Abstract
Electrospun drug-eluting fibers are emerging as a novel dosage form for multipurpose prevention against sexually transmitted infections, including HIV, and unintended pregnancy. Previous work from our lab and others show the versatility of this platform to deliver large doses of physico-chemically diverse agents. However, there is still an unmet need to develop practical fiber formulations for water-soluble small molecule drugs needed at high dosing due to intrinsic low potency or desire for sustained prevention. To date, most sustained release fibers have been restricted to the delivery of biologics or hydrophobic small molecules at low drug loading of typically < 1 wt.%, which is often impractical for most clinical applications. For hydrophilic small molecule drugs, their high aqueous solubility and poor partitioning and incompatibility with insoluble polymers make long-term release even more challenging. Here we investigate several existing strategies to sustain release of hydrophilic small molecule drugs that are highly-loaded in electrospun fibers. In particular, we investigate what is known about the design constraints required to realize multi-day release from fibers fabricated from uniaxial and coaxial electrospinning.
Keywords: Electrospun fibers, Uniaxial, Coaxial, Sustained release, Drug delivery
1. Introduction
Electrospinning is an established technique to produce small diameter fibers in the range of several nanometers to micrometers. Since the advent of using electrostatic forces to produce fibers in the 1930s [1], the process of electrospinning has become widely employed in many engineering fields including filtration [2–4], masking/fabrics [5–9], sensors [10,11], and energy-related applications [12]. The theory of electrospinning, including the role that processing and solution variables have on fiber size, architecture and mechanical properties has been extensively reviewed previously by others [13–15]. In addition, there have been several comprehensive reviews on scale-up processes for electrospinning and their applications in tissue engineering and drug delivery [16–24]. Among the biomedical applications, drug delivery is one of the most promising areas to employ electrospun materials. The advantages of using electrospun fibers in drug delivery include: (1) high drug loading (up to 60%) and encapsulation efficiency (up to 100%) [25–27], (2) polymer diversity to accommodate compatibility with physico-chemically distinct agents [28,29], (3) ability to modulate release [30,31], and (4) process simplicity and cost-effectiveness [32].
Due to the platform versatility of electrospinning, there is an emerging interest to understand the role of electrospun materials in addressing clinical gaps associated with treatment and prevention of bacterial and viral infections. The development of long-acting drug formulations is being sought in many of these applications to overcome challenges with adherence and emerging drug resistance. However, many antimicrobial agents require high and frequent dosing for efficacy due to their low intrinsic potency and short half-lives, when administered in vivo [33]. For example, common antibiotics used to treat bacterial infections are typically dosed at a minimum of hundreds of milligrams per day for several weeks. Long-acting strategies for HIV prevention and treatment require similar dosing regimens for existing antiretroviral drugs currently on the market [34]. The high aqueous solubility of many lead antimicrobial agents further precludes their formulation into long-acting dosage forms by standard techniques such as milling to produce nanocrystalline drug dispersions. A better understanding of the current design space required to achieve sustained release from electrospun fibers will help inform the platform's applicability to address these specific clinical gaps in drug delivery.
The purpose of this review is to highlight current strategies using electrospun fibers for sustained drug release, particularly in applications where high drug dosing may be needed for clinical efficacy. We give specific attention to approaches for sustaining the release of hydrophilic small molecule drugs that are highly loaded in fiber formulations. In particular, we focus on examples of drug-eluting fibers that meet a minimum of 20 wt.% loading and achieve at least daily release of 10-100 mg of drugs for up to a week or longer. Much of the research and development in the field has employed uniaxial fibers or core–shell fibers fabricated by coaxial electrospinning (Table 1), which will be the main emphasis of this review. These studies highlight our current knowledge of the material compositions and solid-state characteristics needed to achieve high dosing and sustained release of agents from electrospun fibers in applications requiring long-acting clinical efficacy.
Table 1.
Strategies for sustained drug release from electrospun fibers.
| Fiber architecture | Agent |
Release (units) |
Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Loading wt.% | Aq. Sol.a
(mg/mL) |
Log Pa | 1 h | 24 h | 7d | 14d | ||
| Uniaxial Fibers | |||||||||
| PLGA | Paclitaxel | 10 | <0.01 | 3.95 | 10% | 22% | 40% | 50% | [54] |
| Cefoxitin sodium | 5 | 0.15 | −0.92 | 70% | 72% | 80% | – | [37] | |
| PLA | Tetracycline hydrochloride | 5 | 0.46 | −0.42b | 35% | 35% | 35% | – | [77] |
| Metronidazole | 40 | 29 | −0.14 | 5% | 25% | 45% | – | [64] | |
| Amoxicillin | 7 | 0.58 | 0.88 | 10% | 15% | 20% | 20% | [78] | |
| PLLA | Paclitaxel | 15 | <0.01 | 3.95 | 0–1% | 0–1% | – | – | [57] |
| Doxorubicin hydrochloride | 1.6 | 1.18b | 1.41b | 70% | 87% | – | – | [57] | |
| Doxorubicin | 1.6 | 0.41 | 0.24 | 20% | 20% | – | – | [57] | |
| Polyurethane | Itraconazole | 40 | <0.01 | 4.99 | 2ug/cm2 | 20ug/cm2 | – | – | [36] |
| Ketanserin | 10 | <0.01 | 3.56 | 2ug/cm2 | 10ug/cm2 | – | – | [36] | |
| Coaxial fibers (core:shell Qc/Qs) |
|||||||||
| PEG:PBS 3/1 | Triclosan | 5 | <0.01 | 5.34 | 75% | – | – | – | [75] |
| Curcumin | 5 | 0.05 | 3.07 | 90% | – | – | – | [75] | |
| 28%Zein:1%Zein 4/1 | Ketoprofen | 10 | 58 | 2.91 | 5% | 100% | – | – | [79] |
| PCL:Gelatin 1/2 | Metronidazole | 33.4 | 29 | −0.14 | 5% | 60% | 95% | 100% | [76] |
| PCL:PVA 1/8 | Metoclopramide hydrochloride | 1 | 0.24b | 2.36b | 5% | 55% | 65% | 68% | [68] |
| PLLA:PVA 1/8 | Metoclopramide hydrochloride | 1 | 0.24b | 2.36b | 2% | 12% | 22% | 25% | [68] |
| PLGA:PVA 1/8 | METOCLOPRAMIDE hydrochloride | 1 | 0.24b | 2.36b | 5% | 38% | 62% | 72% | [68] |
| PCL:PEG | Salicylic acid | 10 | 1000 | 2.01 | 10% | 25% | 40% | – | [80] |
| PEG:PLA 1/4 | Salicylic acid | 15 | 1000 | 2.01 | 0.1 mg/mL | 0.2 mg/mL | – | – | [70] |
| PEG:cellulose acetate and gelatin 1/2 | Amoxicillin | 3.7 | 0.58 | 0.88 | 22% | 100% | – | – | [73] |
| PMMA:Nylon6 1/1 | Ampicillin | 20 | 0.87 | 1.48 | 100ug/mL | 300ug/mL | 600ug/mL | 800ug/mL | [67] |
Scifinder, substance identifier: calculated using Advanced Chemistry Development (ACD/Labs) software v11.02; (accessed Jul 04, 2015).
