Abstract
Trichomoniasis is perhaps the most common curable sexually transmitted disease worldwide, yet few resources are devoted to its control. It is associated with potentially serious complications such as preterm birth and human immunodeficiency virus acquisition and transmission. The immunology of a related organism, Tritrichomonas foetus, which causes disease in cattle, has been investigated to some extent, but more work is needed for the human strain, Trichomonas vaginalis. In addition, although trichomoniasis is easily treated with oral metronidazole, there is concern that the number of strains resistant to this antibiotic are increasing, and currently no alternative is licensed in the United States. As more is appreciated concerning the important public health implications of this common infection, more work will need to be done in understanding the diagnosis, treatment, and immunology of this organism.
INTRODUCTION
Trichomonas vaginalis is the causative agent of trichomoniasis, a common cause of vaginitis. Despite being a readily diagnosed and treatable sexually transmitted disease (STD), trichomoniasis is not a reportable infection, and control of the infection has received relatively little emphasis from public health STD control programs. More recently, however, appreciation of the high rates of disease and of associations of trichomoniasis in women with adverse outcomes of pregnancy and increased risk for human immunodeficiency virus HIV infection suggest a need for increased control efforts. This review discusses the epidemiology, clinical manifestations, diagnosis, treatment, complications, and pathophysiology of this parasitic infection.
STRUCTURE AND LIFE CYCLE
The shape of T. vaginalis in culture is typically pyriform, although amoeboid shapes are evident in parasites adhering to vaginal tissues in vivo (80). T. vaginalis is about 9 by 7 μm, and nondividing organisms have four anterior flagella [9(2) + 2 arrangement]. In addition to the undulating membrane, one recurrent flagellum and the costa originate in the kinetosomal complex at the anterior of the parasite. Internal organelles include a prominent nucleus and a rigid structure, the axostyle, that runs through the cell from the anterior end to the posterior end (42, 80). Unique energy-producing organelles, the hydrogenosomes (62), are present as paraxostylar and paracostal chromatic granules by light microscopy and as osmiophilic granules by electron microscopy (79, 80). The life cycle of T. vaginalis is simple in that the trophozoite is transmitted through coitus and no cyst form is known. The trophozoite divides by binary fission and, in natural infections, gives rise to a population in the lumen and on the mucosal surfaces of the urogenital tracts of humans.
EVOLUTIONARY BACKGROUND
T. vaginalis is an early-diverging parabasalid protozoan that appears to have branched before protozoan genera such as kinetoplastids, some of the earliest protozoa with mitochondria. Recent analysis of the subunit rRNA suppors this view as well and suggests that the taxonomy of some trichomonads may be soon revised (111). Unlike most eukaryotes, T. vaginalis lacks mitochondria an instead uses the hydrogenosome to accomplish fermentative carbohydrate metabolism with hydrogen as the electron acceptor. The hydrogenosome appears to have a common ancestry with mitochondria based on similarities in protein import (10, 25). However, major differences exist between hydrogenosomes and mitochondria in that hydrogenosomes lack cytochromes, mitochondrial respiratory chain enzymes, and DNA.
CLASSIFICATION
T. vaginalis is a parasitic protozoan, and the taxonomic position is based on the classification scheme by Dyer (26), in which protozoa with the “9 + 2” flagellum fall into the phylum Zoomastigina.
Phylum: Zoomastigina—possess flagella.
Class: Parabasalia—presence of a parabasal body: Golgi associated with kinetosomes; axostyle (bundled microtubules); undulating membrane, an extension of the plasma membrane, enveloping the recurrent flagellum; occur in association with animals.
Order: Trichomonadida (Kirby, 1947 emend. Honigberg, 1974)—four to six flagella, free or attached to an undulating membrane; no true cysts.
Family: Trichomonadidae (Wenyon, 1926)—presence of a cytostome, three to five free flagella (one flagellum on the margin of the undulating membrane); axostyle protruding through the posterior of the cell.
Genus: Trichomonas—four free flagella; one recurrent, along the outer margin of the undulating membrane; a costa at the base of the undulating membrane, and an axostyle extending through the cell.
Species: Trichomonas vaginalis (Donné, 1836)
RELATED HUMAN AND NONHUMAN SPECIES
Trichomonas tenax, found in oral gingival and tracheobronchial sites, and Pentatrichomonas hominis, isolated from the intestinal tract, are considered nonpathogenic and occur infrequently in humans. Each human species has specific tropism for its site of infection.
Tritrichomonas foetus is perhaps the nonhuman trichomonad most similar to T. vaginalis. Aside from having three anterior flagella (versus four in T. vaginalis), there are few morphologic differences between the parasites, and T. foetus causes the sexually transmitted disease bovine trichomoniasis. T. foetus displays cytotoxicity toward mammalian cells (14), adheres to mammalian cells (16), and produces an array of hydrolases (64, 71), and the response to T. foetus in the reproductive tract is in many ways similar to the response to T. vaginalis, with a mononuclear infiltration of varying intensity (77, 83). However, unlike T. vaginalis. T. foetus can be invasive to the fetus, having been demonstrated in the placenta, fetal lung, gut, and lymph nodes (15, 90), and is a known cause of abortion in infected cattle. Certain other differences such as difference in infectivity in mice (1, 43, 110) suggest that key differences in the biology of these parasites should be considered when extrapolating between them.
EPIDEMIOLOGY
Humans are the only natural host of T. vaginalis. Trichomoniasis is an extremely common infection in the United States and worldwide. The prevalence of trichomoniasis in inner city U.S. STD clinics typically approaches 25% and may be higher in certain populations (8, 100). In Los Angeles, for instance, the prevalence among African American attendees at a public clinic was 38% (107). A recent estimate of the incidence of STDs in the United States estimated an annual incidence of 7.4 million new cases (116). Although this far exceeds the incidence of chlamydia and gonorrhea, trichomoniasis is not a public health priority, as evidenced by the fact that it is not a reportable disease. The World Health Organization has estimated that this infection accounts for almost half of all curable infections worldwide (17). Various studies of African populations have reported the prevalence of vaginal trichomoniasis to be between 11 and 25% (53, 57, 61). Laga et al. reported an incidence of 38% during a 4-month exposure interval among HIV-infected women in Zaire (57).
Epidemiologically, T. vaginalis infections are commonly associated with other STDs and are a marker of high-risk sexual behavior. Trichomoniasis is frequently seen concomitantly with other STDs, particularly gonorrhea (66). The majority of women with trichomoniasis also have bacterial vaginosis (44, 108, 119). Unlike other STDs, which have a higher prevalence among adolescents and young adults, the rates of trichomoniasis are more evenly distributed among sexually active women of all age groups, further strengthening its potential utility as a marker for risky sexual behavior (66). Although survival on fomites is documented, the organism is thought to be transmitted almost exclusively by sexual activity (118). The incubation period of this infection is unknown; however, in vitro studies suggest an incubation period of 4 to 28 days (38).
Prevention of trichomoniasis has not been a priority due to lack of understanding of its public health implications and lack of resources. Although there has been some discussion of requiring reporting of this infection to state and local health departments and ultimately the Centers for Disease Control and Prevention, it is not reportable at this time. Control efforts would require reporting of cases as well as direction of resources toward screening at-risk individuals, including males, for infection.
CLINICAL MANIFESTATIONS
Women who are symptomatic from trichomoniasis complain of vaginal discharge, pruritus, and irritation. Signs of infection include vaginal discharge (42%), odor (50%), and edema or erythema (22 to 37%). The discharge is classically described as frothy, but it is actually frothy in only about 10% of patients. The color of the discharge may vary (119). Colpitis macularis (strawberry cervix) is a specific clinical sign for this infection but is detected with reliability only by colposcopy and rarely during routine examination (119). Other complaints may include dysuria and lower abdominal pain; the etiology of the latter is unclear. The urethra is also infected in the majority of women (88). Nearly half of all women with T. vaginalis are asymptomatic (31). Therefore, if these women are not screened, the diagnosis will be missed. The extent of the inflammatory response to the parasite may determine the severity of the symptoms. Factors that influence the host inflammatory response are not well understood but may include hormonal levels, the coexisting vaginal flora, and the strain and relative concentration of the organisms present in the vagina.
The prevalence and spectrum of disease in males are less well characterized; the infection appears to usually be asymptomatic, but it has been suggested as an increasingly important cause of non-gonococcal urethritis (56). Reported prevalences of urethral infection with T. vaginalis in males have varied depending on the population studied and the diagnostic techniques used. In his series of sentinel studies, using culture of urine, urethra, coronal sulcus, and semen, Krieger et al. reported a prevalence of 11% among men attending an STD clinic (56). Urethritis was present in half of the men with trichomonas as the sole urethral pathogen (56). In a similar study conducted at an STD clinic in Denver, investigators found a prevalence rate of 2.8% by using urine sediment culture (46). Among men attending an STD clinic in Birmingham, AL, T. vaginalis was detected by PCR in 17% of men attending the clinic for a new-problem visit or screening. There was no significant difference in the detection of the organism between men with and without urethral symptoms (20 and 14.5%, respectively). Among men with nongonococcal urethritis, respectively) nearly as many had T. vaginalis detected as had chlamydia (19.9 and 25.2%, respectively) (98).
HOST RESPONSE AND IMMUNOLOGY
Our current understanding of immunity to T. vaginalis has come largely from observations of responses in human patients and experimentation using in vitro models and animal models of the related species, T. foetus. Natural infection seems to produce immunity that is only partially protective, since reinfection of patients can be 30% on follow-up (78).
Acquired Immunity: Evidence and Mechanism
Antibody responses during infection with T. vaginalis have been demonstrated to occur in infected patients by several types of immunoassays (13). Older experimental work using subcutaneous or intraperitoneal injections in mice suggested that antibodies could be protective, and more recent experiments with immunized mice challenged by intravaginal inoculation indeed indicate some protection (1). Infection in humans results in parasite-specific antibodies in the reproductive tract and, in most instances, circulating antibodies in the serum (41); there is also evidence of lymphocyte priming as detected by antigen-specific proliferation of peripheral blood mononuclear cells (72). Thus, natural infection with T. vaginalis results in priming of acquired immune responses.
Establishing the precise protective effects of parasite-specific immunity has been less straightforward. In vitro analyses of the effects of serum-derived antibodies and monoclonal antibodies to surface antigens have identified antiadhesin antibodies that block the adhesion of T. vaginalis to various human cell lines, such as HeLa (4, 7), and to primary human vaginal epithelial cells (33). Reduced destruction of these mammalian cells was observed, implying that antibody protected against adhesion-dependent host cell destruction. Alternatively, antibodies specific for soluble parasite molecules such as proteases (64, 71, 81), cytoactive molecules (69), or lytic factors such as phospholipases (68) may also be protective. However, proof of the protective nature of antibodies in eliminating infection or limiting pathogenesis in vivo has been hampered by lack of an adequate experimental-animal model for vaginal infection studies. It seems clear that more than antibody is needed for elimination of infection, so that innate immune responses and acquired cellular immune responses are likely to be important. Mice and other laboratory animals are refractory to intravaginal infection with T. vaginalis, with the result that there is not a convenient laboratory animal model for infection. Although experimental infections in mice by intravaginal inoculation of parasites together with lactobacilli have been reported (1), infections are sporadic in this model. Mice have been successfully infected with the related trichomonad T. foetus, a parasite of the reproductive tract of cattle, either after treatment with estradiol (110) or without treatment (77). This model may offers some potential for studies of immunity to trichomonads, provided that immunity to T. vaginalis and immunity to T. foetus operate by similar mechanisms.
Targets of Acquired Immunity
The presence of parasite-specific immunoglobulin G (2) and immunoglobulin A (121) responses also indicates priming of helper T cells, although the relevant antigens are largely unknown, as are the exact effects of antibodies on the parasites. One obvious target of protective antibody could be the ahesin molecules used by the parasite to facilitate close contact to host cells, a process previously shown to lead to efficient host cell destruction (54). The molecular basis of adhesion of T. vaginalis has been investigated, and four antigenic surface molecules have also been implicated in the adhesion of T. vaginalis to vaginal epithelial cells; their expression is being upregulated during attachment to host cells (7). Antibodies to these molecules protected target cells from parasite-mediated cytotoxicity (6), suggesting that antiadhesion immune responses could be important in in vivo protection against the pathogenic effects of T. vaginalis.
However, our current understanding of immunity to T. vaginalis remains unsatisfactory, and it is not clear whether acquired immune responses are required for protection and, if so, what role is played by acquired immunity in containing or eliminating infections. Although there is some evidence that protection may be achieved by immunization of laboratory animals (1), strong protective immunity does not seem to follow natural infection in humans. A recent study of patients infected with T. vaginalis and HIV indicated no evidence of increased levels or longevity of parasite infection in these patients compared to those in patients infected with T. vaginalis but not HIV (21). These observations may indicate that innate immunity involving chemotaxis and subsequent influx of neutrophils is much more important than acquired immunity in controlling infections with T. vaginalis, since neutrophils are often the most numerous leukocytes present in response to infection (119).
BIOCHEMISTRY OF VIRULENCE AND PATHOGENESIS
Although pathogenesis and virulence in human trichomoniasis is not fully understood, progress has been made in identifying parasite products that can damage host cells and tissues.
Molecular Mechanisms of Pathogenesis
Adhesion is thought to play an important role in the pathogenesis of trichomoniasis, and investigations of the molecular basis of adhesion of T. vaginalis to human cells have identified several adhesion molecules [Ad] on the surface of the parasite. Much of the evidence for the role of Ad in pathogenesis has come from coculture experiments in which antibodies to Ad were shown to reduce parasite adhesion and subsequent cytopathic effects [CE] on host cells (27). Additional studies had shown that contact of T. vaginalis with mammalian targets caused upregulation of Ad and that the parasite assumed a flattened shape, essentially laminating itself to the host cell (7). Cysteine proteinases seem to be necessary for efficient Ad-mediated adhesion of parasites to targets (74). Control of the expression of Ad also seems to be under the influence of iron, since transcription induction of ap65-1 (adhesion protein 65,000) was reported to be regulated via certain iron-responsive DNA elements (e.g., AGATAACGA) (109). Thus, the Ad family of molecules appears to be regulated at several levels. These findings suggest that adhesion of T. vaginalis facilitates efficient cytotoxicity toward mammalian cells and probably involves complex interactions similar to the situation for other cell-cell contacts such as those of leukocytes (28).
Additional parasite molecules with functional carbohydrate groups, distinct from Ad, appear to be involved in the adhesion of T. vaginalis to human vaginal epithelial cells (33), although the precise roles of the molecules in parasite adhesion and CE are not known. It will be important to show, by using knockouts or perhaps interfering RNA methods, which adhesins are required for significant CE to occur.
Little is known about the host cell receptors to which parasite adhesion molecules bind, although there is some evidence that laminin may be a target for trichomonad adhesion (103).
Hydrolases
A variety of hydrolases have been described in T. vaginalis, with cysteine proteinases being particularly prevalent. Ranging from 20 to 110 kDa (63), several of the lower-molecular-mass proteinases are released from the cell (63, 64, 81), although a recent report suggests that one cysteine proteinase is probably cytoplasmic since it lacks a signal peptide (60).
There are reports of other parasite products, described as cell-detaching factors, that are released by the parasite (32, 69); although these have received only limited biochemical analysis, it is known that some of them do have trypsin-like activity. These factors are active on human and hamster cells, causing them to detach and round up. The release of cell-detaching factors and proteinases from T. vaginalis clearly implies that these parasite products could degrade proteins such as laminin, vitronectin, and other components of the extracellular matrix, thus effecting the release of host cells from tissue. In addition, the levels of secretory leukocyte proteases inhibitor in patients with T. vaginalis infections are significantly lower than those in uninfected patients (22, 23), suggesting a possible role for parasite proteases in the observed enhanced risk of HIV infection associated with infection with this parasite (107).
The substrate specificity and structure of some of the proteinases of T. vaginalis are now being determined. Parasite proteinases are not inhibited by pepstatin, phenylmethylsulfonyl fluoride, or EDTA, but they are inhibited by iodoacetic acid, antipain, leupeptin and N-α-p-tosyl-l-lysine chloromethyl ketone (TLCK). Proteinases with lower molecular masses are released from the parasite; proteinases of 25, 27 and 34 kDa specifically hydrolyzed synthetic substrates with arginine-arginine residues, whereas other proteinases have activity over a wide substrate range (81). Recent cloning studies of cysteine proteinase genes of T. vaginalis indicate that at least four distinct genes are present and that they have considerable homology (up to 45% identity) to cysteine proteinase genes of Dictyostelium discoideum. Three of the proteinase genes are present as single copies, whereas the other is present as a multiple-copy gene. The amino acid sequence data indicated that these proteinases are of the L-cathepsin H-papain type (71).
Cytotoxic Molecules
Recent evidence suggests that T. vaginalis may produce molecules that are delivered to target cells and mediate cytotoxicity through damage of the target cell plasma membrane. One of these molecules creates pores in erythrocyte membranes as detected by electron microscopy (29), thus displaying perforin-like activity (30).
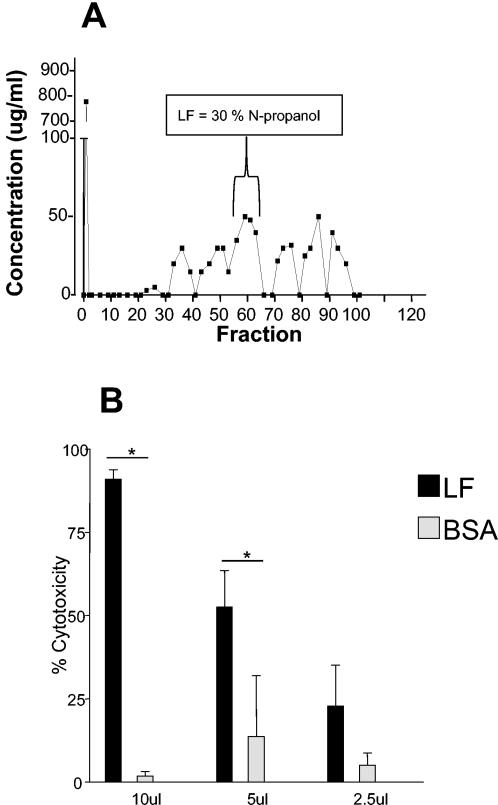
An additional membrane-attacking molecule has recently been detected in T. vaginalis. A lytic factor (LF) is released by T. vaginalis that can destroy nucleated cells and erythrocytes and specifically degrades phosphatidylcholine (Fig. 1), suggesting that it is a phospholipase A2 (68). It will be important to determine the identity of the mechanism that triggers the release of this phospholipase A2 and other virulence factors as they are identified.
FIG. 1.
Purified LF is cytotoxic to WEHI 164 cells. (A) Octyl-Sepharose chromatogram of T. vaginalis extract with LF elution at 30% N-propanol. (B) Cytotoxicity of LF and bovine serum albumin (BSA), purified by octyl-Sepharose chromatography as described in the text, lyophilized, and reconstituted in Hanks' balanced salt solution. Cytotoxicity was measured on WEHI 164 cells as described previously. Data shown are means and standard deviations for three individual experiments. *, significant difference (P < 0.05) between treatment groups.
Other Molecular Pathogenesis Possibilities
The importance of the thioredoxin system as one of the major antioxidant defense mechanisms in trichomonads was confirmed by showing that the parasite responds to environmental changes resulting in increased oxidative stress by upregulating thioredoxin and thioredoxin peroxidases levels. Sequence data indicate that the thioredoxin reductase of trichomonads differs fundamentally in structure from that of its human host and thus may represent a useful drug target (19).
Soon after the initial report of double-stranded RNA (dsRNA) viruses in T. vaginalis (114), the presence of this virus was found to correlate with variation of the expression of certain surface antigens, and loss of the dsRNA accompanied loss of antigen expression (113). Recent reports confirm the presence of dsRNA virus in clinical isolates of T. vaginalis (3), with virus prevalence being as high as 82% in parasite isolates (115). One function ascribed to the dsRNA virus is the regulation of expression of the P270 surface antigen (50). One virus, TVV2-1, codes for an 85-kDa capsid protein, a 160-kDa capsid-polymerase fusion protein, and possibly two additional proteins (9). The precise role of dsRNA viruses in the pathogenesis of T. vaginalis remains to be determined, however.
Sequencing the Genome
Progress in sequencing the genome of T. vaginalis now provides considerable resources that may be used to define genes that are important in the pathogenesis of human trichomoniasis. The recent release of the 5X genome sequence data (http://www.tigr.org/tdb/e2k1/tvg/) makes available the most comprehensive genomic sequence of this parasite thus far.
DIAGNOSIS
The most common means of diagnosis is visualization of the motile trichomonads in a saline preparation of the vaginal fluid. This must be performed within 10 to 20 min of collection of the sample, or the organisms will lose viability. The organisms are about the size of a white blood cell and may be actively motile or may be seen beating their flagella at rest. Although quick and inexpensive, the test has limited sensitivity, ranging from 60 to 70% (55). There are often white blood cells in the vaginal fluid that are indicative of accompanying inflammation. The vaginal pH is elevated (greater than 4.5) in the majority of cases, but it may be normal. The whiff test (addition of potassium hydroxide to vaginal fluid for olfactory detection of amines) is variable.
Currently, the “gold standard” for the diagnosis of trichomoniasis is culture. Traditionally, this has been accomplished though cultivation in Diamond's medium, which is not widely available and thus was used mainly for research purposes. However, a new commercially available culture method composed of liquid medium in a clear pouch has been shown to be as good as the traditional research method (24). This method has been used successfully with both clinician-obtained and self-obtained specimens (101); the latter is becoming quite useful in situations where pelvic examination is not possible or desirable (for example, screening in adolescents or in patients in developing countries). In addition, a delayed-inoculation technique is possible, allowing for initial reading of the wet preparation and then inoculation of the culture pouch if the wet preparation is negative. Swab specimens may sit at room temperature for up to 30 min prior to pouch inoculation (100). Results of the culture test are available in 2 to 5 days. Considering the suboptimal sensitivity of the wet mount, routine screening using culture may soon become included in the diagnostic workup, especially when high-prevalence populations are being screened. Other commercially available methods for diagnosis include an office-based oligonucleotide probe test, which has a sensitivity of 80 to 90% and a specificity of 95% (11). Trichomonads may be seen on Pap smears with a sensitivity of about 60% and a specificity of 95% (117). Survival for up to 24 h in Amies gel agar transport medium has also been documented (100). Self-collected vaginal swab specimens are as sensitive as clinician-obtained for the diagnosis of trichomoniasis (101).
More recently, a point-of-care antigen detection test for the diagnosis of trichomoniasis in women has been licensed (Genzyme Corp. Cambridge, Mass.). In a study of women attending STD clinics in Seattle, Washing and Birmingham, Ala., the enzyme-linked immunosorbent assay demonstrated a sensitivity and specificity of 78.5 and 98.6%, respectively, compared to culture. Rapid test performance did not vary with vaginal symptoms or with the presence of other vaginal or cervical syndromes or infections. The rapid assay was more sensitive than wet-preparation microscopy (78.5 and 72.4%, respectively; P = 0.04) but was less specific (98.6 and 100.00%, respectively; P = 0.001) (56a). This test may be of value in settings where microscopy is not possible.
PCR-based tests for T. vaginalis are currently under development. Several groups of investigators have reported their findings on the development of a PCR technique for diagnosis of trichomoniasis in females. In 1992, Riley et al. published a report of primers (TVA5 and TVA6) for the detection of T. vaginalis (91). Subsequently, many additional primer sets have been described (5, 47, 49, 82). The sensitivity and specificity of these primers in clinical studies with vaginal swab specimens have varied, with sensitivities of 85 to 100% being reported (37, 45, 59, 70, 84, 93, 96, 102). Unlike PCR for infections such as gonorrhea and chlamydia, which appears to have greater sensitivity than culture methods, PCR for trichomoniasis in women does not appear to offer a diagnostic advantage. This may be because T. vaginalis is much less fastidious to culture than Neisseria gonorrhoeae or Chlamydia trachomatis. Successful culture of T. vaginalis requires only the multiplication of a single organism, the same as that needed for PCR. PCR of vaginal swabs may be advantageous in settings where incubation of cultures is not possible and shipping of specimens to a reference laboratory is required. Self-obtained vaginal swab specimens may also be useful for the PCR technique. In addition, PCR may be superior to culture for the diagnosis of T. vaginalis in males (see below). Of note are the many different primers which have been used for the detection of T. vaginalis by PCR. Direct comparisons of these primers, and perhaps the development of new primers, could prove useful with regard to refining the technique and improving its sensitivity.
Diagnosis of this infection is much more difficult for males, with the best culture results yielded by combining urethral swabs and urine sediment for culture (56). PCR appears to have far greater sensitivity in this setting. Among 300 men attending an STD clinic for a new problem or screening, culture of urethral swab and urine sediment detected T. vaginalis in 15 of 300 (5%), in contrast to 52 of 300 (17%) detected by PCR of urine and urethral swab specimens. The sensitivity of PCR with urine specimens in this study was 100%, in contrast to a sensitivity of 80% for swab specimens (99). In a second study, men attending an STD clinic as well as a dermatology clinic in Malawi were studied using wet-mount microscopy and urethral culture as well as PCR detection with urethral swab specimens. The prevalence of infection among symptomatic and asymptomatic men was 21 and 12%, respectively. The sensitivity and specificity of the PCR assay was 82 and 95%, respectively (40). In a larger cohort of the same population, the sensitivity and specificity of urine- based PCR were 92.7 and 88.6%, respectively, with an adjusted specificity of 95.2% (48).
TREATMENT
Until recently, metronidazole was the only efficacious antibiotic available in the United States for the treatment of trichomoniasis. The recommended dose is 2 g orally in a single dose, and the reported cure rate is 97% (65). Sexual partners should also be treated. Metronidazole intravaginal gel has limited efficacy and should not be used. Although there continues to be some controversy about the safety of metronidazole in pregnancy, there has never been a documented case of fetal malformation attributed to its use, even when it is used in the first trimester (92). Recently, controversy has also developed concerning the treatment of trichomoniasis in pregnancy and its relationship to preterm birth. Two studies have recently been published which suggest that treatment of trichomoniasis in pregnancy may actually increase the risk of preterm birth rather than decrease the risk as predicted (52). However, there are limitations to both of these studies. One of the studies used much higher doses of metronidazole than are recommended. In addition, the study was stopped prematurely because of the trend toward preterm birth that was seen, and so the number of women enrolled fell short of the number needed for a definitive analysis (52). The second study was a subanalysis of a study designed to answer questions relating to STD and HIV risk, therefore, it was not designed primarily to answer questions regarding the risks of preterm birth associated with treatment of trichomoniasis in pregnancy (51). Since the publication of these papers, the Centers for Disease Control and Prevention has not revised recommendations for treatment during pregnancy. Pregnant women may be treated with the 2-g single dose of metronidazole (18). Occasionally patients are allergic to metronidazole. Since there is no effective alternative, desensitization is the only option (18, 85). Another therapeutic dilemma involves metronidazole resistance in T. vaginalis. The mechanism of development of anaerobic resistance to metronidazole also is controlled by hydrogenosomes, in that metronidazole competes for H+ as an electron acceptor. In metronidazole-resistant T. vaginalis, the expression levels of the hydrogenosomal enzymes pyruvateferredoxin oxidoreductase, ferridoxin, malic enzyme, and hydrogenase are reduced dramatically, which probably eliminates the ability of the parasite to activate metronidazole (58).
It is estimated that approximately 2.5 to 5% of all cases of trichomoniasis display some level of resistance to treatment with metronidazole (97). This resistance is relative and can usually be overcome with higher doses of oral metronidazole (67). For example, marginal resistance, defined as an aerobic minimal lethal concentration (MLC) of metronidazole of 50 μg/ml, requires a total treatment dose of 10 g administered over several days for cure wheareas high-level resistance (MLC, ≥400 ug/ml) requires 40 g. Intravenous formulations offer no advantage over the oral drug. Some authorities have recommended higher doses of oral medication in combination with pharmacy- prepared intravaginal preparations. There are limited anecdotal reports of success with paromomycin cream; however, there may also be a high incidence of local side effects associated with this therapy (105). Tinidazole (see below) may be useful for resistant infections. Women with asymptomatic infection should be treated. If left untreated, they may later become symptomatic, and they continue to transmit the infection while untreated.
Tinidazole is a 5-nitroimidazole compound that is chemically related to metronidazole and has been widely used outside of the United States for treatment of trichomonas. It was recently licensed for the treatment of trichomoniasis in the United States. It has a plasma half-life twice that of metronidazole (12 to 14 h for tinidazole versus 6 to 7 h for metronidazole) (89, 120) and may have a lower incidence of adverse effects than metronidazole. Against trichomoniasis, a 2-g oral dose of tinidazole has overall clinical efficacy equal to metronidazole (90 to 100%) (95).
Tinidazole may be a good option for patients with infection resistant to metronidazole. The Centers for Disease Control and Prevention tested 195 metronidazole-resistant T. vaginalis clinical isolates submitted for MLC testing for both metronidazole and tinidazole. The mean aerobic metronidazole MLC was 400 μg/ml, compared to an aerobic tinidazole MLC of 100 μg/ml (H. Gillette, G. Schmid, D. Mosure, J. Lossick, E. Secor, L. Narcisi, and P. Garnard, Abstr. ISSTDR, abstr. O67, 1999). Six clinical studies have evaluated various doses of tinidazole for treatment of metronidazole-resistant trichomoniasis (35, 94, 106, 112; Gillette et al., Abstr. ISSTDR, abstr. O67, 1999). The largest series of patients was reported by Sobel et al. (106). In this study, 20 patients with clinically refractory trichomoniasis (failure to respond to therapy with oral metronidazole at at least 500 mg twice a day for 7 days) were treated with high doses of oral and vaginal tinidazole (2 to 3 g orally plus 1 to 1.5 g intravaginally for 14 days). The cure rate was 92% (22 of 24); no patients discontinued therapy due to side effects (106).
COMPLICATIONS
Long considered a “minor” STD with few associated complications, infection with T. vaginalis has recently been implicated as a cause of preterm delivery in several studies. In a large multicenter study, after adjusting for demographic, behavioral, and microbiological variables, T. vaginalis was significantly associated with low birth weight, premature rupture of membranes, and preterm delivery (relative risk, 1.4) (20). Similarly, Minkoff et al. also documented a significant correlation between trichomoniasis and premature rupture of membranes (75). In that study, the incidence of this complication at term was 27.5% in women with T. vaginalis infection versus 12.8% in those without (P < 0.03) (75). In another study of pregnant adolescents, T. vaginalis was independently associated with prematurity and low birth weight (36).
Veterinary data further support a contribution of Trichomonas infections to adverse outcomes of pregnancy. Bovine venereal trichomoniasis is a cause of abortion in cattle, and a Trichomonas vaccine reduces the occurrence of this complication (104). The exact linkages between colonization or infection of the lower tract in pregnancy and prematurity remain speculative. The leading hypothesis is that infection triggers local cytokine release, which in turn triggers the onset of labor (76), a hypothesis for which data continue to accumulate. Several studies have found associations between the presence of biochemical substances (which may be involved in the initiation of labor) in the vaginal fluid of pregnant women and lower genital tract infections. These substances include phospholipase A2, sialidases, endotoxin, and interleukin-1 alpha (11, 12, 73, 86). Infections associated with elevated levels of these substances within the vaginal or cervical fluids have included trichomoniasis as well as bacterial vaginosis and C. trachomatis infection (11, 12, 73, 86). Investigators have also shown a relationship between the presence of elevated cytokine levels in the amniotic fluid and preterm labor. Hillier et al. studied afebrile patients with intact membranes who presented with preterm labor at or before 34 weeks of gestation. They found that the presence of cytokines in the amniotic fluid was related to amniotic fluid infection, histologic chorioamnionitis, and premature delivery (39). In summary, lower genital tract infections, including trichomoniasis, have been linked to elevated levels of enzymes and cytokines within the vaginal fluid and the presence of cytokines within the amniotic fluid has been linked to chorioamnionitis and premature delivery.
Prospective studies of treatment of trichomoniasis during pregnancy for the prevention of preterm birth have yielded disappointing results. Among women with asymptomatic infection who were treated with metronidazole during the second and third trimesters of pregnancy, a trend toward increased preterm delivery was seen compared to the placebo group. However, the dose of metronidazole used was four times the recommended dose. In addition, the study was stopped prematurely due to a slow accrual of subjects and to the trend for increased risk of preterm delivery in the treatment group (52). A second study, conducted in Uganda, also found that treatment of trichomoniasis during pregnancy resulted in an increase in the incidence of preterm birth. However, this study was actually a subgroup analysis of a larger trial and was not properly designed to answer the question of the effect of treatment of T. vaginalis during pregnancy on preterm birth (51). Therefore, the question remains unanswered.
Acquisition of HIV has been associated with trichomoniasis in several African studies, possibly as a result of the local inflammation often caused by the parasite. Leroy et al. found a significant difference between the prevalence of trichomoniasis among a cohort of HIV-infected and noninfected pregnant women in Rwanda (20.2 and 10.9%, respectively; P = 0.0007) (61). In a prospective study by Laga et al., the incidence of trichomoniasis was significantly associated with HIV seroconversion (odds ratio, 1.9) in a multivariate analysis of a cohort of women in Zaire (57). The associations between HIV and trichomoniasis, as well as other STDs, may relate to (i) increased shedding of HIV as a result of the local inflammation produced by the STD, (ii) increased susceptibility to HIV as a result of the macro- or microscopic breaks in mucosal barriers caused by the STD, (iii) a higher prevalence of STDs among HIV-infected individuals as a result of common risk factors for both infections, and/or (iv) an increased susceptibility to STDs as a result of the immunosuppression associated with HIV infection. Given the higher prevalence and incidence of trichomoniasis than most other treatable STDs in most studies to date, the attributable fraction of HIV acquisitions due to trichomoniasis may eclipse the relative contribution of other STDs (107).
Transmission of HIV is enhanced by coinfection with T. vaginalis. In a study conducted in Malawi, the median HIV RNA concentration in the seminal fluid of men with urethritis was significantly higher in the men with trichomoniasis than in those with symptomatic urethritis due to an unidentified cause (40). In addition, successful treatment of trichomonal urethritis reduced the levels of HIV RNA so that they were similar to those seen in uninfected controls (87).
CONCLUSIONS
Trichomoniasis is an extremely common infection worldwide and is associated with important public health problems, including amplification of HIV transmission. Current treatment with metronidazole is reliable and inexpensive; however, the number of strains resistant to metronidazole may be increasing. Important questions remain concerning immunology, complications of pregnancy, accurate diagnosis, and public health control of this infection.
REFERENCES
- 1.Abraham, M., C. M. Desjardins, L. G. Filion, and G. E. Garber. 1996. Inducible immunity to Trichomonas vaginalis in a mouse model of vaginal infection. Infect. Immun. 64:3571-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addis, M., P. Rappelli, A. M. Pinto De Andrade, F. M. Rita, M. M. Colombo, P. Cappuccinelli, and P. L. Fiori. 1999. Identification of Trichomonas vaginalis alpha-actinin as the most common immunogen recognized by sera of women exposed to the parasite. J. Infect. Dis. 180:1727-1730. [DOI] [PubMed] [Google Scholar]
- 3.Alderete, J., K. A. Wendel, A. M. Rompalo, E. J. Erbelding, M. Benchimol, and T. H. Chang. 2003. Trichomonas vaginalis: evaluating capsid proteins of dsRNA viruses and dsRNA virus within patients attending a sexually tranmitted disease clinic. Exp. Parasitol. 103:44-50. [DOI] [PubMed] [Google Scholar]
- 4.Alderete, J. F., R. Arroyo, and M. W. Lehker. 1995. Analysis for adhesins and specific cytoadhesion of Trichomonas vaginalis. Methods Enzymol. 253:407-414. [DOI] [PubMed] [Google Scholar]
- 5.Alderete, J. F., J. L. OBrien, R. Arrayo, et al. 1995. Cloning and molecular characterization involved in Trichomonas vaginalis cytoadherence. Mol. Microbiol. 17:69-83. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo, R., J. Engbring, and J. Alderete. 1992. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol. Microbiol. 6:853-862. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo, R., A. Gonzalez-Robles, A. Martinez-Palomo, and J. F. Alderete. 1993. Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol. Microbiol. 7:299-309. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann, L., I. Lewis, R. Allen, J. Schwebke, L. Leviton, H. Siegal, and E. Hook III. 2000. Risk and prevalence of treatable sexually transmitted diseases at a Birmingham substance abuse treatment facility. Am. J. Public Health 90:1615-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bessarab, I., H. W. Liu, C. F. Ip, and J. H. Tai. 2000. The complete cDNA sequence of a type II Trichomonas vaginalis virus. Virology 267:350-359. [DOI] [PubMed] [Google Scholar]
- 10.Bradley, P., C. J. Lahti, E. Plumper, and P. J. Johnson. 1997. Targeting and translocation of proteins in to the hydrogenosome of the protist Trichomonas: similarites with mitochondrial protein import. EMBO J. 16:3484-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briselden, A. M., and S. H. Hillier. 1994. Evaluation of Affirm VP microbial identification test for Gardnerella vaginalis and Trichomonas vaginalis. J. Clin. Microbiol. 32:148-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briselden, A. M., B. J. Moncla, C. E. Stevens, and S. L. Hillier. 1992. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis- associated microflora. J. Clin. Microbiol. 30:663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess, D. 1998. Trichomonads and intestinal flagellates, p. 203-214. In F. Cox, J. P. Krier, and D. Wakelin (ed.), Topley and Wilson's microbiology and microbial infections, 9th ed. University Press, New York, N.Y.
- 14.Burgess, D., K. F. Knoblock, T. Daugherty, and N. P. Robertson. 1990. Cytotoxic and hemolytic effects of Tritrichomonas foetus on mammalian cells. Infect. Immun. 58:3627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess, D., and K. F. Knoblock. 1989. Identification of Tritrichomonas foetus in sections of bovine placental tissue with monoclonal antibodies. J. Parasitol. 75:977-980. [PubMed] [Google Scholar]
- 16.Burgess, D., and C. M. McDonald. 1992. Analysis of adhesion and cytotoxicity of Tritrichomonas foetus to mammalian cells by use of monoclonal antibodies. Infect. Immun. 60:4253-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cates, W., and The American Social Health Association Panel. 1999. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex. Transm. Dis. 26:52-57. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2002. 2002 Sexually transmitted diseases treatment Guidelines. Morb. Mortal. Wkly. Rep. 51(RR-6):44-45. [Google Scholar]
- 19.Coombs, G., G. D. Westrop, P. Suchan, G. Puzova, R. P. Hirt, T. M. Embley, J. C. Mottram, and S. Muller. 2004. The amitochondrial eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. J. Biol. Chem. 279:5249-5256. [DOI] [PubMed] [Google Scholar]
- 20.Cotch, M. F., J. G. Pastorek, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Reegan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, and G. G. Rhoads. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:361-362. [DOI] [PubMed] [Google Scholar]
- 21.Cu-Uvin, S., H. Ko, J. W. Jamieson, Hogan, P. Schuman, J. Anderson, R. S. Klein, and the HIV Epidemiology Research Study (HERS) Group. 2002. Prevalence, incidence, and persistence or recurrence of trichomoniasis among human immunodeficiency virus (HIV)-positive women and among HIV-negative women at high risk for HIV infection. Clin. Infect. Dis. 34:1406-1411. [DOI] [PubMed] [Google Scholar]
- 22.Draper, D., W. Donohoe, L. Mortimer, and R. P. Heine. 1998. Cysteine proteases of Trichomonas vaginalis degrade secretory leukocyte protease inhibitor. J. Infect. Dis. 178:815-819. [DOI] [PubMed] [Google Scholar]
- 23.Draper, D., D. V. Landers, M. A. Krohn, S. L. Hillier, H. C. Wieseneld, and R. P. Heine. 2000. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am. J. Obstet. Gynecol. 183:1243-1248. [DOI] [PubMed] [Google Scholar]
- 24.Draper, D., R. Parker, E. Patterson, W. Jones, M. Beutz, J. French, K. Borchardt, and J. McGregor. 1993. Detection of Trichomonas vaginalis in pregnant women with the InPouch TV system. J. Clin. Microbiol. 31:1016-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyall, S., and P. J. Johnson. 2000. Origins of hydrogenosomes and mitochondria: evolution and organelle biogenesis. Curr. Opin. Microbiol. 3:404-411. [DOI] [PubMed] [Google Scholar]
- 26.Dyer, B. 1990. Phylum Zoomastigina Class Parabasalia, p. 252-258. In L. Margulis, J. O. Corliss, M. Melkonian, and D. J. Chapman (ed.), Handbook of Protoctista. Jones and Bartlett, Boston, Mass.
- 27.Engbring, J., and J. F. Alderete. 1998. Characterization of Trichomonas vaginalis AP33 adhesin and cell surface interactive domains. Microbiology 144:3011-3018. [DOI] [PubMed] [Google Scholar]
- 28.Fais, S., and W. Malorni. 2003. Leukocycte uropod formation and membrane/cytoskeleton linkage in immune interactions. J. Leukoc. Biol. 73:556-563. [DOI] [PubMed]
- 29.Fiori, P., P. Rappelli, A. Rocchigiani, and P. Cappuccinelli. 1993. Trichomonas vaginalis haemolysis: evidence of functional pores formation on red cell membranes. FEMS Microbiol. Lett. 13-18. [DOI] [PubMed]
- 30.Fiori, P., M. F. Rappelli, and M. F. Addis. 1999. The flagellated parasite Trichomonas vaginalis: new insights into cytopathogenicity mechanisms. Microb. Pathog. 1:149-156. [DOI] [PubMed] [Google Scholar]
- 31.Fouts, A. C., and S. J. Kraus. 1980. Trichomonas vaginalis: re-evaluation of its clinical presentation and laboratory diagnosis. J. Infect. Dis. 141:137-143. [DOI] [PubMed] [Google Scholar]
- 32.Garber, G. E., L. T. Lemchuk-Favel, and W. R. Bowie. 1989. Isolation of a cell-detaching factor of Trichomonas vaginalis. J. Clin. Microbiol. 27:1548-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert, R., G. Elia, D. H. Beach, S. Klaessig, and B. N. Singh. 2000. Cytopathogenic effect of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect. Immun. 68:4200-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reference deleted.
- 35.Hamed, K., and A. Studemeister. 1992. Successful response of metronidazole-resistant trichomonal vaginitis to tinidazole. Sex. Transm. Dis. 19:339-340. [PubMed] [Google Scholar]
- 36.Hardy, P., J. Hardy, E. Nell, and D. Graham. 1984. Prevalence of six sexually transmitted disease agents among pregnant inner-city adolescents and pregnancy outcome. Lancet ii:333-337. [DOI] [PubMed] [Google Scholar]
- 37.Heine, R. P., H. C. Wiensfeld, R. L. Sweet, and S. S. Witkin. 1997. Polymerase chain reaction analysis of distal vaginal specimens: a less invasive strategy for detection of Trichomonas vaginalis. Clin. Infect. Dis. 24:985-987. [DOI] [PubMed] [Google Scholar]
- 38.Hesseltine, H. 1942. Experimental human vaginal trichomoniasis. J. Infect. Dis. 71:127. [Google Scholar]
- 39.Hillier, S. L., S. S. Wilken, M. A. Krohn, D. H. Watts, N. B. Kiviat, and D. A. Eschenbach. 1993. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis and chorioamnion infection. Obstet. Gynecol. 81:941-948. [PubMed] [Google Scholar]
- 40.Hobbs, M., P. Kazembe, A. Reed, W. Miller, and E. Nkata. 1999. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex. Transm. Dis. 26:381-387. [DOI] [PubMed] [Google Scholar]
- 41.Honigberg, B., and D. E. Burgess. 1994. Trichomonads of importance in human medicine including Dientamoeba fragilis, p. 1-109. In J. P. Kreier (ed.), Parasitic protozoa, vol. 9. Academic Press, Inc., New York, N.Y.
- 42.Honigberg, B., and V. M. King. 1964. Structure of Trichomonas vaginalis Donne. J. Parasitol. 50:345-364. [PubMed] [Google Scholar]
- 43.Hook, R. J., M. St. Claire, L. K. Riley, C. I. Franklin, and C. L. Besch-Williford. 1997. Mouse strain and age affect susceptibility to experimentally induced genital trichomoniasis. Lab. Anim. Sci. 47:324-326. [PubMed] [Google Scholar]
- 44.James, J. A., J. L. Thomason, S. M. Gelbart, P. Osypowski, P. Kaiser, and L. Hanson. 1992. Is trichomoniasis often associated with bacterial vaginosis in pregnant adolescents? Am. J. Obstet. Gynecol. 166:859-863. [DOI] [PubMed] [Google Scholar]
- 45.Jeremias, J., D. Draper, M. Ziegert, et al. 1994. Detection of Trichomonas vaginalis using the polymerase chain reaction in pregnant and non-pregnant women. Infect. Dis. Obstet. Gynecol. 2:16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joyner, J., J. Douglas, S. Ragsdale, M. Foster, and F. Judson. 2000. Comparative prevalence of infection with Trichomonas vaginalis among men attending a sexually transmitted diseases clinic. Sex. Transm. Dis. 27:236-240. [DOI] [PubMed] [Google Scholar]
- 47.Katiyar, S. K., and T. D. Edlind. 1994. β-Tubulin genes of Trichomonas vaginalis. Mol. Biochem. Parasitol. 64:33-42. [DOI] [PubMed] [Google Scholar]
- 48.Kaydos-Daniels, S., W. C. Miller, I. Hoffman, T. Banda, W. Dzinyemba, F. Martinson, M. S. Cohen, and M. M. Hobbs. 2003. Validation of a urine-based PCR-enzyme-linked immunosorbent assay for use in clinical reseach settings to detect Trichomonas vaginalis in men. J. Clin. Microbiol. 41:318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kengne, P., F. Veas, N. Vidal, J. L. Rey, and G. Cuny. 1994. Trichomonas vaginalis: repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell. Mol. Biol. 40:819-831. [PubMed]
- 50.Khoshnan, A., and J. F. Alderete. 1994. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J. Virol. 68:4035-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kigozi, G., H. Brahmbhatt, F. Wabwire-Mangen, M. J. Wawer, D. Serwaqdda, N. Sewankambo, and R. H. Gray. 2003. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am. J. Obstet. Gynecol. 189:1398-1400. [DOI] [PubMed] [Google Scholar]
- 52.Klebanoff, M., J. Carey, J. Hauth, S. L. Hillier, R. Nugent, E. Thom, J. Ernest, R. Heine, R. Wapner, W. Trout, A. Moawad, K. Leveno, M. Miodovnik, B. Sibai, J. Van Dorsten, M. Dombrowski, M. O'Sullivan, M. Varner, O. Langer, D. McNellis, and J. Roberts. 2001. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N. Engl. J. Med. 345:487-493. [DOI] [PubMed] [Google Scholar]
- 53.Klouman, E., E. J. Massenga, K. I. Klepp, N. E. Sam, W. Nkya, and C. Nlya. 1997. HIV and reproductive tract infections in a total village population in rural Kilimanjaro, Tanzania: women at increased risk. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 14:163-168. [DOI] [PubMed] [Google Scholar]
- 54.Krieger, J., J. I. Ravdin, and M. F. Rein. 1985. Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect. Immun. 50:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krieger, J. N., M. R. Tam, C. E. Stevens, I. O. Nielsen, J. Hale, N. B. Kiviat, and K. K. Holmes. 1988. Diagnosis of trichomoniasis. JAMA 259:1223-1227. [DOI] [PubMed] [Google Scholar]
- 56.Krieger, J. N., M. Verdon, N. Siegel, and K. K. Holmes. 1993. Natural history of urogenital trichomoniasis in men. J. Urol. 149:1455-1458. [DOI] [PubMed] [Google Scholar]
- 56a.Kurth, A., W. L. H. Whittington, M. R. Golden, K. K. Thomas, K. K. Holmes, and J. Schwebke. 2004. Performance of a new, rapid assay for detection of Trichomonas vaginalis. J. Clin. Microbiol. 42:2940-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, and M. Alary. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95-102. [DOI] [PubMed] [Google Scholar]
- 58.Land, K., M. G. Delgadillo-Correa, J. Tachezy, S. Vanacova, C. L. Hsieh, R. Sutak, and P. J. Johnson. 2004. Targeted gene replacement of a ferredoxin gene in Trichomonas vaginalis does not lead to metronidazole resistance. Mol. Microbiol. 51:115-122. [DOI] [PubMed] [Google Scholar]
- 59.Lawing, L., S. Hedges, and J. Schwebke. 2000. Detection of trichomonosis in vaginal and urine specimens from women by culture and PCR. J. Clin. Microbiol. 38:3585-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leon-Sicairos, C., J. Leon-Felix, and R. Arroyo. 2004. tvcp12: a novel Trichomonas vaginalis cathepsin L-like cystein proteinase-encoding gene. Microbiology 150:1131-1138. [DOI] [PubMed] [Google Scholar]
- 61.Leroy, V., A. De Clercq, J. Ladner, J. Bogaerts, P. Van de Perre, and F. Dabis. 1995. Should screening of genital infections be part of antenatal care in areas of high HIV prevalence? A prospective cohort study from Kigali, Rwanda, 1992-1993. Genitourin. Med. 71:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindmark, D., and M. Muller. 1973. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J. Biol. Chem. 248:7724-7728. [PubMed] [Google Scholar]
- 63.Lockwood, B., M. J. North, K. I. Scott, A. F. Bremner, G. H. Coombs. 1987. The use of a highly sensitive electophoretic method to compare the proteinases of trichomonads. Mol. Biochem. Parasitol. 24:89-95. [DOI] [PubMed] [Google Scholar]
- 64.Lockwood, B., M. J. North, and G. H. Coombs. 1984. Trichomonas vaginalis. Tritrichomonas foetus, and Trichomitus batrachorum: comparative proteolytic activity. Exp. Parasitol. 58:245-253. [DOI] [PubMed] [Google Scholar]
- 65.Lossick, J. 1980. Single-dose metronidazole treatment for vaginal trichomoniasis. Obstet. Gynecol. 56:508-510. [PubMed] [Google Scholar]
- 66.Lossick, J. G. 1989. Epidemiology of urogenital trichomoniasis, p. 311-323. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, N.Y.
- 67.Lossick, J. G., M. Muller, and T. E. Gorrell. 1986. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J. Infect. Dis. 153:948-955. [DOI] [PubMed] [Google Scholar]
- 68.Lubick, K., and D. E. Burgess. 2004. Purification and analysis of phospholipaseA2-like lytic factor of Trichomonas vaginalis. Infect. Immun. 72:1284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lushbaugh, W., A. C. Turner, G. A. Gentry, and P. C. Klykken. 1989. Characterization of a secreted cytoactive factor from Trichomonas vaginalis. Am. J. Trop. Med. Hyg. 41:18-28. [PubMed] [Google Scholar]
- 70.Madico, G., T. C. Quinn, A. Rompalo, K. T. McKee, Jr., and C. A. Gaydas. 1998. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J. Clin. Microbiol. 36:3205-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mallinson, D. J., B. C. Lockwood, G. H. Coombs, and M. J. North. 1994. Identification and molecular cloning of four cysteine proteinase genes from the pathogenic protozoon Trichomonas vaginalis. Microbiology 140:2725-2735. [DOI] [PubMed] [Google Scholar]
- 72.Mason, P., and B. A. Patterson. 1985. Proliferative response of human lymphocytes to secretory and cellular antigens of Trichomonas vaginalis. J. Parasitol. 71:265-268. [PubMed] [Google Scholar]
- 73.McGregor, J. A., J. I. Franch, W. Jones, R. Parker, E. Patterson, and D. Draper. 1992. Association of cervicovaginal infections with increased vaginal fluid phospholipase A2 activity. Am. J. Obstet. Gynecol. 167:1588-1594. [DOI] [PubMed] [Google Scholar]
- 74.Mendoza-Lopez, M., C. Becerril-Garcia, L. V. Fattel-Facenda, L. Avila- Gonzalez, M. E. Ruiz-Tachiquin, J. Ortega-Lopez, and R. Arroyo. 2000. CP30, a cysteine proeinase involved in Trichomonas vaginalis cytoadherence. Infect. Immun. 68:4907-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minkoff, H., A. N. Grunebaum, R. H. Schwarz, J. Feldman, M. Cummings, M. Crombleholme, and W. Clark. 1984. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am. J. Obstet. Gynecol. 150:965-972. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell, M. D., D. W. Branch, S. Lundin-Schiller, R. J. Romero, R. A. Daynes, and D. J. Dudley. 1991. Immunologic aspects of preterm labor. Semin. Perinatol. 15:210-224. [PubMed] [Google Scholar]
- 77.Mutwiri, G., and L. B. Corbeil. 1998. Genital and systemic immune responses in a murine model of Tritrichomonas foetus infection. J. Parasitol. 84:321-327. [PubMed] [Google Scholar]
- 78.Niccolai, L., J. J. Kopicko, A. Kassie, H. Petros, R. A. Clark, and P. Kissinger. 2000. Incidence and predictors of reinfection with Trichomonas vaginalis in HIV-infected women. Sex. Transm. Dis. 27:284-288. [DOI] [PubMed] [Google Scholar]
- 79.Nielsen, M. 1976. In vitro effect of metronidazole on the ultrastructure of Trichomonas vaginalis Donne. Acta Pathol. Microbiol. Scand. Sect. B 84:93-100. [DOI] [PubMed] [Google Scholar]
- 80.Nielsen, M., and R. Nielsen. 1975. Electron microscopy of Trichomonas vaginalis Donne: interaction with vaginal epithelium in human trichomoniasis. Acta Pathol. Microbiol. Scand. Sect. B 83:305-320. [DOI] [PubMed] [Google Scholar]
- 81.North, M., C. D. Robertson, and G. H. Coombs. 1990. The specificity of trichomonad proteinases analyzed using fluorogenic substrates and specific inhibitors. Mol. Biochem. Parasitol. 39:183-194. [DOI] [PubMed] [Google Scholar]
- 82.Paces, J., V. Urbankova, and P. Urbanek. 1992. Cloning and characterization of a repetitive DNA sequence specific for Trichomonas vaginalis. Mol. Biochem. Parasitol. 54:247-256. [DOI] [PubMed] [Google Scholar]
- 83.Parsonson, I., B. I. Clark, and J. H. Duffy. 1976. Early pathogenesis and pathology of Tritrichomonas foetus infection in virgin heifers. J. Comp. Pathol. 86:59-66. [DOI] [PubMed] [Google Scholar]
- 84.Paterson, B. A., S. N. Tabrizi, S. M. Garland, C. K. Fairley, and F. J. Bowden. 1998. The tampon test for trichomoniasis: a comparison between conventional methods and a polymerase chain reaction for Trichomonas vaginalis in women. Sex. Transm. Infect. 74:136-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pearlman, M., C. Yashar, S. Ernst, and W. Solomon. 1996. An incremental dosing protocol for women with severe vaginal trichomoniasis and adverse reactions to metronidazole. Am. J. Obstet. Gynecol. 174:934-936. [DOI] [PubMed] [Google Scholar]
- 86.Platz-Christensen, J. J., I. Mattsby-Baltzer, P. Thomsen, and N. Wiqvist. 1993. Endotoxin and interleukin-1 alpha in the cervical mucus and vaginal fluid of pregnant women with bacterial vaginosis. Am. J. Obstet. Gynecol. 169:1161-1166. [DOI] [PubMed] [Google Scholar]
- 87.Price, M., D. Zimba, I. F. Hoffman, S. C. Kaydos-Daniels, W. C. Miller, F. Martinson, D. Chilongozi, E. Kip, E. Msowoya, M. M. Hobbs, P. N. Kazembe, and M. S. Cohen. 2003. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial. Sex. Transm. Dis. 30:516-522. [DOI] [PubMed] [Google Scholar]
- 88.Rein, M. F., and M. Muller. 1990. Trichomonas vaginalis and trichomoniasis. Sex. Transm. Dis. 481-492. [DOI] [PubMed]
- 89.Reynolds, J. (ed.). 1996. Martindale: the extra pharmacopoeia, 21st ed., p. 632-633. Royal Pharmaceutical Society, London, United Kingdom.
- 90.Rhyan, J., K. L. Wilson, D. E. Burgess, L. L. Stackhouse, and W. J. Quinn. 1995. Immunohistochemical detection of Tritrichomonas foetus in formalin-fixed, paraffin-embedded sections of bovine placenta and fetal lung. J. Vet. Diagn. Investig. 7:98-101. [DOI] [PubMed] [Google Scholar]
- 91.Riley, D. E., M. C. Roberts, T. Takayama, and J. N. Krieger. 1992. Development of a polymerase chain reaction based diagnosis of Trichomonas vaginalis. J. Clin. Microbiol. 30:465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roe, F. 1985. Safety of nitroimidazoles. Scand. J. Infect. Dis. Suppl. 46:72-81. [PubMed] [Google Scholar]
- 93.Ryu, J., H. Chung, D. Min, Y. Cho, Y. Ro, and S. Kim. 1999. Diagnosis of trichomoniasis by polymerase chain reaction. Yonsei Med. J. 40:56-60. [DOI] [PubMed] [Google Scholar]
- 94.Saurina, G., L. DeMao, and W. McCormack. 1998. Cure of metronidazole- and tinidazole-resistant trichomoniasis with the use of high-dose oral and intravaginal tinidazole. Clin. Infect. Dis. 26: [DOI] [PubMed]
- 95.Sawyer, P., R. N. Brogden, R. M. Pinder, T. M. Speight, and G. S. Avery. 1976. Tinidazole: a review of its antiprotozoal activity and therapeutic efficacy. Drugs 11:423-440. [DOI] [PubMed] [Google Scholar]
- 96.Schee, C., A. Belkum, L. Zwijgers, et al. 1999. Improved diagnosis of Trichomonas vaginalis infection by PCR using vaginal swabs and urine specimens compared to diagnosis by wet mount microscopy, culture, and fluorescent staining. J. