Abstract
The GRAPPA-OMERACT Psoriatic Arthritis (PsA) working group is in the process of updating the PsA core domain set to improve and standardize the measurement of PsA outcomes. Work streams comprise literature reviews of domains and outcome measurement instruments, an international qualitative research project with PsA patients to generate domains important to patients, outcome measurement instrument assessment, conduct of domain consensus panels with patients and physicians, and evidence-based selection of instruments. Patient Research Partners are involved in each of the projects. The working group will present findings and seek endorsement for the new PsA core domain set, outcome measurement set, and research agenda at the OMERACT Meeting in May 2016.
Key indexing terms: psoriatic arthritis, core set, outcome measures
INTRODUCTION
In order to standardize measurements of disease used in randomized clinical trials, disease-specific groups within the Outcome Measures in Rheumatology organization (OMERACT) have developed Core Domain Sets and Core Outcome Measurement Sets. A Core Outcome Measurement Set defines the minimum measurements that should be collected in randomized controlled trials (RCTs) as well as other studies to inform patients, physicians, and others about the status of patients and efficacy of medication. The core set is recommended for RCTs, and is applicable to longitudinal observational studies and to clinical practice. Before developing a Core Outcome Measurement Set, working groups must first define the “domains” or constructs of most interest to stakeholder groups, i.e., the Core Domain Set. Then measurement instruments can be identified and assessed for each domain. OMERACT published specific methodological standards and step-by-step recommendations to guide disease-specific groups in drafting disease-specific core sets, which could then achieve multi-stakeholder consensus at OMERACT meetings.(1, 2)
The existing psoriatic arthritis (PsA) core domain set for clinical trials, endorsed at the OMERACT meeting in 2006, contains the following domains: peripheral joint activity, skin activity, patient global, pain, physical function, and health-related quality of life.(3) Since the endorsement of the 2006 PsA Core Set,(3, 4) new PsA outcome measures for clinical trials and clinical care have been developed. Patient Research Partners (PRPs) have been included in evaluating the completeness of the core set (4–6) and development of measures.(7) Additionally, OMERACT has developed a new “Filter 2.0” framework, which outlines four core areas to be covered in each core set. These core areas are relevant across all health conditions and need to be matched with disease-specific domains.(2) The PsA OMERACT Working Group is now updating the PsA core domain set with these objectives: 1) increase patient involvement in elaboration of the core set, and 2) integrate the use of the OMERACT Filter 2.0 methodology, adopted in 2014.(2, 8)
The United Kingdom (UK) is leading a synergistic initiative where focus groups will be conducted within the “early detection to imPRove OutcoMe in people with undiagnosed Psoriatic arthriTis” (PROMPT) programme. PROMPT will determine whether early detection improves outcome in patients with undiagnosed PsA and will ensure that outcome measures encompass aspects of early disease. Focus groups will be held to identify the outcomes important to PsA patients. Outcomes will then be ranked by patients and mapped with the existing core set of domains and composite measures of disease to identify omissions within both. Finally, existing patient-reported outcome measures will be identified to address these omissions and inform revised full and shortened versions of composite measures. A follow-up study within PROMPT, assessment of modified COMPosite disease meAsures in REcently diagnosed PsA (COMPARE), will validate these modified composite measures.
As summarized in this report, the PsA Working Group has made significant progress toward their objectives since the May 2014 OMERACT meeting.
PLENARY PRESENTATIONS
Four plenary presentations were made at the 2015 annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): 1) an overview of the multiple ongoing projects aimed at achieving patient and clinician consensus on preliminary PsA core sets of domains and outcome measures (Figure 1); 2) a summary of the development of the patient-derived and disease-specific PsA Impact of Disease (PsAID) (9) outcome measure; 3) a presentation of the generic Patient Reported Outcomes Measurement Information System (PROMIS®) measures and applicability to PsA; and 4) a patient and clinician focus group project in the United States (US) that identifies how patients and physicians prioritize PsA domains and asks patients about the content validity of PsA outcome measures.
Figure 1.
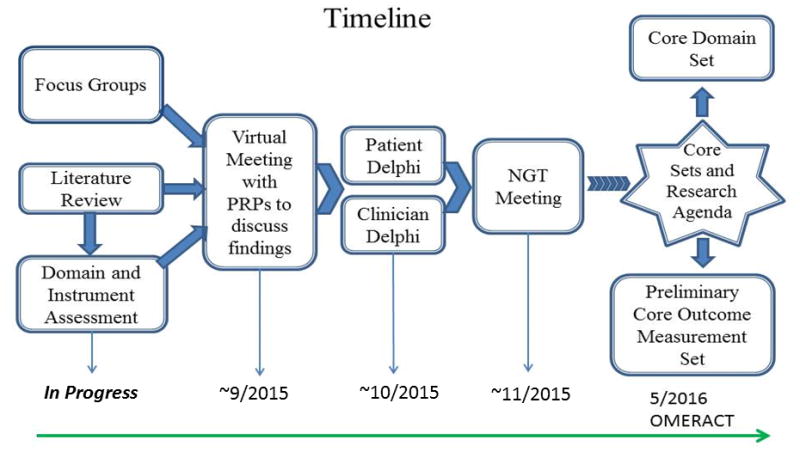
Timeline of the GRAPPA OMERACT Psoriatic Arthritis Core Set Working Group Activities
1. Overview of GRAPPA-OMERACT PsA Working Group activities
Drs. Ana-Maria Orbai, Alexis Ogdie, and Umut Kalyoncu presented the framework, timeline, activities, and preliminary results from the working group. Ongoing projects include 1) two systematic literature reviews (SLR); 2) conduct of international focus groups; 3) outcome measures assessment in clinical trial datasets; and two domain prioritization projects: 4) Delphi exercises separately with patients and physicians; and 5) a face-to-face nominal group technique consensus meeting with both patients and physicians. At least two PRPs are involved in each work stream and a total of five PRPs are part of the working group. The PsA working group also includes two fellows who will be actively involved in conducting the outcome measure literature review, and coordinating the consensus process. Projects are outlined below.
1) Systematic Literature Reviews
Systematic Literature Review 1
In addition to the existing SLR of outcomes measured in PsA RCTs from 2006 to 2010,(10) an SLR of PsA RCTs from 2010 to 2015 is ongoing (SLR1) to generate lists of domains and outcome measures. We presented preliminary results of SLR1. Most of the domains identified in PsA RCTs mapped not only to the existing 2006 PsA core set domains (3) but also to other domains such as “Resource Use,” a core area under the OMERACT Filter 2 framework.(2) Some clinical trial domains mapped to more than one core area, e.g., “patient global” mapped to both pathophysiologic manifestations and life impact and “productivity” to both life impact and resource use. The SLR1 will be expanded to include data from longitudinal observational studies. Further, any additional domains identified from the PsAID outcome measure,(9) previous International Classification of Functioning PsA mapping studies,(11, 12) and the ongoing PsA Flare study (13) will also be included to generate a comprehensive list of candidate domains for the updated PsA core domain set.
Systematic Literature Review 2
This second SLR (SLR2) will focus specifically on psychometric properties of outcome measures.(14) The objective is to synthesize data on truth/validity, feasibility, discrimination, availability of meaningful cutoffs, and patient involvement for each PsA outcome measure.(2) SLR2 will follow methodology developed by the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) group to identify all available studies on the measurement properties of all available outcome measures in PsA.(15, 16) Using the COSMIN checklist for critical appraisal of the measurement properties of each outcome measure will reveal any potential gaps among existing instruments and the need to revise or develop new outcome measurement instruments.
2) Qualitative Research
A multi-national qualitative research project is ongoing in seven countries with two focus groups in each country (US, Netherlands, Australia, Brazil, Canada, France, and Singapore) and five to eight patients in each focus group. The objective is to determine domains of greatest importance to patients with PsA. Qualitative data will be translated into English and analyzed by a core qualitative research team from the US and the Netherlands with input from all investigators and PRPs. Domains identified in focus groups with PsA patients will be added to the comprehensive list of candidate domains, which will be subject to Delphi rounds and nominal group technique meeting (below), for the updated PsA core set.
3) Outcome Measurement Instrument Assessment
A thorough assessment of available outcome measures to measure candidate core domains in PsA is also underway. Clinical trial datasets have been requested from five pharmaceutical companies for the purpose of assessing outcome measure content and construct validity. This will determine additional domains to be included in the Delphi procedures, a draft set of candidate outcome measures, and subsequent steps required to identify candidate responder index/indices.
4) Delphi Exercises to Narrow Candidate Domains
A single comprehensive list of domains will be created by merging domains identified through the aforementioned work streams. This list will be discussed with PRPs and subsequently the entire PsA working group. The discussion with PRPs will center on face validity and completeness of the initial domain list, redundancy, and inclusion of missing domains as needed. The final draft list of domains will be the basis for two parallel domain-ranking Delphi exercises with patients and rheumatologists, using a web-based platform. Diverse international representation will be ensured, with 100 participants in each group. PRPs will help to evaluate and optimize comprehensibility for the patient Delphi, using up to three rounds of surveys. At the conclusion of the Delphi rounds, the most highly ranked domains will be shown on two lists, one each from patients and physicians.
5) Consensus meeting with patients and healthcare providers
A face-to-face consensus meeting including 12 patients and 12 rheumatologists is planned for mid-March 2016. The meeting will be moderated by a methodologist not involved in the working group and using a modified nominal group technique to ensure there is no bias in including both the PRP and rheumatologist perspectives. The objective of the meeting is to reconcile the two domain lists and to define a preliminary core domain set for presentation, consensus, and endorsement at the OMERACT meeting in May 2016.
2. Psoriatic Arthritis Impact of Disease (PsAID)
Dr. Laure Gossec presented the development and validation of the European League against Rheumatism (EULAR) PsAID outcome measure.(9) The PsAID was patient-derived with active involvement of patients on different levels.(7) Domains were identified by PRPs from 11 European countries who participated in a meeting to prioritize PsA health domains. These domains were then subject to prioritization by 139 patients to exclude the four lowest prioritized domains of the initial 16 domains. There are two versions of the PsAID questionnaire: one with 12 domains recommended for clinical care, and one with nine domains recommended for clinical trials. The PsAID was validated in a sample of 447 people with PsA from different European countries. The relation with other well-known outcome measures was evaluated cross-sectionally, and reliability and sensitivity to change in smaller samples was validated longitudinally (N=80 and 71, respectively). The measures appeared to perform well, and reliability was high (ICC=0.95, 95%CI 0.92–0.96). The PsAID questionnaires are free to use and available from the EULAR website in several languages (http://www.eular.org/tools_products.cfm). External validation is ongoing.
3. Patient Reported Outcomes Measurement Information System (PROMIS®)
Dr. Ana-Maria Orbai summarized the steps and methodology used in the development of the Patient Reported Outcomes Measurement Information System (PROMIS®). PROMIS, developed with US National Institutes of Health support, is a library of generic health measures meant to be used across chronic health conditions. PROMIS uses state-of-the-art qualitative, quantitative, and psychometric methodology from health concept definition to outcome measure testing and validation in a large US population sample (n=21,000). Each item was tested in about 900 people from the general population and 500 people living with a chronic disease. PROMIS measures are free to use (http://assessmentcenter.net) and are being translated and validated in multiple languages by the PROMIS International organization.(17, 18) PROMIS measure implementation and expansion is currently focused on validation studies in specific health conditions,(19–23) including testing in PsA in an ongoing longitudinal project at Johns Hopkins.(24)
4. Project Focus Groups with Patients and Physicians
Dr. Philip Mease presented the plan for a US multicenter qualitative study to identify how patients and physicians prioritize health domains in PsA. A second objective is to examine patient perceptions of outcome measures that are either currently being used or are candidate measures for use in PsA clinical trials. The project addresses the content validity of these measures and will inform outcome measure selection for the PsA core outcome measurement instrument set.
DISCUSSION
An update of the 2006 PsA Core Domain Set is underway to ensure that it incorporates the patients’ perspectives and reflects the subsequent accumulated knowledge in the PsA field. For example, we now have a better understanding of patient preferences and priorities from development of new outcome measures for PsA as well as PsA pathophysiology since the discovery and approval of new therapeutics. Researchers in the GRAPPA-OMERACT PsA working group are using OMERACT Filter 2.0 methodology (2, 8, 25) to build on prior work through SLRs and secondary data analyses of outcome measures used in clinical trial datasets. The qualitative research work stream with PsA patients is pivotal in eliciting concepts of importance to patients and ensuring PsA assessments are based on a valid and complete conceptual framework for PsA domains. Equal input from patients and healthcare providers in deciding on core domains through Delphi and consensus meeting components is essential because their priorities complement each other. This is exemplified by the OMERACT 2006 patient perspective workshop (26) and the Rheumatoid Arthritis (RA) Flare Delphi exercises (27) where PRP participation led to the inclusion of fatigue in RA assessments and of additional domains for RA flare assessment. The findings in RA parallel the evolution of PsA data related to fatigue, where fatigue was the third most important domain prioritized by patients (after pain and skin) in the PsAID questionnaire,(9) but has yet to be included in the current PsA core domain set. This situation may be similar for other PsA domains. Concurrently, PsA outcome measurement instruments are being evaluated for their completeness as well as fulfillment of OMERACT Filter 2.0 standards.
Acknowledgments
Grant support: Supported in part by research grant P30-AR053503 (RDRCC Human Subjects Research Core) from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the Camille J. Morgan Arthritis Research and Education Fund. The international focus group study was supported by Celgene and Janssen. The nominal group technique meeting was supported by Abbvie, Celgene, and Pfizer. The patient and physician focus group study was supported by Novartis. A.M. Orbai was supported by a Scientist Development Award from the Rheumatology Research Foundation and by the Johns Hopkins Arthritis Center Discovery Fund. A. Ogdie was supported by research grant K23 AR063764 from NIAMS.
Footnotes
Disclosures: U. Kalyoncu has received fees for speaking and/or consulting from AbbVie, BMS, MSD, Pfizer, Roche, and UCB; W. Tillett has received fees for speaking and/or consulting from AbbVie, Celgene, and Pfizer/Wyeth; L. Gossec has received fees for speaking and/or consulting from AbbVie, BMS, Celgene, Janssen, MSD, Novartis, Pfizer/Wyeth, Roche, and UCB; N.J. McHugh has received fees for speaking and/or consulting from AbbVie, Celgene, Novartis, and Pfizer.
Contributor Information
Ana-Maria Orbai, Division of Rheumatology, Johns Hopkins University, Baltimore USA.
Philip J Mease, Rheumatology Research, Swedish Medical Center and University of Washington School of Medicine, Seattle, WA, USA.
Maarten de Wit, Patient Research Partner, VU Medical Centre, Amsterdam, The Netherlands.
Umut Kalyoncu, Division of Rheumatology, Johns Hopkins University, Baltimore USA, and Department of Internal Medicine, Division of Rheumatology, Hacettepe University Ankara, Turkey.
Willemina Campbell, Patient Research Partner, Toronto Western Hospital, Toronto, Ontario, Canada.
William Tillett, Royal National Hospital for Rheumatic Diseases, Bath, UK.
Lihi Eder, Toronto Western Hospital, Toronto, Ontario, Canada.
Musaab Elmamoun, Dept of Rheumatology, St. Vincents University Hospital and Conway Institute for Biomolecular Research, University College Dublin, Ireland.
Oliver FitzGerald, Newman Clinical Research Professor, Dept of Rheumatology, St. Vincents University Hospital and Conway Institute for Biomolecular Research, University College Dublin, Ireland.
Dafna D Gladman, Professor of Medicine, University of Toronto; Senior Scientist, Krembil Research Institute; Director, Psoriatic Arthritis Program, University Health Network; Toronto, Ontario, Canada.
Niti Goel, Patient Research Partner, Quintiles, Duke University School of Medicine. Durham, NC, USA.
Laure Gossec, Sorbonne Universités, UPMC Univ Paris 06, Institut Pierre Louis d’Epidémiologie et de Santé Publique, GRC-UPMC 08 (EEMOIS); AP-HP, Pitié Salpêtrière Hospital, Department of Rheumatology, Paris, France.
Chris A Lindsay, Patient Research Partner, Thousand Oaks, CA, USA.
Ingrid Steinkoening, Patient Research Partner, Cleveland Clinic, Cleveland, OH, USA.
Philip S Helliwell, University of Leeds, Leeds, UK, and Bradford Hospitals NHS Foundation Trust, Bradford, UK.
Neil McHugh, Royal National Hospital for Rheumatic Diseases, Bath, UK.
Vibeke Strand, Division of Immunology, Stanford University, Palo Alto, CA, USA.
Alexis Ogdie, University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.Wells G, Beaton DE, Tugwell P, Boers M, Kirwan JR, Bingham CO, 3rd, et al. Updating the OMERACT filter: discrimination and feasibility. J Rheumatol. 2014;41:1005–10. doi: 10.3899/jrheum.131311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d’Agostino MA, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2. 0. J Clin Epidemiol. 2014;67:745–53. doi: 10.1016/j.jclinepi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Mease PJ, Strand V, Healy P, Helliwell PS, Fitzgerald O, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167–70. [PubMed] [Google Scholar]
- 4.de Wit M, Campbell W, FitzGerald O, Gladman DD, Helliwell PS, James J, et al. Patient participation in psoriasis and psoriatic arthritis outcome research: a report from the GRAPPA 2013 Annual Meeting. J Rheumatol. 2014;41:1206–11. doi: 10.3899/jrheum.140171. [DOI] [PubMed] [Google Scholar]
- 5.de Wit M, Campbell W, Orbai AM, Tillett W, Fitzgerald O, Gladman DD, et al. Building Bridges between Researchers and Patient Research Partners: A Report from the GRAPPA 2014 Annual Meeting. J Rheumatol. 2015;42:1021–26. [Google Scholar]
- 6.Tillett W, Eder L, Goel N, De Wit M, Gladman DD, FitzGerald O, et al. Enhanced Patient Involvement and the Need to Revise the Core Set - Report from the Psoriatic Arthritis Working Group at OMERACT 2014. J Rheumatol. 2015 doi: 10.3899/jrheum.141156. [DOI] [PubMed] [Google Scholar]
- 7.de Wit M, Kvien T, Gossec L. Patient participation as an integral part of patient reported outcomes development guarantees the representativeness of the patient voice – A case-study from the field of rheumatology. RMD Open. 2015;1:e000129. doi: 10.1136/rmdopen-2015-000129. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tillett W, Adebajo A, Brooke M, Campbell W, Coates LC, FitzGerald O, et al. Patient involvement in outcome measures for psoriatic arthritis. Curr Rheumatol Rep. 2014;16:418. doi: 10.1007/s11926-014-0418-7. [DOI] [PubMed] [Google Scholar]
- 9.Gossec L, de Wit M, Kiltz U, Braun J, Kalyoncu U, Scrivo R, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73:1012–9. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 10.Palominos PE, Gaujoux-Viala C, Fautrel B, Dougados M, Gossec L. Clinical outcomes in psoriatic arthritis: A systematic literature review. Arthritis Care Res (Hoboken) 2012;64:397–406. doi: 10.1002/acr.21552. [DOI] [PubMed] [Google Scholar]
- 11.Stamm TA, Nell V, Mathis M, Coenen M, Aletaha D, Cieza A, et al. Concepts important to patients with psoriatic arthritis are not adequately covered by standard measures of functioning. Arthritis Rheum. 2007;57:487–94. doi: 10.1002/art.22605. [DOI] [PubMed] [Google Scholar]
- 12.Taylor WJ, Mease PJ, Adebajo A, Nash PJ, Feletar M, Gladman DD. Effect of psoriatic arthritis according to the affected categories of the international classification of functioning, disability and health. J Rheumatol. 2010;37:1885–91. doi: 10.3899/jrheum.091315. [DOI] [PubMed] [Google Scholar]
- 13.Moverley AR, Vinall-Collier KA, Helliwell PS. It’s not just the joints, it’s the whole thing: qualitative analysis of patients’ experience of flare in psoriatic arthritis. Rheumatology (Oxford) 2015;54:1448–53. doi: 10.1093/rheumatology/kev009. [DOI] [PubMed] [Google Scholar]
- 14.Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S64–85. doi: 10.1002/acr.20577. [DOI] [PubMed] [Google Scholar]
- 15.Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res. 2012;21:651–7. doi: 10.1007/s11136-011-9960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terwee CB, Jansma EP, Riphagen II, de Vet HC. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res. 2009;18:1115–23. doi: 10.1007/s11136-009-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso J, Bartlett SJ, Rose M, Aaronson NK, Chaplin JE, Efficace F, et al. The case for an international patient-reported outcomes measurement information system (PROMIS(R)) initiative. Health Qual Life Outcomes. 2013;11:210. doi: 10.1186/1477-7525-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haverman L, Grootenhuis MA, Raat H, van Rossum MA, van Dulmen-den Broeder E, Hoppenbrouwers K, et al. Dutch-Flemish translation of nine pediatric item banks from the Patient-Reported Outcomes Measurement Information System (PROMIS) Qual Life Res. 2015 doi: 10.1007/s11136-015-0966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broderick JE, Schneider S, Junghaenel DU, Schwartz JE, Stone AA. Validity and reliability of patient-reported outcomes measurement information system instruments in osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:1625–33. doi: 10.1002/acr.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ. Validation of PROMIS (R) Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471:3466–74. doi: 10.1007/s11999-013-3097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen RE, Potosky AL, Reeve BB, Hahn E, Cella D, Fries J, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. 2015;24:2333–44. doi: 10.1007/s11136-015-0992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32:793–801. doi: 10.1002/jor.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senders A, Hanes D, Bourdette D, Whitham R, Shinto L. Reducing survey burden: feasibility and validity of PROMIS measures in multiple sclerosis. Mult Scler. 2014;20:1102–11. doi: 10.1177/1352458513517279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orbai AM, Bartlett SJ, Duncan T, De Leon E, Jones MRK, Bingham CO., 3rd Multidimensional Health Related Quality of Life Assessment Using PROMIS Measures in Psoriatic Arthritis Flares [abstract] Ann Rheum Dis. 2014;73(Suppl2):1048–9. [Google Scholar]
- 25.Tillett W, Eder L, Goel N, De Wit M, Ogdie A, Orbai AM, et al. Review of the Psoriatic Arthritis Working Group at OMERACT 12: A report from the GRAPPA 2014 Annual Meeting. J Rheumatol. 2015;42:1048–51. [Google Scholar]
- 26.Kirwan JR, Minnock P, Adebajo A, Bresnihan B, Choy E, de Wit M, et al. Patient perspective: fatigue as a recommended patient centered outcome measure in rheumatoid arthritis. J Rheumatol. 2007;34:1174–7. [PubMed] [Google Scholar]
- 27.Bartlett SJ, Hewlett S, Bingham CO, 3rd, Woodworth TG, Alten R, Pohl C, et al. Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Ann Rheum Dis. 2012;71:1855–60. doi: 10.1136/annrheumdis-2011-201201. [DOI] [PubMed] [Google Scholar]


