Abstract
Sarcocystis species are intracellular protozoan parasites with an intermediate-definitive host life cycle based on a prey-predator relationship. Asexual stages develop in intermediate hosts after they ingest the oocyst stage from definitive-host feces and terminate with the formation of intramuscular cysts (sarcocysts). Sarcocysts in meat eaten by a definitive host initiate sexual stages in the intestine that terminate in oocysts excreted in the feces. Most Sarcocystis species infect specific hosts or closely related host species. For example, humans and some primates are definitive hosts for Sarcocystis hominis and S. suihominis after eating raw meat from cattle and pigs, respectively. The prevalence of intestinal sarcocystosis in humans is low and is only rarely associated with illness, except in volunteers who ingest large numbers of sarcocysts. Cases of infection of humans as intermediate hosts, with intramuscular cysts, number less than 100 and are of unknown origin. The asexual stages, including sarcocysts, can stimulate a strong inflammatory response. Livestock have suffered acute debilitating infections, resulting in abortion and death or chronic infections with failure to grow or thrive. This review provides a summary of Sarcocystis biology, including its morphology, life cycle, host specificity, prevalence, diagnosis, treatment, and prevention strategies, for human and food animal infections.
INTRODUCTION
Sarcocystis was first reported in 1843 by Miescher as white threadlike cysts in striated muscles of a house mouse, without a scientific name. For the following 20 years, the parasite was simply referred to as Meischer's tubules. In 1865 similar structures were found in pig muscle, but another 34 years passed until the name Sarcocystis meischeriana was proposed to identify them (9). Subsequently, when intramuscular cysts were found in a new host, a new species name was proposed. During much of this time, scientists debated whether Sarcocystis species were protozoa or fungi. The possibility that Sarcocystis were fungi arose because only the sarcocyst stage was known and, when sarcocysts and their contents were placed in various culture media, hyphae and mycelia (now recognized to be a result of contamination) were sometimes found several days later. It was not until 1967, 124 years after the first report of Sarcocystis, that the spindle- or crescent-shaped bodies (bradyzoites) in the sarcocysts were studied by electron microscopy and organelles were observed like those seen in other apicomplexan protozoa such as Toxoplasma and Eimeria (46). The life cycle and all other stages remained unknown until 1970, when bradyzoites from sarcocysts in bird muscles were inoculated into cultured mammalian cells and underwent development into sexual stages and oocysts (10, 11). Transmission studies involving Sarcocystis fusiformis, the species name applied to three distinct morphological types of sarcocysts found in cattle, provided further clarification of the biology of this once enigmatic group of protozoan parasites. After sarcocysts were fed to different potential definitive hosts—dogs, cats, and humans—S. fusiformis was found to encompass three species, and the new species names S. bovicanis. S. bovifelis, and S. bovihominis (named for the intermediate and definitive hosts) were proposed (20, 41, 42). These collective findings provide the current basis for understanding the sources of infectious organisms, the transmission dynamics, the criteria for identifying and naming species of Sarcocystis, and the biology critical to prevention and treatment strategies.
LIFE CYCLES
Sarcocystis species are intracellular protozoan parasites with a requisite two-host life cycle based on a prey-predator (intermediate-definitive) host relationship.
Stages in the Intermediate (Prey) Host
Early stages of development have not been observed in human intermediate hosts. The following description of early development is based on studies of S. cruzi in cattle (12, 15). After oocysts or free sporocysts from the definitive host are ingested by a susceptible intermediate host, they pass to the small intestine (Fig. 1). The plates forming the sporocyst walls separate, releasing the four sporozoites held inside. Motile sporozoites migrate through the gut epithelium, eventually entering endothelial cells in small arteries throughout the body (Fig. 2). Here they undergo the first of four asexual generations (called schizogony or merogony), producing numerous merozoites (cells morphologically similar to sporozoites and bradyzoites) about 15 to 16 days after ingestion of sporocysts. Subsequent generations of merozoites develop downstream in the direction of blood flow to arterioles, capillaries, venules, and veins throughout the body and then develop the final asexual generation in muscles. Merozoites constituting the second generation (of Sarcocystsis cruzi) were observed in the peripheral blood 27 days after ingestion of sporocysts. Some were single with a single nucleus or with two nuclei, whereas others were seen in developing pairs. Some appeared extracellular (Fig. 3), while others were in unidentified mononuclear cells. The third asexual generation appeared as multinucleate schizonts in capillaries throughout the body (Fig. 2) but were most abundant in the renal glomeruli. Merozoites from this generation enter muscle cells, round up to form metrocytes (mother cells), and initiate sarcocyst (Greek: sarkos = flesh, kystis = bladder) formation. Sarcocysts begin as unicellular bodies containing a single metrocyte. Through repeated asexual multiplication, numerous metrocytes accumulate and the sarcocyst increases in size (Fig. 4). As sarcocysts mature, the small, rounded, noninfectious metrocytes give rise to infectious, crescent-shaped bodies called bradyzoites (Greek: brady = slow, zoite = small animal) (Fig. 5 to 7). Maturation varies with each species and takes 2 months or more until bradyzoites form and sarcocysts become infectious for the definitive host. Sarcocysts can persist for months or years. Mature sarcocysts of each species vary in size from microscopic to macroscopic, vary in length and circumference, and develop structurally distinct sarcocyst walls that vary in thickness and organization of villar protrusions, but all contain numerous bradyzoites. At least seven structurally distinct wall patterns have been found by electron microscopy of specimens isolated from humans. By light microscopy, often the most one can distinguish is whether the wall is thick or thin. Sarcocysts are found in virtually all striated muscles of the body including the tongue, esophagus, and diaphragm, as well as cardiac muscle and, to a lesser extent, smooth muscle (Fig. 4 to 6). Sarcocysts have also been found in small numbers in neural tissue such as spinal cord and brain and Purkinje fibers of the heart. As intermediate hosts with sarcocysts developing in striated muscles, humans apparently are accidental hosts, for there is little or no opportunity to maintain a life cycle in which humans are frequently eaten by and also exposed to feces from a carnivore definitive host.
FIG. 1.
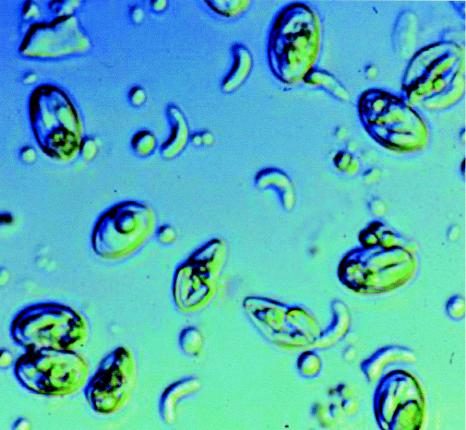
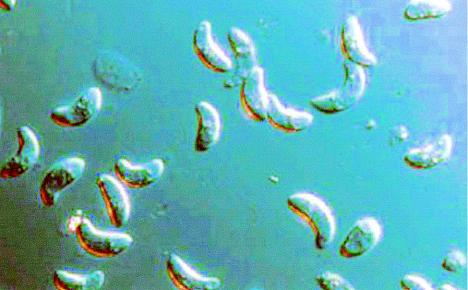
S. cruzi. Differential interference contrast microscopy shows intact oocysts containing two adjacent sporocysts, free sporocysts each containing four sporozoites, and free sporozoites. Magnification, ×1,000.
FIG. 2.
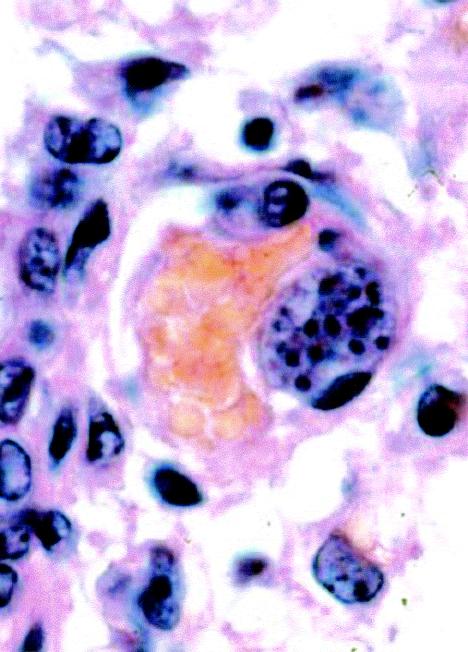
Asexual multinucleate stage (schizont) of S. cruzi in an endothelial cell protruding into the lumen of a small blood vessel in the lung. Hematoxylin and eosin stain. Magnification, ×1,000.
FIG. 3.
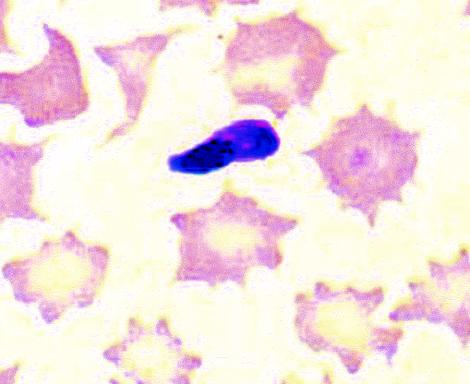
Merozoite of S. cruzi in a blood smear. Wright's stain. Magnification, ×2,400.
FIG. 4.
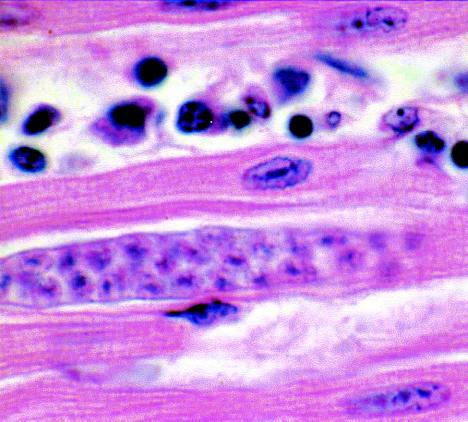
Immature sarcocyst of S. cruzi in skeletal muscle. The sarcocyst contains noninfectious metrocytes (mother cells) but no bradyzoites. Hematoxylin and eosin stain. Magnification, ×500.
FIG. 5.
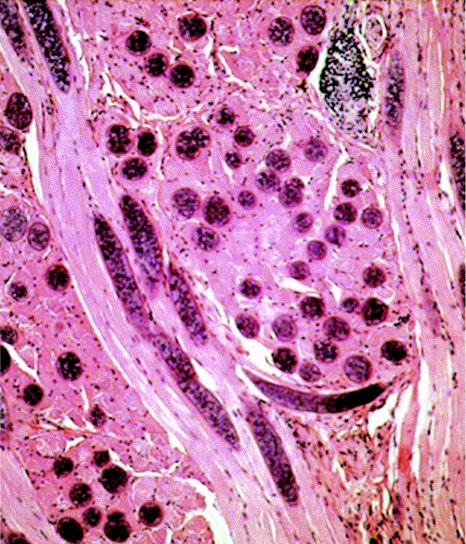
Numerous sarcocysts in longitudinal and cross section in muscles of sheep tongue. Note the lack of direct inflammatory response to sarcocysts. Hematoxylin and eosin stain. Magnification, ×100.
FIG. 7.
S. cruzi. Differential interference contrast microscopy shows bradyzoites released from a sarcocyst. Magnification, ×1,000.
FIG. 6.
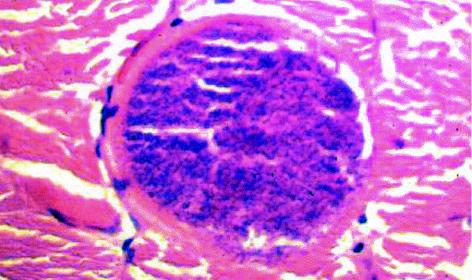
Cross section of a sarcocyst in a skeletal muscle biopsy specimen from a human. Hematoxylin and eosin stain. Magnification, ×340.
Stages in the Definitive (Predator) Host
After sarcocysts are eaten by a susceptible definitive host and the wall is mechanically ruptured or digested, bradyzoites become motile, leave the sarcocyst, and enter cells of the intestinal lamina propria. Each intracellular bradyzoite develops into a male or female stage. Some form multinucleated microgametocytes from which sperm-like microgametes develop. Others form macrogametes resembling uninucleate ova. After these gametes fuse, the cytoplasm within the macrogamete undergoes sequential development (sporogony) into a mature oocyst containing two sporocysts. Oocysts pass into the intestinal lumen and then pass from the body in the feces. Intact oocysts are usually observed only in the first few days of patency and appear as two adjacent sporocysts with the oocyst wall barely visible, if visible at all. The thin oocyst wall often breaks, releasing individual sporocysts, often the only stage observed in feces (Fig. 1). Sporocysts of most species measure approximately 10 by 15 μm, contain four sporozoites and a discrete granular residual body (Fig. 1), and are infectious for susceptible intermediate hosts. They are morphologically indistinguishable from sporocysts described many years earlier as Isospora hominis and are found in the villi of the small intestine of humans (41).
DETECTION, IDENTIFICATION, AND HOST SPECIFICITY
Oocysts with two sporocysts or, more frequently, individual sporocysts in human feces are diagnostic for intestinal infection. Oocysts often appear as two adjacent sporocysts with no apparent surrounding oocyst wall. Oocysts of S. suihominis measure 12.3 to 14.6 μm by 18.5 to 20.0 μm. Sporocysts contain four sporozoites and a granular residual body. Sporocysts of S. hominis average 9.3 by 14.7 μm, and those of S. suihominis average 10.5 by 13.5 μm (9). They cannot be distinguished from one another or from sporocysts shed by other hosts.
Most sarcocysts in humans have been found in skeletal muscle and cardiac muscle, but sarcocysts have also been found in muscles in the larynx, pharynx, and upper esophagus (32). Sarcocysts of S. hominis are microscopic in the muscles of cattle, whereas those of S. suihominis are macroscopic in muscles of swine. Sarcocysts in the muscles of these intermediate hosts can be detected by microscopy of hematoxylin-and-eosin-stained histological sections (Fig. 4). Sarcocysts have distinctive physical features that aid in species identification such as overall size, presence or absence of septa, and ultrastructural morphology of the wall. However, these features vary with the age of the sarcocyst, the host cell type, and the methods of fixation. Walls are positively stained by the periodic acid-Schiff (PAS) reaction. As many as 24 wall types have been identified for 62 species (9). For example, walls of S. hominis and S. suihominis sarcocysts are both type 10. The wall of S. hominis sarcocysts is up to 6 μm thick and appears radially striated from villar protrusions up to 7 μm long; bradyzoites are 7 to 9 μm long (9). The wall of S. suihominis sarcocysts is 4 to 9 μm thick, with villar protrusions up to 13 μm long; bradyzoites are 15 μm long (9).
Molecular methods have been used for species identification. S. hirsuta. S. hominis, and S. cruzi from cattle and bison were identified by sequencing 18S ribosomal RNA gene PCR products (17). Using 18S rRNA gene sequences, Sarcocystis from a water buffalo was found to be nearly identical to S. hominis (0.1% difference), indicating that multiple ruminant species serve as intermediate hosts and potential sources of human infection for this parasite (52), but molecular methods have not been used to determine the species of sarcocysts found in human tissues.
Specificity for Intermediate Hosts
Like most other species of Sarcocystis. S. hominis and S. suihominis are genetically programmed to complete their life cycles in specific intermediate hosts or within closely related host species. For example, sporocysts of S. hominis infect cattle but not pigs whereas those of S. suihominis infect pigs but not cattle. Sporocysts of S. ovifelis from cats and S. ovicanis from dogs infect sheep but not cattle or goats. Sporocysts of S. hirsuta from cats infect cattle but not sheep. However, S. cruzi from dogs can infect cattle (Bos taurus), water buffalo (Bubalus bubalis), and bison (Bison bison) (13). Similarly, humans appear to serve as intermediate hosts for several unidentified species of Sarcocystis, perhaps acquiring infections by ingesting sporocysts excreted by predators of nonhuman primates. To determine the species responsible for acute fulminant infection in a captive-born rhesus monkey with schizonts in endothelial cells throughout the body and mature sarcocysts in muscle, 18s rRNA gene sequences were examined (27). Homology of 95 to 96% was found to several species of Sarcocystis, but complete identity was lacking. That report indicates the susceptibility of a primate to life-threatening infection with unknown species of Sarcocystis even in the apparent absence of a typical definitive host. Other species appear less host specific. A water buffalo that was fed sporocysts from a human volunteer who had ingested S. hominis cysts from naturally infected cattle was necropsied 119 days later, and large numbers of sarcocysts were found in skeletal muscles (6). Sarcocysts from this buffalo were infective when ingested by two human volunteers, indicating that buffalo as well as cattle can serve as intermediate hosts for S. hominis.
Specificity for Definitive Hosts
Similar specificity relationships have been found for definitive hosts of some species. Dogs and coyotes serve as definitive hosts for S. cruzi, but humans and cats do not (28). Humans, baboons, and rhesus monkeys can serve as definitive hosts for S. hominis (19), and humans, chimpanzees, and rhesus and cynomolgus monkeys can serve as definitive hosts for S. suihominis (14). No other definitive hosts have been identified for S. hominis or S. suihominis.
PREVALENCE
Few large-scale population surveys have been conducted for Sarcocystis in humans. Prevalence data for Sarcocystis infections primarily reflect case reports and findings of physicians, public health workers, and scientists with specific interests. Consequently, many infections go unreported.
Muscular Sarcocystosis in Humans
The name Sarcocystis lindemanni was once proposed for all intramuscular sarcocysts in humans, but it was not clearly described, and evidence of multiple morphologically different cysts suggests that there probably are several species of Sarcocystis involved in human infections (9). Therefore, the name is considered a nomen nudum and is no longer used. Sarcocystosis has been reported to affect a wide age range of humans, from a 26-day-old infant to a 75-year-old man (32). Most cases have been found in persons living in tropical or subtropical environments. Of approximately 46 cases reported by 1990 (9) most were from tropical or subtropical countries in Asia and Southeast Asia. An additional 46 cases, based on histologic findings, include 1 from China; 2 from Malaysians of Indian origin; 2 others of undetermined origin; 4 each from Africa, Europe, and the United States; 5 from Central and South America; 11 from India; and 13 from Southeast Asia. An outbreak involving 7 of 15 military personnel in Malaysia is the largest cluster case on record (1). A seroepidemiological survey in West Malaysia, found that 19.7% of 243 persons had antibodies to Sarcocystis (49). Titers were highest among the Orang Aslis (aboriginals) followed by Malays, Indians, and Chinese, possibly reflecting food habits and environmental sanitation levels.