DrugBank v4.3: calculated using ALOGPS v2.1; (accessed Jul 04, 2015).
2. Material compositions for sustained drug release from electrospun fibers
2.1. Polymers and excipients
The choice of polymer represents a key component in the development of sustained release electrospun fibers (Table 1). The seemingly unlimited number of natural and synthetic polymers that can be electrospun highlights the versatility of this platform. Therefore, the intended application for the sustained release fibers often dictates the polymer selection. Both non-biodegradable and biodegradable polymers have been used for sustained release from electrospun fibers [35, 36]. The most prevalent polymers used in electrospun fibers for sustained release are biodegradable polyesters such as polylactic acid (PLA), polyglycolic acid (PGA), poly (lactic-co-glycolic) acid (PLGA), and polycaprolactone (PCL) [37]. Common non-degradable synthetic polymers employed for sustained drug release included polyurethane (PU), polycarbonate, and nylon-6. Verreck et al. [36] demonstrated controlled release of two hydrophobic drugs loaded at 10–40 wt.% in PU electrospun fibers. While some naturally occurring polymers such as silk, collagen, gelatin, alginate, and chitosan have been investigated for sustained drug release from fibers, their shear-thinning properties make them challenging to electrospin alone or at high yield. These polymers have been reviewed by Piskin et al. [38] on their ability to be electrospun into nanofibers.
The mechanism of drug release from fibers with these polymeric compositions can be attributed to a combination of diffusive processes, polymer degradation, drug partitioning in polymers, and drug dissolution. In both degradable and non-degradable polymers that are also non-swellable, drugs must diffuse through the solid polymer matrix before diffusion into the bulk. The rate of drug diffusion out of the polymer matrix reflects a number of processes including the rate of water diffusion into the polymer, partitioning and solubility of the drug between the polymer and the bulk, and diffusivity of the drug in the polymer. For non-degradable polymers, the average distance a drug diffuses through the polymer matrix is dependent on a fixed geometry. In contrast, the geometry for a biodegradable polymer changes with time and the average distance of diffusion will vary depending on the rate of polymer degradation.
Semi-crystalline and glassy polymers are often used for sustained drug release due to the slower rate of water diffusion into these materials. Cross-linked polymers can also impede water penetration, which make them useful materials for some sustained release applications [39]. Yohe et al. [40] demonstrated that controlling the hydrophobicity of PCL fibers with a dopant is an effective strategy to tune drug release by affecting the rate of water diffusion into the electrospun porous fiber network. In contrast, other excipients such as glycerol have been used to enhance the wetting process and accelerate drug release [25]. Properties of the drug and its final form in the solid dispersion with the polymer fiber will also greatly impact the observed release kinetics. As will be discussed in detail below, compatibility of the drug with the solvent, as well as the evaporation rate of the solvent during electropsinning, can influence the solid-state characteristics of the formulation. In general, the electrospinning platform allows for a number of interconnected factors to be controlled but care must be taken to achieve designs for sustained drug release.
2.2. Hydrophilic agents for sustained release
Although a wide variety of agents can be incorporated into electrospun fibers [41], most examples of sustained drug release out to at least 7 days have been primarily limited to hydrophobic small molecule drugs or large biological macromolecules. These agents are more amenable to sustained release due to their poor solubility, large size, or preferential partitioning into insoluble polymers. In contrast, hydrophilic small molecule drugs represent a major challenge in sustained release because of high solubility with the release media, poor partitioning, and low compatibility with many hydrophobic polymers. Drug-polymer compatibility can correlate with the ability to fully encapsulate drugs and accomplish sustained release. Hydrophilic small molecule drugs that have low solubility with a nonpolar solvent-polymer system will more likely partition to the fiber surface and result in burst release.
A majority of the studies investigating hydrophilic small molecule drug loading and release from electrospun fibers have focused on antibiotics and some antiviral compounds (Table 1). The physico-chemical diversity of small molecule drugs with respect to parameters such as aqueous solubility, partition coefficient, ionization and pKa, molecular dipole, glass transition and melt temperature, are all important factors that will contribute to its interaction with the solvent and polymer both in solution and in the final solid dispersion. As such, using model hydrophilic compounds to extrapolate structure-function relationships between the fiber formulation characteristics and drug release kinetics should be interpreted with caution. For example, a recent study by Carson et al. [42] demonstrated the ability to tune the release 10–40 wt.% tenofovir from 24 h out to 30 days, using PCL/PLGA electrospun fibers. The study showed that higher PCL or PLGA content yielded faster or slower diffusional release of tenofovir, respectively. The goal was to generalize the PCL/PLGA electrospun fiber platform to other hydrophilic small molecule drugs. However, the release of azidothymidine, maraviroc, raltegravir, and tenofovir disoproxil fumarate was much faster compared to tenovir, using equivalent PCL/PLGA fiber formulations. This suggests that even slight differences within the chemical structures of these compounds compared to tenofovir, can affect release rates. Therefore, a deeper understanding of the drug-polymer–solvent interactions at all stages of the electrospinning process will require availability and access to lead clinical compounds.
The large drug loading needed for clinical applications that require high daily dosing (10–100 mg/dose) presents additional challenges for sustaining drug release from fibers. Higher drug loading often results in increased burst release due to larger amounts of surface-associated drug and the high surface area of fibers. In fact, sustained release of small molecule drugs from fibers has been typically performed with low drug loading (< 1 wt.%), which limits clinical applications for treating or preventing many bacterial and viral infections. For example, Ball et al. [28] fabricated nanofibers using various biodegradable polymers for sustained release. In their studies, several microbicides such as maraviroc, azidothymidine, acyclovir, and glycerylmonolaurate were successfully incorporated into the nanofibers without any toxicity to various cells and explants. Although the nanofibers provided sustained release for some of these agents, they were all loaded at only 1 wt.%, which is not clinically relevant for the proposed applications [28]. In a separate example for the same clinical purpose, Huang et al. [43] loaded tenofovir disoproxil fumarate, a water-soluble antiretroviral prodrug, into fibers electrospun from a polymer that would undergo dissolution in response to a pH change induced by semen. However, they did not demonstrate sustained release of the prodrug or provide any analytical data describing the solid drug dispersion in the finished fibers.
2.3. Analytical methods to characterize drug-polymer fiber dispersions
Analytical techniques can be employed at different stages of the electrospinning process to investigate the structure-function relationship between formulation properties and performance attributes of the final drug-loaded polymer fibers. Solution properties of the solvent containing drug, polymer, and other excipients impact both the electrospinning process and resulting fibers. A critical solution viscosity is needed to maintain molecular chain entanglement to form fibers during the electrospinning process and is measured with a rheometer. Although the solution viscosity will depend on the polymer and other factors, typical values of viscosity are reported in the range of 0.5-2 Pa·s [44,45]. Drug–polymer interactions in solution can also induce a change in fluid surface tension, which is measured using a tensiometer, and affect Taylor cone formation and jet initiation. A drug–polymer solution with low surface tension, achieved by using nonpolar or weakly polar solvents, favors the formation of a Taylor cone at a much lower electrospinning voltage [46]. Furthermore, the conductivity of the solution can influence the ability to electrospin nanofibers as well as the morphology of the resulting fibers. In general, characterization of the electrospinning solution can probe drug–polymer–solvent interactions that may inform the properties of the final solid dispersion and release kinetics.
Several techniques are also employed to investigate the bulk properties of the fiber mats or fabrics that emerge from the electrospinning process. The physical properties of a fabric can be accessed by the measurement of density (basis weight), uniformity, surface roughness, and mechanical strength. These properties can influence both drug release kinetics and also user perceptions of a product in clinical trials. Other than these physical properties, specific fiber properties have a direct association to the release mechanism. For example, fiber size, morphology, arrangement, and surface features individually and alone can impact the drug release kinetics [47]. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are standard techniques used to measure fiber size and size distribution, as well as alignment. Microscopy techniques can also be used to visualize crystalline drug dispersed within the polymer fibers. X-ray photoelectron spectroscopy (XPS) can be used to probe the surface chemistry of a fiber and quantify the amount of drug enriched at the fiber surface, which can result in burst release kinetics. Other analytical techniques used to characterize electrospun nanofibers include differential scanning calorimetry (DSC) and X-ray diffraction (XRD), which can probe drug–polymer compatibility and crystallinity in the finished fibers. Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy are also useful tools to examine the molecular configurations of the drug and polymer in the resulting fibers. In general, these analytical techniques are useful for probing the bulk properties of a fabric to understand their effect on drug release from the final solid dispersions.
3. Uniaxial fiber design considerations for sustained release
3.1. Uniaxial electrospinning process and solution properties
Uniaxial electrospun fibers represent a relatively simple and scalable method to achieve rapid and sustained release of encapsulated drugs. A bench scale uniaxial electrospinning setup requires a micropump, syringe, syringe needle, power generator, metal collector, and polymer solution. Briefly, a micropump is used to extrude a viscous polymer solution containing drugs and other excipients through a syringe needle. The polymer fluid is charged using an applied external voltage that results in a single instable Taylor cone, which emenates in a fluid jet that is whipped toward a grounded collector. This process causes rapid evaporation of solvent yielding small-diameter solid polymer fibers.
Processing parameters and formulation properties represent important design elements that can impact the development of sustained release fibers. Changing the polymer concentration, electric field strength, feed rate, and other factors can potentially alter the final finished fiber solid drug dispersion, which in turn can affect the drug release kinetics. Xie and Buschle–Diller [48] showed that fiber diameter, which can be tuned by electrospinning processing and solution parameters, can affect diffusional release of 2 wt.% tetracycline from PDLLA fibers. In the study, the group incorporated increasing amounts of methanol as a cosolvent to control the resulting fiber diameters. A 1:16 or 1:4 ratio of cosolvent/solvent produced ~800 nm and ~200 nm fibers, respectively. Release studies indicated that larger diameter fibers yielded slower release compared to smaller fibers. After 24 h, smaller diameter fibers released threefold more tetracycline than larger diameter fibers. However, the group also studied the effect of fiber diameter on release of 2 wt.% loaded chlorotetracycline from PDLLA electrospun fibers. Results showed that ~1550 nm fibers release chlorotetracycline faster than ~200 nm fibers. These differences in drug release as a function of fiber diameter may be a result of swelling behavior and drug solubility in the different fiber formulations. Other studies have also shown differing effects of fiber diameter on release behavior [49,50]. Verreck et al. [50] posited that smaller diameter fibers are more tightly packed, inhibiting the rate of matrix swelling. It would be expected that for pure diffusional release, larger diameter fibers would create a longer path length for drug to diffuse, but these studies remind us that drug release mechanisms are often very complex. Formulation properties such as polymer concentration and solvent choice can directly affect electrospin processing and resulting drug dispersion in the fibers [50]. Therefore, formulation properties and processing parameters must be considered simultaneously when developing uniaxial sustained electrospun fibers.
3.2. Effect of polymer and drug dispersion on sustained release
In contrast to burst release fibers, sustained release fibers require extensive design considerations depending on the desired drug release application. Because burst release fibers generally incorporate fast dissolving polymers, which quickly hydrate in aqueous solution and dump their drug load [25–27,51,52], design of these systems are relatively simple when compared to design constraints necessary for sustained release applications. Some design considerations include polymer choice, drug physiochemical properties, drug–polymer compatibility, formulation properties, and electrospinning processing parameters. The interplay of each aforementioned design parameters can be complex and significantly affect the release profile of drug loaded-electrospun fibers. However, the rate of matrix hydration and drug diffusion out of polymer has been the primary focus of many studies [40,53]. In this section, we discuss how each of these aspects contributes to the design of sustained release uniaxial electrospun fibers.
As mentioned previously, PLGA is a well-established electrospun fiber system for sustained release of hydrophobic and hydrophilic compounds. Xie and Wang [54] showed the sustained release of paclitaxel, a lipophilic anti-cancer drug, out to 60 days from PLGA electrospun fibers loaded at 10 wt.%. The same PLGA fiber used by Kim et al. [55] demonstrated controlled release of cefoxitin sodium salt, a hydrophilic antibacterial drug incorporated at 5 wt.%. However, in this study the results showed that 1 wt.% and 5 wt.% loaded cefoxitin sodium salt resulted in a significant burst portion within the first hour of release. Incorporation of PLA/PEG-b-PLA into PLGA yielded continuous sustained release of 27% of loaded drug over one week, after an initial burst phase. These examples show the use of PLGA for long-term release of a hydrophobic drug out to two months and sustained release of a hydrophilic drug out to one week. However, significant gaps in sustained release remain, particularly related to the sustained release of hydrophilic molecules. The study by Kim et al. [55] still shows significant burst release (~50–70% in first hour), and the 5 wt.% drug loading is likely insufficient for many clinical applications that require high doses and realistic total dosage sizes.
Yohe et al. [40] demonstrated that the use of superhydrophic electrospun fibers could control air-displacement and sustain release of a 1 wt.% small molecule hydrophobic drug past 60 days. This study focused on controlling the rate of matrix hydration, by first displacing air, as a means of sustaining drug release. The high glass transition temperatures of some polymers can be used for sustaining drug release by hindering diffusing through the polymer chains. This mechanism hinges on uniform dispersion of drug molecules through polymer chain networks within single electrospun fibers. Lyu et al. [56] showed the effect of polymer Tg on drug release. In the study, hydrophobic dexamethasone was loaded at 10 wt.% into two polyurethanes with low and high glass transition temperatures. The results showed that drug release was much slower for the high Tg polyurethane and much faster in the low Tg polyurethane. Furthermore, blends of the two polymers yielded intermediate release of the hydrophobic drug. The examples above incorporating hydrophobic drugs into fibers showed sufficient sustained release but again are limited by the relatively low drug loading used in the fibers.
An important factor associated with sustained release from uniaxial matrix fibers is drug-polymer compatibility [57]. Drug polymer compatibility refers to the physical interaction between drug molecules and polymer chains, which will influence the drug distribution in the final solid drug dispersion. This compatibility is primarily dependent on drug solubility in the polymer–solvent system. Zeng et al. [57] demonstrated the effect of drug compatibility on release by loading 15 wt.% lipophilic paclitaxel and 1.6 wt.% hydrophilic doxorubicin hydrochloride into PLLA electrospinning solution. Paclitaxel was highly soluble in the organic solvent used in the formulations whereas doxorubicin hydrochloride showed low solubility. As a result, during electrospinning the rapid evaporation of solvent allowed paclitaxel to be fully dispersed and encapsulated within the finished solid fibers. Because doxorubicin hydrochloride was not soluble, phase separation occurred during the spinning process that resulted in a large portion of the drug on the surface of the fibers, causing higher burst release. Conversion of doxorubicin hydrochloride to a lipophilic drug by addition of ammonia resulted in better solubility in solution and improved encapsulation in the finished fiber. Seif et al. [58] further investigated drug–polymer compatibility and how it affects drug crystallization and subsequent release. The study investigated the tendency of ~ 10 wt.% hydrophilic caffeine loaded in PCL to crystallize based on both the polymer–drug compatibility as well the solvent selection. The results of the study demonstrated that release of caffeine from PCL was dampened in fibers without surface crystallized drug compared to fibers with no drug crystals.
The ionization state of incorporated drugs can also influence drug distribution within the finished fibers. The study presented by Kim et al. above suggested that a major portion of incorporated cefoxitin sodium salt localized to the surface because of the high ionic strength of the drug [55]. Ball and Woodrow [32] used XPS analysis to demonstrate enrichment of highly loaded maraviroc (28 wt.%) to the surface of PVP fibers. In this study it was suggest that physico-chemical properties of the drug as well as drug loading influenced the propensity of maraviroc to localize to the fiber surface. A formulation with low drug–polymer compatibility resulting from drug solubility, ionization state, and loading will result in drug partitioning to the surface of the fibers which can then lead to burst release. Drug loading also plays a key role in the propensity of drug to localize to the surface of fibers and several studies have shown that higher drug loading increases burst release [59–61]. Therefore, sustained release of small molecules has been accomplished by using low loadings and hydrophobic compounds to improve compatibility with the required solution properties of sustained release polymers [53,62]. Recent advances have led to the development of more complex platforms to sustain release of compounds [63,64]. However, sustained release from uniaxial electrospun fibers is strongly influenced by drug–polymer–solvent compatibility as it relates to the hydration of the fiber matrix and drug dissolution.
As discussed previously, sustained release from electrospun uniaxial fibers can be significantly manipulated through a number of platform and formulation characteristics (Fig. 1 [40,54]). Polymer choice is the first step in developing a sustained release system where its hydrophobicity and Tg value substantially influence drug release kinetics. Drug compatibility with the polymer–solvent system is also an important design parameter for sustained release from uniaxial fibers. Low drug solubility in the polymer–solvent system can cause preferential localization of drug to the surface of fibers during electrospinning even at low loading, which leads to burst release. Drug loading and ionization state can also influence encapsulation of drug in fibers. Higher drug loading and ionic content have been shown to lead to surface localization of drug. Processing parameters can also influence the morphology of finished fibers, which can affect drug release. The complexity of these design parameters has limited the ability to demonstrate sustained release with a wide variety of drugs, especially at high loadings. Therefore, much of the sustained release observed from uniaxial fibers has been achieved with low loadings and hydrophobic molecules. An optimized combination of the above mentioned design parameters could provide ideal sustained release for both hydrophilic and hydrophobic small molecule drugs at high loadings.
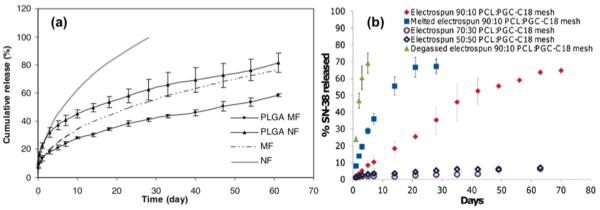
Fig. 1.
Examples of sustained release curves from electrospun uniaxial fibers: (a) 10 wt.% loaded paclitaxel in PLGA microfibers (PLGA MF) and nanofibers (PLGA NF) and first order release curve fitting for microfibers (MF) (k = 0.119, n = 0.45) and nanofibers (NF) (k = 0.223, n = 0.45). Release of paclitaxel sustained for 60 days to 80% cumulative release in PLGA NF and 60% cumulative release in PLGA MF. Fiber diameter significantly affected the release profile. [54]; (b) Release curves of SN-38, a bioactive agent, from blends of PCL and poly (glycerol monostearate-co-ε-caprolactone) (PGC-C18) at ratios of 90:10, 70:30, 50:50 PCL:PGC-C18. Effect of air displacement was minimal for the degassed 90:10 PCL:PGC-C18 mesh comparing to the native one. Melted mesh (at 80 °C for 1 min) reduced the pores and further eliminated time required for air displacement comparing to the native one. Higher PGC-C18 doping in the mesh significantly reduce the release rate. The effect on air displace has a direct influence on the wetting of the mesh and thus affects the release rate [40].
4. Coaxial fiber design considerations for sustained release
4.1. Coaxial electrospinning process and solution properties
Core–shell fibers produced by coaxial electrospinning are an alternative strategy that has been explored to achieve sustained drug release from electrospun materials. Coaxial electrospinning is similar to the process for forming uniaxial fibers except for the nozzle configuration. A coaxial nozzle comprises two concentric but separate nozzles, which are individually controlled by a separate syringe pump. This setup allows for different solutions to be used in each nozzle as well as separate flow rate control. The core–shell fiber architecture is a result of the compound Taylor cone formed by this coaxial nozzle geometry. For sustained release applications, drugs are typically loaded into the polymer solution that forms the inner core whereas the outer shell consists of a polymer that serves as a diffusive barrier for the drugs. Since coaxial electrospinning involves the use of two polymer solutions, the miscibility of the polymers and the solvents used in the core and the shell solution becomes a very important factor that impacts the integrity of the final core–shell architecture. Another important variable is the solution flow rate of the shell and core polymers, which can be controlled to set the shell thickness and core diameter. In general, electrospun coaxial fibers often have the advantage of achieving sustained drug release by localizing drug within the inner core and by tuning the shell thickness.
4.2. Effect of core–shell composition and architecture on sustained release
As mentioned previously (Section 4.1), coaxial fibers have the ability to tune the release profile over a wide range of time scales due to the core–shell structure (Fig. 2 [71,76]). Although a number of reports have described the fabrication and application of various core–shell fibers for drug release (Table 1), the process can require significant optimization to realize sustained release from different core, shell and drug compositions. Here, we consider factors that are most important to the structure of the coaxial fibers and their sustained drug release.
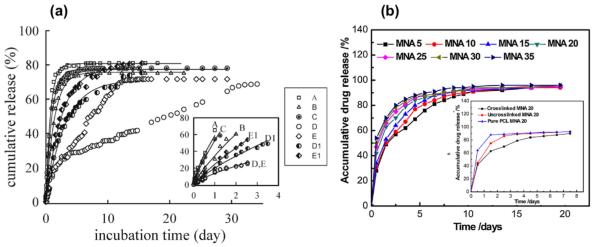
Fig. 2.
Examples of sustained release curves from electrospun coaxial fibers: (a) Cumulative release of DMOG from electrospun fibers. Figure legends: A (PLA), B (PHB), C (PLA-core PHB-shell; Qc/Qs = 0.5/1.5; drug in shell), D (PLA-core PHB-shell; Qc/Qs = 0.5/1.5; drug in core), E (PLA-core PHB-shell; Qc/Qs = 2.0/1.5; drug in core), D1 (PHB-core PLA-shell; Qc/Qs = 1.5/0.5; drug in core), E1 (PHB-core PLA-shell; Qc/Qs = 1.5/2.0; drug in core). Fiber type D showed a sustained release of 70% of DMOG over 30 days. Switching core and shell polymers resulted in burst release due to different hydrophobicity of PLA and PHB. Shell thickness of fiber type D is twice thicker than fiber type E resulting in significant effect in release rate. [71]; (b) Cumulative release of metronidazole (MNA) loaded from 5 wt.% to 35 wt.% in the core of PCL-core gelatin-shell fibers (all gelatin shells were cross-linked). Higher drug loading increased the release rate. Insert showed the release curves on the effect gelatin shell crosslinking. Crosslinking of the gelatin shell resulted in changes of hydrophobicity and therefore changing the release behavior [76].
4.2.1. Integrity of the core–shell structure
The release behavior of drugs from core–shell fibers is highly dependent on the distribution of the drug within the core or shell phase, as well as the final morphology of these compound fibers. Solvent volatility and evaporation rate are of particular importance to produce uniform fiber diameter and regular morphology of core–shell fibers. Katsogiannis et al. [65] describe solvent mixtures of chloroform, dichloromethane, tetrahydrofuran, and formic acid with DMSO to produce PCL fibers with a porous structure. Volatile solvents have faster evaporation rates than DMSO. After the remaining DMSO evaporates, a porous fiber structure is obtained. Since coaxial fibers usually consist of two different types of polymer solutions and solvents, the mixture of solvent at the Taylor cone is unavoidable. Dayal and Kyu [66] have demonstrated that when core and shell solutions are immiscible, and the shell solvent evaporates faster than the core solvent, a hollow fiber is obtained. Even if a hollow fiber structure is avoided, insufficient interfacial compatibility of the core and shell solutions often results in delamination at the interface. By contrast, if the two solvents are miscible and the evaporation rate of the shell solvent is faster than the core solvent, the entrapped core solvent may dissolve some portion of the shell polymer and produce a porous shell structure. Therefore, choosing compatible solvents and polymer solutions is a crucial step to form coaxial fibers with great integrity.
The shell layer of coaxial fibers can often contain some amount of drugs from the core as a result of mixing of the core–shell solvents during electrospinning or due to defects in the core–shell structure. These flaws in the integrity of the core–shell architecture can compromise sustained release and result in burst release of the drug entrapped within the core. For example, Sohrabi et al. [67] showed that the release of the ampicillin loaded in the core of the poly (methyl methacrylate)–nylon6 (core–shell) fibers exhibited an initial burst release of 30%. They suggested that the initial burst release is most likely due to the accumulation of drug molecules at or near the surface of the fibers during coaxial electrospinning. In addition, higher concentrations of ampicillin in the core magnify the accumulation of the drug molecules at or near the surface of the fibers resulting in the initial burst. In a separate example, Tiwari et al. [68] showed the release of metoclopramide hydrochloride from PVA–PCL (core–shell) fibers exhibited an initial burst to about 55% of the total release. They suggested that the burst effect is most likely due to the presence of pores (either micron or nano-sized) in the PCL shell, which is similar to the release mechanism found by others [69,70]. Therefore, the shell structure and integrity are important to achieve sustained release from core–shell fibers.
4.2.2. Shell thickness and composition
Once the structural integrity of the core–shell architecture is achieved to localize the drug in a core that is surrounded by a uniform and smooth shell, control of shell thickness and composition can be further explored to modulate release. In core–shell fibers, most reports suggest that the sustained release profiles are usually a contribution from the ratelimiting effect of drug diffusion through the shell polymer [25,70–74]. Therefore, the thickness and composition of the shell in coaxial fibers play important roles since both affect how well the drug is encapsulated in the core and the wetting behavior of the coaxial fibers during release. Wang et al. [71] used hydrophobic PLA and poly (3-hydroxy butyrate) (PHB) to produce coaxial fibers loaded with dimethyloxalylglycine (DMOG). They observed that coaxial fibers made of PHB-core and PLAshell exhibited a burst release whereas those made of PLA-core and PHB-shell exhibited a two-phase sustained release over 30 days. The first phase of the release was shown to be independent of the shell thickness. However, the second phase showed a linear release that was sustained over 30 days and was dependent on the shell thickness. For example, thin shells (~120 nm) showed ~ 70% cumulative drug release in 11 days, whereas thick shell (~230 nm) required > 30 days to reach the same cumulative percent release. In this example, sustained release of hydrophilic small molecules from coaxial fibers strongly depends on the hydrophobicity of the shell layer and the thickness of the shell.
Llorens et al. [75] compared the release of triclosan (an antibacterial drug) and curcumin (an anti-carcinogenic drug) loaded in the core of coaxial fibers made from PEG and poly (butylene succinate). Their results showed that drug release was higher from poly (butylene succinate)-core and PEG-shell fibers than core-shell fibers of the opposite composition. In addition, they observed that drug releases from core–shell fibers were associated with a controlled wetting mechanism. This was supported by a significant increase in the amount of triclosan and curcumin release upon use of low surface tension release media such as mixtures containing ethanol [75]. In another study reported by He et al. [76], metronidazole was loaded up to 35 wt.% in PCL-core fibers surrounded by a gelatin-shell. Contact angle measurements showed that the gelatin-shell could be made more hydrophobic by crosslinking, and this resulted in sustained release of metronidazole for up to 6 days. In contrast, an uncross-linked gelatin-shell resulted in 80% of cumulative release after 1 day. Overall, there is also a significant dependence on the surface hydrophilic–hydrophobic properties of the coaxial fibers and the sustained release of drugs.
5. Conclusions and future perspectives
We have highlighted the design considerations used to achieve sustained release from electrospun uniaxial and coaxial fibers. In particular, we focused our discussion on current strategies that may be promising for weekly or monthly release of hydrophilic drugs at multimilligram quantities per day. In addition, several design constrains need to be considered for fibers that contain high loading of drugs. For example, sustained release from uniaxial fibers can be greatly affected by polymer choice (where the most important factors are hydrophobicity and Tg of the polymer), polymer and drug compatibility, drug loading and ionization state, and processing parameters. In general, it appears that it is very difficult to reach the specified targets (20 wt.% loading, 10–100 mg daily release for greater than a week) listed for prolonged release of small molecule hydrophilic drugs from uniaxial fibers. Uniaxial electrospun fiber strategies in the future will likely need to focus on blended compositions to realize the complex design constraints associated with sustained release of small water-soluble molecules. In contrast, sustained release from coaxial fibers is greatly influenced by the integrity of the coaxial fiber structure and the shell composition and thickness. Based on the mechanisms of release associated with the two fiber types described above, coaxial fibers are more likely to achieve the required targets for sustained release of small hydrophilic drugs. The ability to load high amounts of drug in a core with a tunable shell is a major advantage when compared to sustained release efforts from uniaxial fibers. In general, electrospinning shows promise for developing sustained release materials with tunable release kinetics that depend upon material surface chemistry, drug loading, and processing parameters. Our mechanistic understanding of hydrophilic drug release from uniaxial and coaxial fibers offers insights to develop more robust electrospun drug release systems.
Acknowledgment
This work is supported by grants from the US National Institutes of Health (AI094412, AI112002) and a grant from the Bill and Melinda Gates Foundation (1067729) awarded to K.A.W.
References
- [1].Furmhals A. US patent 1,975,504 1934
- [2].Gopal R, Kaur S, Ma Z, Chan C, Ramakrishna S, Matsuura T. Electrospun nanofibrous filtration membrane. J. Membr. Sci. 2006;281:581–586. [Google Scholar]
- [3].Aussawasathien D, Teerawattananon C, Vongachariya A. Separation of micron to sub-micron particles from water: electrospun nylon-6 nanofibrous membranes as pre-filters. J. Membr. Sci. 2008;315:11–19. [Google Scholar]
- [4].Schreuder-Gibson H, Gibson P, Senecal K, Sennett M, Walker J, Yeomans W, Ziegler D, Tsai PP. Protective textile materials based on electrospun nanofibers. J. Adv. Mater. 2002;34:44–55. [Google Scholar]
- [5].Gu, Xu Q. Cosmetic mask based on electrospinning nanometer fiber for skin, comprises electrospinning nanometer fiber nonwoven fabric or nonwoven felt. CN101390814-A [Google Scholar]
- [6].Kusamoto N, Tajima T. US patent, US2009031691-A1 Fiber comprises an eggshell membrane component useful for producing a fiber assembly, which is used as a wound dressing or a cosmetic sheet.
- [7].Chung SE, Park CH. US patent KR2010048661-A Manufacture of shape memory polyurethane electrospun web for e.g. protective clothing, involves electrospinning solution obtained by adding poly(caprolactone diol), diphenylmethane diisocyanate and butanediol to polyurethane.
- [8].Lee S, Obendorf SK. Developing protective textile materials as barriers to liquid penetration using melt-electrospinning. J. Appl. Polym. Sci. 2006;102:3430–3437. [Google Scholar]
- [9].Gorji M, Jeddi AAA, Gharehaghaji AA. Fabrication and characterization of polyurethane electrospun nanofiber membranes for protective clothing applications. J. Appl. Polym. Sci. 2012;125:4135–4141. [Google Scholar]
- [10].Rojas R, Pinto NJ. Using electrospinning for the fabrication of rapid response gas sensors based on conducting polymer nanowires. IEEE Sens. J. 2008;8:951–953. [Google Scholar]
- [11].Kowalczyk T, Nowicka A, Elbaum D, Kowalewski TA. Electrospinning of bovine serum albumin. Optimization and the use for production of biosensors. Biomacromol. 2008;9:2087–2090. doi: 10.1021/bm800421s. [DOI] [PubMed] [Google Scholar]
- [12].Dong Z, Kennedy SJ, Wu Y. Electrospinning materials for energy-related applications and devices. J. Power Sources. 2011;196:4886–4904. [Google Scholar]
- [13].Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- [14].Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- [15].Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005;96:557–569. [Google Scholar]
- [16].Zhou FL, Gong RH, Porat I. Mass production of nanofibre assemblies by electrostatic spinning. Polym. Int. 2009;58:331–342. [Google Scholar]
- [17].Persano L, Camposeo A, Tekmen C, Pisignano D. Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol. Mater. Eng. 2013;298:504–520. [Google Scholar]
- [18].Luo CJ, Stoyanov SD, Stride E, Pelan E, Edirisinghe M. Electrospinning versus fibre production methods: from specifics to technological convergence. Chem. Soc. Rev. 2012;41:4708–4735. doi: 10.1039/c2cs35083a. [DOI] [PubMed] [Google Scholar]
- [19].Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010;21:77–95. [Google Scholar]
- [20].Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. B. 2003;67B:675–679. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
- [21].Rieger KA, Birch NP, Schiffman JD. Designing electrospun nanofiber mats to promote wound healing—a review. J. Mater. Chem. B. 2013;1:4531–4541. doi: 10.1039/c3tb20795a. [DOI] [PubMed] [Google Scholar]
- [22].Pillay V, Dott C, Choonara YE, Tyagi C, Tomar L, Kumar P, du Toit LC, Ndesendo VMK. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013:1–22. [Google Scholar]
- [23].Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue. engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- [24].Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009;61:1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- [25].Ball C, Woodrow KA. Electrospun solid dispersions of maraviroc for rapid intravaginal preexposure prophylaxis of HIV. Antimicrob. Agents Chemother. 2014;58:4855–4865. doi: 10.1128/AAC.02564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blakney AK, Krogstad EA, Jiang YH, Woodrow KA. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int. J. Nanomed. 2014;9:2967–2978. doi: 10.2147/IJN.S61664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int. J. Pharm. 2014;475:282–291. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS One. 2012;7:e49792. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blakney AK, Ball C, Krogstad EA, Woodrow KA. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral Res. 2013;100:S9–S16. doi: 10.1016/j.antiviral.2013.09.022. [DOI] [PubMed] [Google Scholar]
- [30].Sundararaj SC, Thomas MV, Peyyala R, Dziubla TD, Puleo DA. Design of a multiple drug delivery system directed at periodontitis. Biomaterials. 2013;34:8835–8842. doi: 10.1016/j.biomaterials.2013.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Falde EJ, Freedman JD, Herrera VLM, Yohe ST, Colson YL, Grinstaff MW. Layered superhydrophobic meshes for controlled drug release. J. Control. Release. 2015;214:23–29. doi: 10.1016/j.jconrel.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ball C, Woodrow KA. In: Electrospun Fibers for Microbicide Drug Delivery. das Neves J, Sarmento B, editors. Pan Stanford Publishing Pte. Ltd.; 2014. pp. 459–499. [Google Scholar]
- [33].Owens RC, Shorr AF. Rational dosing of antimicrobial agents: pharmacokinetic and pharmacodynamic strategies. Am. J. Health-Syst. Pharm. 2009;66:S23–S30. doi: 10.2146/090087d. [DOI] [PubMed] [Google Scholar]
- [34].Spreen WR, Margolis DA, Pottage JC., Jr. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr. Opin. HIV AIDS. 2013;8:565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ranjbar-Mohammadi M, Zamani M, Prabhakaran MP, Hajir Bahrami S, Ramakrishna S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng. C. 2016;58:521–531. doi: 10.1016/j.msec.2015.08.066. [DOI] [PubMed] [Google Scholar]
- [36].Verreck G, Chun I, Rosenblatt J, Peeters J, Dijck AV, Mensch J, Noppe M, Brewster ME. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release. 2003;92:349–360. doi: 10.1016/s0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- [37].McDonald PF, Lyons JG, Geever LM, Higginbotham CL. In vitro degradation and drug release from polymer blends based on poly(DL-lactide), poly(L-lactideglycolide) and poly(e-caprolactone) J. Mater. Sci. 2010;45:1284–1292. [Google Scholar]
- [38].Piskin E, Bolgen N, Egri S, Isoglu IP. Electrospun matrices made of poly(alpha-hydroxy acids) for medical use. Nanomedicine. 2007;2:441–457. doi: 10.2217/17435889.2.4.441. [DOI] [PubMed] [Google Scholar]
- [39].Zhang JJ, Liu J, Yu H, Zhang Y, Zhu MF, Chen YM. Crosslinked electrospun UPM/ PHBV/PVP fibers for sustained drug release. Mater. Sci. 2009:1331–1334. Forum 610–613. [Google Scholar]
- [40].Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for tunable drug release: using displacement of air to control delivery rates. J. Am. Chem. Soc. 2012;134:2016–2019. doi: 10.1021/ja211148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zamani M, Prabhakaran MP, Ramakrishna S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013;8:2997–3017. doi: 10.2147/IJN.S43575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carson D, Jiang Y, Woodrow KA. Tunable release of multiclass anti-HIV drugs that are water soluble and loaded at high drug content in polyester blended electrospun fibers. Pharm. Res. 2015:1–12. doi: 10.1007/s11095-015-1769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huang C, Soenen S, van Gulck E, Vanham G, Rejman J, Van Calenbergh S, et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33:962–969. doi: 10.1016/j.biomaterials.2011.10.004. [DOI] [PubMed] [Google Scholar]
- [44].Graessley WW. The entanglement concept in polymer rheology. Adv. Polym. Sci. 1974;16:1–179. [Google Scholar]
- [45].Stijnman AC, Bodnar I, Tromp RH. Electrospinning of food-grade polysaccharides. Food Hydrocoll. 2011;25:1393–1398. [Google Scholar]
- [46].Szentivanyi A, Assmann U, Schuster R, Glasmacher B. Production of biohybrid protein/PEO scaffolds by electrospinning. Materialwiss. Werkst. 2009;40:65–72. [Google Scholar]
- [47].Meng ZX, Zheng W, Li L, Zheng YF. Fabrication, characterization and in vitro drug release behavior of electrospun PLGA/chitosan nanofibrous scaffold. Mater. Chem. Phys. 2011;125:606–611. [Google Scholar]
- [48].Xie Z, Buschle-Diller G. Electrospun poly(D, L-lactide) fibers for drug delivery: the influence of cosolvent and the mechanism of drug release. J. Appl. Polym. Sci. 2010;115:1–8. [Google Scholar]
- [49].Chen SC, Huang XB, Cai XM, Lu J, Yuan J, Shen J. The influence of fiber diameter of electrospun poly(lactic acid) on drug delivery. Fibers Polym. 2012;9:1120–1125. [Google Scholar]
- [50].Verreck G, Chun I, Peeters J, Rosenblatt J, Brewster ME. Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm. Res. 2003;20:810–817. doi: 10.1023/a:1023450006281. [DOI] [PubMed] [Google Scholar]
- [51].Yu DG, Shen XX, Branford-White C, White K, Zhu LM, Bligh SWA. Oral fastdissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology. 2009;20:055104. doi: 10.1088/0957-4484/20/5/055104. [DOI] [PubMed] [Google Scholar]
- [52].Li X, Kanjwal MA, Lin L, Chronakis IS. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B. 2013;103:182–188. doi: 10.1016/j.colsurfb.2012.10.016. [DOI] [PubMed] [Google Scholar]
- [53].Fredenber S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—a review. Int. J. Pharm. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- [54].Xie J, Wang CH. Electrospun microand nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm. Res. 2006;23:1817–1825. doi: 10.1007/s11095-006-9036-z. [DOI] [PubMed] [Google Scholar]
- [55].Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)based electrospun nanofibrous scaffolds. J. Control. Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- [56].Lyu S, Sparaer R, Hobot C, Dang K. Adjusting drug diffusivity use miscible polymer blends. J. Control. Release. 2005;102:679–687. doi: 10.1016/j.jconrel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [57].Zeng J, Yang LX, Liang QZ, Zhang XF, Guan HL, Xu XL, Chen XS, Jing XB. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control. Release. 2005;105:43–51. doi: 10.1016/j.jconrel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- [58].Seif S, Franzen L, Windbergs M. Overcoming drug crystallization in electrospun fibers — elucidating key parameters and developing strategies for drug delivery. Int. J. Pharm. 2015;478:390–397. doi: 10.1016/j.ijpharm.2014.11.045. [DOI] [PubMed] [Google Scholar]
- [59].Natu MV, de Sousa HC, Gil MH. Effects of drug solubility, state, and loading on controlled release in bicomponent electrospun fibers. Int. J. Pharm. 2010;397:50–58. doi: 10.1016/j.ijpharm.2010.06.045. [DOI] [PubMed] [Google Scholar]
- [60].Zamani M, Morshed M, Varshosaz J, Jannesari M. Controlled release of metronidazole benzoate from poly e-caprolactone electrospun nanofibers for periodontal diseases. Eur. J. Pharm. Biopharm. 2010;2:179–185. doi: 10.1016/j.ejpb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [61].Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J. Investigation of drug release and matrix degradation of electrospun poly(dl-lactide) fibers with paracetanol inoculation. Biomacromolecules. 2006;7:1623–1629. doi: 10.1021/bm060057z. [DOI] [PubMed] [Google Scholar]
- [62].Xie C, Li X, Luo X, Yang Y, Cui W, Zou J, Zhou S. Release modulation and cytotoxicity of hydroxycamptothecin-loaded electrospun fibers with 2hydroxypropylbeta-cyclodextrin inoculations. Int. J. Pharm. 2010;391:55–64. doi: 10.1016/j.ijpharm.2010.02.016. [DOI] [PubMed] [Google Scholar]
- [63].Zhang YZ, Wang X, Feng Y, Li J, Lim CT, Ramakrishna S. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(e-caprolactone) nanofibers for sustained release. Biomacromolecules. 2006;7:1049–1057. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]
- [64].He CL, Huang ZM, Han XJ, Liu L, Zhang HS, Chen LS. Coaxial electrospun poly(llactic acid) ultrafine fibers for sustained drug delivery. J. Macromol. Sci. Part B Phys. 2006;45:515–524. [Google Scholar]
- [65].Katsogiannis KAG, Vladisavljević GT, Georgiadou S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015;69:284–295. [Google Scholar]
- [66].Dayal P, Kyu T. Porous fiber formation in polymer–solvent system undergoing solvent evaporation. J. Appl. Phys. 2006;100:043512. [Google Scholar]
- [67].Sohrabi A, Shaibani PM, Etayash H, Kaur K, Thundat T. Sustained drug release and antibacterial activity of ampicillin incorporated poly(methyl methacrylate) Polymer. 2013;54:2699–2705. [Google Scholar]
- [68].Tiwari SK, Tzezana R, Zussman E, Venkatraman SS. Optimizing partition-controlled drug release from electrospun core–shell fibers. Pharm. Nanotechnol. 2010;392:209–217. doi: 10.1016/j.ijpharm.2010.03.021. [DOI] [PubMed] [Google Scholar]
- [69].Srikar R, Yarin AL, Megaridis CM, Bazilevsky AV, Kelley E. Desorption-limited mechanism of release from polymer nanofibers. Langmuir. 2008;24:965–974. doi: 10.1021/la702449k. [DOI] [PubMed] [Google Scholar]
- [70].Nguyen TTT, Ghosh C, Hwang SG, Chanunpanich N, Park JS. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int. J. Pharm. 2012;439:296–306. doi: 10.1016/j.ijpharm.2012.09.019. [DOI] [PubMed] [Google Scholar]
- [71].Wang C, Yan KW, Lin YD, Hsieh PCH. Biodegradable core/shell fibers by coaxial electrospinning: processing, fiber characterization, and its application in sustained drug release. Macromolecules. 2010;43:6389–6397. [Google Scholar]
- [72].Viry L, Moulton SE, Romeo T, Suhr C, Mawad D, Cook M, Wallace GG. Emulsioncoaxial electrospinning: designing novel architectures for sustained release of highly soluble low molecular weight drugs. J. Mater. Chem. 2012;22:11347–11353. [Google Scholar]
- [73].Kiatyongchai T, Wongsasulak S, Yoovidhya T. Coaxial electrospinning and release characteristics of cellulose acetate–gelatin blend encapsulating a model drug. J. Appl. Polym. Sci. 2014;131 [Google Scholar]
- [74].Malcolm RK, Forbes CJ, Geer L, Veazey RS, Goldman L, Klasse PJ, Moore JP, et al. Pharmacokinetics and efficacy of a vaginally administered maraviroc gel in rhesus macaques. J. Antimicrob. Chemother. 2013;68:678–683. doi: 10.1093/jac/dks422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Llorens E, Ibañez H, del Valle LJ, Puiggalí J. Biocompatibility and drug release behavior of scaffolds prepared by coaxial electrospinning of poly(butylene succinate) and polyethylene glycol. Mater. Sci. Eng. C. 2015;49:472–484. doi: 10.1016/j.msec.2015.01.039. [DOI] [PubMed] [Google Scholar]
- [76].He M, Xue J, Geng H, Gu H, Chen D, Shi R, Zhang L. Fibrous guided tissue regeneration membrane loaded with anti-inflammatory agent prepared by coaxial electrospinning for the purpose of controlled release. Appl. Surf. Sci. 2015;335:121–129. [Google Scholar]
- [77].Kenawy ER, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE. Release of tetracycline hydrochloride from electrospun poly(ethylene-covinylacetate), poly(lactic acid), and a blend. J. Control. Release. 2002;81:57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- [78].Reise M, Wyrwa R, Muller U, Zylinski M, Volpel A, Schnabelrauch M, Berg A, Jandt KD, Watts DC, Sigusch BW. Release of metronidazole from electrospun poly(Llactide-co-D/L-lactide) fibers for local periodontitis treatment. Dent. Mater. 2011;28:179–188. doi: 10.1016/j.dental.2011.12.006. [DOI] [PubMed] [Google Scholar]
- [79].Yang JM, Zha LS, Yu DG, Liu J. Coaxial electrospinning with acetic acid for preparing ferulic acid/zein composite fibers with improved drug release profiles. Colloids Surf. B. 2013;102:737–743. doi: 10.1016/j.colsurfb.2012.09.039. [DOI] [PubMed] [Google Scholar]
- [80].Yu H, Jia Y, Yao C, Lu Y. PCL/PEG core/sheath fibers with controlled drug release rate fabricated on the basis of a novel combined technique. Int. J. Pharm. 2014;469:17–22. doi: 10.1016/j.ijpharm.2014.04.045. [DOI] [PubMed] [Google Scholar]