar]
- Rukh G, Sonestedt E, Melander O, Hedblad B, Wirfalt E, Ericson U, Orho-Melander M. Genetic susceptibility to obesity and diet intakes: association and interaction analyses in the Malmo Diet and Cancer Study. Genes Nutr. 2013;8:535–47. doi: 10.1007/s12263-013-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silventoinen K, Kaprio J. Genetics of tracking of body mass index from birth to late middle age: evidence from twin and family studies. Obes Facts. 2009;2:196–202. doi: 10.1159/000219675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Hu X, Peng Y. An analytical comparison of the principal component method and the mixed effects model for association studies in the presence of cryptic relatedness and population stratification. Hum Hered. 2013;76:1–9. doi: 10.1159/000353345. [DOI] [PubMed] [Google Scholar]
- Welter D, Macarthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014a;42:D1001–6. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014b;42:D1001–6. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S, Henning E, Blackburn H, Loos RJ, Wareham NJ, O'Rahilly S, Hurles ME, Barroso I, Farooqi IS. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat Genet. 2013;45:513–7. doi: 10.1038/ng.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.