Muscular Sarcocystosis in Animals
Humans acquire intestinal sarcocystosis from eating Sarcocystis-infected meat. Based on examination of tissues from abattoirs, a high percentage of cattle worldwide are infected with sarcocysts, with those of S. cruzi (infectious from cattle to canines) being the most prevalent and easiest to identify histologically (51). Most studies have not attempted to differentiate species of sarcocysts found in meat. The prevalence of Sarcocystis in Japanese and imported beef was reported, but the species were not identified (37). Because S. hominis (infectious from cattle to humans) and S. hirsuta (infectious from cattle to felines) are difficult to distinguish except by electron microscopy, some prevalence data may be erroneous, S. hominis has not been detected in the United States, whereas up to 63% of cattle in Germany have been reported to be infected. Of 238 cattle carcasses examined in Madhya Pradesh, India, over 80% contained sarcocysts (21). Of these, 186, 31, and 29 were identified as S. cruzi. S. hirsuta, and S. hominis, respectively. In Brazil, all 50 samples of raw beef prepared as kibbe in 25 Arabian restaurants in Sao Paulo contained sarcocysts (39). Based on wall structure, 94, 70, and 92% of these samples contained, S. hominis. S. hirsuta, and S. cruzi, respectively. The overall prevalence of Sarcocystis in pigs appears low, at 3 to 36% worldwide. S. suihominis was more prevalent in Germany than Austria, but little information is available from other countries. S. suihominis and S. hominis have been found in slaughtered pigs and cattle raised in Japan (43, 44).
Intestinal Sarcocystosis in Humans
Based on limited surveys, intestinal sarcocystosis in humans was found more frequently in Europe than other continents (9). Of fecal specimens examined from children in Poland and Germany, 10.4 and 7.3% were found positive, respectively. Of 1,228 apprentices from the Hanoi-Haiphong area of Viet Nam who worked in Central Slovakia in 1987 to 1989, 14 (1.1%) were positive (48). After raw beef containing S. hominis was prepared as kibbe and fed to seven human volunteers, six excreted sporocysts and two developed diarrhea (39). After eating raw beef, a patient in Spain with abdominal discomfort and loose stools was diagnosed with S. hominis oocysts in his feces (7). In Tibet, Sarcocystis was detected in 42.9% of beef specimens examined from the marketplace, and S. hominis and S. suihominis were found in stools from 21.8 and 0 to 7% of 926 persons, respectively (53).
TRANSMISSION FROM ANIMALS TO HUMANS
Eating raw or undercooked beef and pork containing mature sarcocysts of S. hominis and S. suihominis, respectively, has resulted in humans acquiring intestinal sarcocystosis. Based on histologic examination of intestinal lesions from persons in Thailand having eaten undercooked meat from Bos indicus cattle (4) and possibly other animals (unpublished data), there could be other species of Sarcocystis from which humans acquire intestinal sarcocystosis. S. cruzi, the species most frequently found in cattle muscle, infects dogs but not humans (28), but several species of domesticated meat animals harbor sarcocysts infective for unknown definitive hosts. These include camels, llamas, water buffalo, yaks, and species of pigs other than the domesticated Sus scrofa. Meat from many reptiles, birds, and species of wild mammals that harbor sarcocysts is eaten in various parts of the world with unknown consequences. Therefore, there remain many potential but unknown sources of human intestinal sarcocystosis.
Sarcocystis causing muscular infection has been found in fewer than 100 humans. In such cases, humans harbor the sarcocyst stage and therefore are the intermediate host. It follows, from all other Sarcocystis life cycles, that infected human tissues would have to be eaten by a carnivore to complete the life cycle. Because there is no known predatory or scavenging cycle in nature in which human tissues are eaten regularly by carnivores, humans most probably become infected by eating food or drinking water contaminated with feces from a predator of nonhuman primates involving unknown species of Sarcocystis. Similar conclusions were reached in reviews of human cases in which sarcocysts were found in muscle tissues (3, 38). In routine examinations of muscle tissues from life-long or long-time residents of Malaysia, sarcocysts were detected as incidental findings (38). Many species of local animals, including nonhuman primates, harbored sarcocysts. Locally known predators such as cats, dogs, and pythons (24) could excrete infectious sporocysts that find their way through contaminated food or water, eventually infecting humans. In tropical areas where most human cases have been reported and nonhuman primates are present, 79 (21%) of 375 wild-caught monkeys examined, comprising 14 species, had sarcocysts whereas none of 369 laboratory-born monkeys had sarcocysts (25).
SYMPTOMS
Human Definitive Hosts
Symptoms of sarcocystosis are summarized in Table 1. Human volunteers in Germany who ate raw beef containing S. hominis became infected and shed oocysts in their feces (2, 41). One person became ill. Signs that appeared 3 to 6 h after eating the beef included nausea, stomach ache, and diarrhea; these were transient and lasted about 36 h. Volunteers in China consumed 1,567 to 14,740 sarcocysts of S. hominis from experimentally infected buffalo meat (5). They had abdominal pain, distension, watery diarrhea, and eosinophilia starting 1 week and ending 4 weeks after ingesting the sarcocysts and were spontaneously cured without treatment. This is an exceedingly large number of sarcocysts and would rarely be found in naturally infected meat. Six persons in Thailand who reportedly ate spiced raw beef from zebu (but who possibly had eaten a variety of other animal products [unpublished data]) developed segmental necrotizing enteritis requiring surgical intervention (4). Histology of intestine samples from these patients revealed sexual stages attributed to Sarcocystis and gram-positive bacilli.
TABLE 1.
Parasite development and disease manifestations in humans
| Characteristic | Muscular infection | Intestinal infection |
|---|---|---|
| Source of infection | Water or food contaminated with feces from unknown carnivore or omnivore | Raw or undercooked meat |
| Infective stage | Oocyst or free sporocysts | Sarcocyst containing bradyzoites |
| Developmental stages | Intravascular schizonts (not seen); intramuscular sarcocysts | Sexual stages in lamina propria; oocysts excreted in feces |
| Time from ingestion of infective stage to symptoms | Weeks to months, lasting months to years | 3-6 h, lasting 36 h |
| Symptoms | Musculoskeletal pain, fever, rash, cardiomyopathy, bronchospasm, subcutaneous swelling | Nausea, loss of appetite, vomiting, stomach ache, bloat, diarrhea, dyspnea, and tachycardia |
| Diagnosis | Biopsy specimen containing sarcocyst; antibodies to bradyzoites | Oocysts or sporocysts in feces, beginning 5-12 days after ingestion |
| Therapy (none approved) | Co-trimoxazole, furazolidone, albendazole, anticoccidials, pyrimethamine, anti-inflammatories | None |
Volunteers in Germany who ate raw pork containing S. suihominis became infected, shed oocysts, and had dramatic symptoms 6 to 48 h later, including bloat, nausea, loss of appetite, stomach ache, vomiting, diarrhea, difficulty in breathing, and rapid pulse (18, 41). Volunteers who ate well-cooked meat from the same pigs remained asymptomatic (18). In a subsequent study involving 17 volunteers at the University of Bonn (Germany), 14 persons ate raw pork from a pig that was experimentally infected with S. suihominis and killed 175 days later (26). During the first 2 days after the volunteers had eaten the infected meat, they presented with the same symptoms as volunteers in the earlier study. Symptoms appeared to be related to the quantity of meat consumed, but individual reactions varied considerably.
Sporocysts, possibly of S. suihominis, were detected in the feces of two men in China (54). One was a 48-year-old resident of Xiaguan, who complained of abdominal pain and distension, alternating diarrhea and constipation, mild stomach ache, and dyspnea. He had eaten raw pork for many years and had done so 13, 23, and 65 days before the stool examination. In contrast, a Chinese scientist, who infected himself with S. suihominis by eating raw pork from a pig killed 144 days after experimental infection, began excreting sporocysts 12 days later and continued to excrete sporocysts for more than 120 days with no appreciable symptoms (33).
Animal Intermediate Hosts
At about 2 weeks after cattle and sheep ingest Sarcocystis sporocysts from dogs fed with raw beef or lamb, respectively, merozoites develop in endothelial cells of small arteries and the body temperature is elevated for a day. At approximately 4 weeks after ingestion of sporocysts, a subsequent asexual generation matures in vascular endothelial cells with an accompanying acute inflammatory reaction. This reaction is characterized by massive perivascular infiltration of mononuclear cells and multiorgan petechial hemorrhage associated with weakness, fever, abortion in pregnant animals, and sometimes death (22, 23, 31, 40). The severity of infection is dependent on the number of sporocysts ingested. Some animals fail to fully recover from the acute phase, and the infection becomes chronic, characterized by inappetence, weight loss, loss of hair or wool breakage, poor or stunted growth, muscle atrophy, lethargy, and weakness. Histologic examination often reveals widespread myositis, including glossitis and inflammation of cardiac muscle.
Human Intermediate Hosts
All human cases have been identified by the presence of intramuscular cysts, most without any symptoms or inflammatory response and none with the intravascular asexual stages. However, eight cases of Sarcocystis with vasculitis and/or myositis have been reported (34). A 40-year-old man in California, who had traveled extensively in Asia 4 years earlier, had painful swellings about 1 to 2 cm in diameter on his extremities when first examined (34). Intermittently for the next 16 months he had similar lesions on his trunk, on his upper and lower extremities proximal and distal to the knees and elbows, and on the plantar surface of his feet. These began as subcutaneous masses associated with overlying erythema and subsided spontaneously about 2 weeks later. Histologic examination of biopsy specimens from nodules revealed vasculitis in capillaries, venules, and arterioles, consisting primarily of perivascular lymphocytes and/or neutrophils. There were scattered clusters of thin-walled sarcocysts in striated muscle fibers without significant myositis (Fig. 5 and 6). Sarcocystis was not unequivocally determined to be responsible for the vasculitis. Because the patient felt well except for the nodules, no treatment was attempted.
In India, sarcocysts were found in biopsy specimens from four persons with lumps or pain in their limbs (35). Of 15 American military personnel in Malaysia, 7 developed acute fever, myalgias, bronchospasm, pruritic rashes, lymphadenopathy, and subcutaneous nodules associated with eosinophilia, elevated erythrocyte sedimentation rate, and elevated creatinine kinase levels (1). Sarcocysts were found in biopsy specimens from the index case, whose symptoms were ameliorated by treatment with albendazole but lasted for more than 5 years. Symptoms in five others were mild to moderate and self-limited, and one person with abnormal blood chemistries was asymptomatic. Fever, chronic myositis, and eosinophilia were also reported in a patient in the Netherlands (50).
DIAGNOSIS
Presumptive diagnosis of human intestinal sarcocystosis is based on symptoms and a history of recently having eaten raw or undercooked meat. Definitive diagnosis, requiring identification of sporocysts in feces (Fig. 1), might require several stool examinations beginning several days after having eaten the meat. Sporocysts of S. hominis are first excreted 14 to 18 days after ingesting beef, and those of S. suihominis are excreted 11 to 13 days after ingesting pork. Sporocysts can be seen by bright-field microscopy in a fecal flotation wet mount just beneath the coverslip. Flotation based on high-density solutions incorporating sodium chloride, cesium chloride, zinc sulfate, sucrose, Percoll, Ficoll-Hypaque, and other such density gradient media is preferred to formalin-ethyl acetate and other sedimentation methods. Because sporocysts of different species overlap in size and shape, species cannot be distinguished from one another solely by microscopy.
Intramuscular sarcocystosis would be suspected based on various combinations of criteria including persistent myalgia, episodic weakness, subcutaneous nodules, dermatomyositis, eosinophilia, and elevated muscle creatinine kinase levels. In some cases, such clinical findings, often linked to a history of residence in or travel to tropical locations have led to biopsy of the affected muscle. Sarcocystis sarcocysts in muscle biopsy specimens can be identified by microscopic examination of histologic sections stained with hematoxylin and eosin (Fig. 4) and other stains such as the PAS reaction (Fig. 5 and 6). However, variability in staining can be expected. In some tissue sections, the sarcocyst wall may be very thin or not clearly visible, and in others the intensity of staining may not be sufficient to clearly determine that the wall is PAS positive. Inflammatory cells have infrequently been found in direct contact with sarcocysts, but myositis, myonecrosis, perivascular and interstitial inflammation, vasculitis, and eosinophilic myositis have been recognized in some cases in association with intramuscular sarcocystosis (1, 34).
Sarcocystis can be detected in meat by direct observation of macroscopic sarcocysts or microscopic examination of histologic sections. Larger quantities of meat can be inspected by grinding meat, artificially digesting it in a solution of pepsin and hydrochloric acid, centrifuging the digest, and microscopically examining the pellet for the presence of bradyzoites. For many years, eosinophilic myositis, observed as a blue-green tint on the surface of a fresh animal carcass, was thought to be associated with Sarcocystis infection because sarcocysts were usually found in histologic sections of the muscles. However, many cattle harbor sarcocysts but show no cellular immune response. Furthermore, numerous experimental infections of livestock have failed to result in eosinophilic myositis (22, 23, 40, 47). One report describes eosinophilic myositis in humans as chronic myositis and eosinophila diagnosed by muscle biopsy (50).
TREATMENT
There is no known prophylaxis or therapeutic treatment for intestinal sarcocystosis. Infections are self-limiting, of short duration, and often asymptomatic. The efficacy of co-trimoxazole (8) or furazolidone (36) remains to be demonstrated. For six persons in Thailand with segmental necrotizing enteritis associated with sexual stages of Sarcocystis and gram-positive bacilli, surgical resection of the small intestine was followed by antibiotic treatment (4). This extremely aggressive course of treatment has not been applied in other cases.
Neither prophylaxis nor therapeutic treatment for myositis, vasculitis, or related lesions in humans has been approved. Prophylaxis was achieved in experimental animal studies (see “Prevention” below), but data for treatment of established infections are lacking. The efficacy of albendazole (1) remains to be substantiated in controlled tests. Whether immunosuppressive therapy for vasculitis or myositis might reduce the severity of the inflammatory reaction or facilitate parasite proliferation is unknown. The usefulness of pyrimethamine or other drugs known to be effective against related protozoa such as Toxoplasma is also unknown. Because of the paucity of reported treatment cases and the lack of any controlled studies, there is no basis for evaluation, and therefore no course of treatment can be recommended as superior to any other at this time.
PREVENTION
Intestinal sarcocystosis can be prevented by thoroughly cooking or freezing meat to kill bradyzoites in the sarcocysts. Sarcocysts in pig muscles were rendered noninfectious for puppies after cooking meat at 60, 70, and 100°C for 20, 15, and 5 min, respectively (45). Freezing at −4 and −20°C for 48 and 24 h, respectively, also rendered bradyzoites in pork noninfectious (45). Beef and beef products purchased from a supermarket reflected the laboratory results of cooking and freezing (28). Fresh chuck roast and round steak, as well as rare roast beef and hamburger, contained bradyzoites infectious for dogs. Cooked products such as beef bologna and beef frankfurters, as well as frozen hamburger and frozen flaked sandwich steaks, were not infectious for dogs.
Chemoprophylaxis using the anticoccidial drugs amprolium and salinomycin was effective in preventing severe illness and death in experimentally infected calves and lambs (16, 29, 30). There is no report of attempted prophylaxis in humans.
To prevent infection of food animals, they must be prevented from ingesting the sporocyst stage from human feces in contaminated water, feed, and bedding. When such preventative measures cannot be assured and meat might be harboring cysts, it should be thoroughly frozen for 2 days or more or thoroughly cooked to kill infectious bradyzoites. These measures will prevent the development of intestinal stages where humans might serve as definitive hosts. To prevent humans from becoming infected as intermediate hosts, ingestion of sporocysts must be prevented. The most likely source of sporocysts is water contaminated with feces from a carnivore or omnivore or foods washed or irrigated with contaminated water. Where contaminated drinking water is suspected, boiling is the best method to ensure disinfection. Where contaminated foods are suspected, they should be thoroughly washed or cooked before being eaten.
TABLE 2.
Encysted protozoan parasites in human feces, differentiated by general size, shape, and features
| Parasite | General size (μm) | Shape | Features |
|---|---|---|---|
| Sarcocystis hominis and S. suihominis | Excreted sporulated | ||
| Oocysts | 15-19 by 15-20 | Spherical | Contain 2 sporocysts |
| Sporocysts | 15-19 by 8-10 | Oval | Contain 4 sporozoites |
| Isospora belli | 20-33 by 10-19 | Ovoid with tapered ends | Excreted unsporulated |
| Cyclospora cayetanensis | 7.7-10 | Spherical | Excreted unsporulated |
| Giardia duodenalis | 11-14 | Ovoid to ellipsoid | 4 nuclei |
| Balantidium coli | 50-70 | Spherical to oval | Large macronucleus, thick wall |
| Cryptosporidium hominis and C. parvum | 4.5 by 5.0 | Nearly spherical | Excreted sporulated, but sporozoites hard to see |
| Entamoeba histolytica and E. dispar | 12-15 | Spherical | Uni- and binucleate, usually with distinct karyosome |
REFERENCES
- 1.Arness, M. K., J. D. Brown, J. P. Dubey, R. C. Neafie, and D. E. Granstrom. 1999. An outbreak of acute eosinophilic myositis due to human Sarcocystis parasitism. Am. J. Trop. Med. Hyg. 1:548-553. [DOI] [PubMed] [Google Scholar]
- 2.Aryeetey, M. E., and G. Piekarski. 1976. Serologische Sarcocystis-studien an Menschen und Ratten. Z. Parasitenkd. 50:109-124. [DOI] [PubMed] [Google Scholar]
- 3.Beaver, P. C., R. K. Gadgil, and P. Morera. 1979. Sarcocystis in man: a review and report of five cases. Am. J. Trop. Med. Hyg. 28:819-844. [PubMed] [Google Scholar]
- 4.Bunyaratvej, S., P. Bunyawongwiroj, and P. Nitiyanant. 1982. Human intestinal sarcosporidiosis: report of six cases. Am. J. Trop. Med. Hyg. 31:36-41. [DOI] [PubMed] [Google Scholar]
- 5.Chen, X., Y. Zuo, and W. Zuo. 1999. Observation on the clinical symptoms and sporocysts excretion in human volunteers experimentally infected with Sarcocystis hominis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17:2527. (In Chinese.) [PubMed] [Google Scholar]
- 6.Chen, X. W., Y. X. Zuo, and J. J. Hu. 2003. Experimental Sarcocystis hominis infection in a water buffalo (Bubalus bubalis). J. Parasitol. 89:393-394. [DOI] [PubMed] [Google Scholar]
- 7.Clavel, A., O. Doiz, M. Varea, S. Morales, F. J. Castillo, M. C. Rubio, and R. Gomez-Lus. 2001. Molestias abdominales y heces blandas en consumidor habitual de carne de vacuno poco cocinada. Enferm. Infec. Microbiol. Clin. 19:29-30. [DOI] [PubMed] [Google Scholar]
- 8.Croft, J. C. 1994. Nonamebic protozoal enteridities. p. 769-774. In D. Hoeprich, M. C. Jordan, and A.R. Ronald (ed.), Infectious processes, 5th ed. Lippincott, Philadelphia, Pa.
- 9.Dubey, J. P., C. A. Speer, and R. Fayer. 1989. Sarcocystis of animals and man. CRC Press, Inc., Boca Raton, Fla.
- 10.Fayer, R. 1970. Sarcocystis: development in cultured avian and mammalian cells. Science 168:1104-1105. [DOI] [PubMed] [Google Scholar]
- 11.Fayer, R. 1972. Gametogony of Sarcocystis sp. in cell culture. Science 175:65-67. [DOI] [PubMed] [Google Scholar]
- 12.Fayer, R. 1979. Multiplication of Sarcocystis bovicanis in the bovine bloodstream. J. Parasitol. 65:980-982. [PubMed] [Google Scholar]
- 13.Fayer, R., J. P. Dubey, and R. G. Leek. 1982. Infectivity of Sarcocystis spp. from bison, elk, moose, and cattle via sporocysts from coyotes. J. Parasitol. 68:681-685. [PubMed] [Google Scholar]
- 14.Fayer, R., A. O. Heydorn, A. J. Johnson, and R. G. Leek. 1979. Transmission of Sarcocystis suihominis from humans to swine to nonhuman primates (Pan troglodytes. Macaca mulatta. Macaca irus). Z. Parasitenkd. 59:15-20. [DOI] [PubMed] [Google Scholar]
- 15.Fayer, R., and A. J. Johnson. 1973. Development of Sarcocystis fusiformis in calves infected with sporocysts from dogs. J. Parasitol. 59:1135-1137. [PubMed] [Google Scholar]
- 16.Fayer, R., and A. J. Johnson. 1975. Effect of amprolium on acute sarcocystosis in experimentally infected calves. J. Parasitol. 61:932-936. [PubMed] [Google Scholar]
- 17.Fischer, S., and K. Odening. 1998. Characterization of bovine Sarcocystis species by analysis of their 18S ribosomal DNA sequences. J. Parasitol. 84:50-54. [PubMed] [Google Scholar]
- 18.Heydorn, A. O. 1977. Sarkosporidien enfiziertes Fleisch als mogliche Krankheitsurache fur den Menschen. Arch. Lebensmittelhyg. 28:27-31. [Google Scholar]
- 19.Heydorn, A. O., R. Gestrich, and K. Janitschke. 1976. Beitrage zum Lebenszyklus der Sarkosporidien. VIII. Sporozysten von Sarcocystis bovihominis in den Fazes von Rhesusaffen (Macaca rhesus) und Pavianen (Papio cynocephalus). Berl. Muench. Tieraerztl. Wochenschr, 89:116-120. [PubMed] [Google Scholar]
- 20.Heydorn, A. O., and M. Rommel. 1972. Beitrage zum Lebenszyklus der Sarkosporidien. II. Hund und Katze als Ubertrager der Sarkosporidien des Rindes. Berl. Muench. Tieraerztl. Wochenschr. 85:121-123. [PubMed] [Google Scholar]
- 21.Jain, P. C., and H. L. Shah. 1987. Sarcocystis hominis in cattle in Madhya Pradesh and its public health importance. Indian Vet. J. 64:650-654. [Google Scholar]
- 22.Johnson, A. J., R. Fayer, and P. K. Hildebrandt. 1974. The pathology of experimental sarcosporidiosis in the bovine. Lab. Investig. 30:377-378. [Google Scholar]
- 23.Johnson, A. J., P. K. Hildebrandt, and R. Fayer. 1975. Experimentally induced Sarcocystis infection in calves: pathology. Am. J. Vet. Res. 3:995-999. [PubMed] [Google Scholar]
- 24.Kan, S. P. 1985. A review of sarcocystosis with special reference to human infection in Malaysia. Trop. Biomed. 2:167-175. [Google Scholar]
- 25.Karr, S. L., and M. M. Wong. 1975. A survey of Sarcocystis in nonhuman primates. Lab. Anim. Sci. 25:641-645. [PubMed] [Google Scholar]
- 26.Kimmig, P., G. Piekarski, and A. O. Heydorn. 1979. Zu Sarkosporidiose (Sarcocystis suihominis) des Menschen. Immun. Infekt. 7:170-177. [PubMed] [Google Scholar]
- 27.Lane, J. H., K. G. Mansfield, L. R. Jackson, R. W. Diters, K. C. Lin, J. J. MacKey, and V. G. Sassevelle. 1998. Acute fulminant sarcocystosis in a captive-born rhesus macaque. Vet. Pathol. 35:499-505. [DOI] [PubMed] [Google Scholar]
- 28.Leek, R. G., and R. Fayer. 1978. Infectivity of Sarcocystis in beef and beef products from a retail food store. Proc. Helminthol. Soc. Wash. 45:135-136. [Google Scholar]
- 29.Leek, R. G. and R. Fayer. 1980. Amprolium for prophylaxis of ovine Sarcocystis. J. Parasitol. 66:100-106. [PubMed] [Google Scholar]
- 30.Leek, R. G., and R. Fayer. 1983. Experimental Sarcocystis ovicanis infection in lambs: salinomycin chemoprophylaxis and protective immunity. J. Parasitol. 69:271-276. [PubMed] [Google Scholar]
- 31.Leek, R. G., R. Fayer, and A. J. Johnson. 1977. Sheep experimentally infected with Sarcocystis from dogs. Disease in young lambs. J. Parasitol. 63:642-650. [PubMed] [Google Scholar]
- 32.Lele, V. R., P. V. Dhopavkar, and A. Kher. 1986. Sarcocystis infection in man. Indian J. Pathol. Microbiol. 29:87-90. [PubMed] [Google Scholar]
- 33.Li, Y., and Z. Lian. 1986. Studys on man-pig cyclic infection of Sarcocystis suihominis found in Yunnan province, China. Acta Zool. Sin. 32:329-334. (In Chinese.) [Google Scholar]
- 34.McLeod, R., R. N. Hirabayashi, W. Rothman, and J. R. Remington. 1980. Necrotizing vasculitis and Sarcocystis: a cause and effect relationship? South. Med. J. 73:1380-1383. [DOI] [PubMed] [Google Scholar]
- 35.Mehrotra, R., D. Bisht, P. A. Singh, S. C. Gupta, and R. K. Gupta. 1996. Diagnosis of human Sarcocystis infection from biopsies of the skeletal muscle. Pathology 28:281-282. [DOI] [PubMed] [Google Scholar]
- 36.Mensa, J., J. M. Gatell, Jimenez de Anta, and G. Prats. 1999. Guia e terapeutica antimicrobiana, 9th ed. Masson, S. A., Barcelona, Spain.
- 37.Ona, M., and T. Ohsumi. 1999. Prevalence of Sarcocystis spp. cysts in Japanese and imported beef (Loin: Musculus longissimus). Parasitol. Int. 48:91-94. [DOI] [PubMed] [Google Scholar]
- 38.Pathanathan, P., and S. P. Kan. 1981. Human Sarcocystis infection in Malaysia. Southeast Asian J. Public Health Trop. Med. 12:247-250. [Google Scholar]
- 39.Pena, H. F., S. Ogassawara, and I. L. Sinhorini. 2001. Occurrence of Cattle Sarcocystis species in raw kibbe from Arabian food establishments in the city of Sao Paolo, Brazil, and experimental transmission to humans. J. Parasitol. 87:1459-1465. [DOI] [PubMed] [Google Scholar]
- 40.Proctor, S. J., D. Barnett, O. H. V. Stalheim, and R. Fayer. 1976. Pathology of Sarcocystis fusiformis in cattle, p. 329-336. Proceedings of the 19th Annual Conference of the American Association of Veterinary Laboratory Diagnosticians.
- 41.Rommel, M., and A. O. Heydorn. 1972. Beitrage zum Lebenszyklus der Sarkosporidien. III. Isospora hominis (Railiet und Lucet, 1891) Wenyon, 1923, eine Dauerform des Sarkosporidien des Rindes und des Schweins. Berl. Muench. Tieraerztl. Wochenschr. 85:143-145. [PubMed] [Google Scholar]
- 42.Rommel, M., A. O. Heydorn, and F. Gruber. 1972. Beitrage zum Lebenszyklus der Sarkosporidien. I. Die Sporozyste von S. tenella in den Fazes der Katze. Berl. Muench. Tieraerztl. Wochenschr. 85:101-105. [PubMed] [Google Scholar]
- 43.Saito, M., Y. Shibata, A. Ohno, M. Kubo, K. Shimura, and H. Itagaki. 1998. Sarcocystis suihominis detected for the first time from pigs in Jap. J. Vet. Med. Sci. 60:307-309. [DOI] [PubMed] [Google Scholar]
- 44.Saito, M., Y. Shibata, M. Kubo, I. Sakakibara, A. Yamada, and H. Itagaki. 1999. First isolation of Sarcocystis hominis from cattle in Japan. Jpn. J. Vet. Med.Sci. 61:307-309. [DOI] [PubMed] [Google Scholar]
- 45.Saleque, A., P. D. Juyal, and B. B. Bhatia. 1990. Effect of temperature on the infectivity of Sarcocystis meischeriana cysts in pork. Vet. Parasitol. 36:343-346. [DOI] [PubMed] [Google Scholar]
- 46.Senaud, J. 1967. Contribution a l'etude des sarcosporidies et des toxoplasmes Toxoplasma. Protistologica 3:169-232. [Google Scholar]
- 47.Stalheim, O. H., S. J. Proctor, R. Fayer, and M. Lunde. 1976. Death and abortion in cows experimentally infected with Sarcocystis from dogs. 19th Ann. Proc. Vet. Lab. Diagnost. p. 317-327.
- 48.Straka, S., J. Skracikova, I. Konvit, M. Szilagyiova, and L. Michal. 1991. Sarcocystis species in Vietnamese workers. Cesk. Epidemiol. Mikrobiol. Immunol. 40:204-208. [PubMed] [Google Scholar]
- 49.Thomas, V., and A. S. Dissanaike. 1978. Antibodies to Sarcocystis in Malaysians. Trans. R. Soc. Trop. Med. Hyg. 72:303-306. [DOI] [PubMed] [Google Scholar]
- 50.Van den Enden, E. M. Praet, R. Joos, A. Van Gompel, and P. Gigasse. 1995. Eosinophilic myositis resulting from sarcocystosis. J. Trop. Med. Hyg. 98:273-276. [PubMed] [Google Scholar]
- 51.Van Knapen, F., D. Bouwmann, and E. Greve. 1987. Study on the incidence of Sarcocystis spp. in Dutch cattle using various methods. Tijdschr. Diergeneeskd. 112:1095-1100. [PubMed] [Google Scholar]
- 52.Yang, Z. Q., Y. X. Zuo, B. Ding, X. W. Chen, J. Luo, and Y. P. Zhang. 2001. Identification of Sarcocystis hominis-like (Protozoa: Sarcocystidae) cyst in water buffalo (Bubalus bubalis) based on 18s rRNA gene sequences. J. Parasitol. 87:934-937. [DOI] [PubMed] [Google Scholar]
- 53.Yu, S. 1991. Field survey of Sarcocystis infection in the Tibet autonomous region. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 13:29-32. (In Chinese.) [PubMed] [Google Scholar]
- 54.Zuo, Y.-X., F.-Q. Chen, and W.-Y. Li. 1982. Two patients with Sarcocystis infection, p. 52-53. In J. B. Jiang, K. Arnols, and K. P. Chang (ed.), Malaria and other protozoal infections. Proceedings of the Chinese Society of Protozoologists. Zhongshan University, Guongzhou, China.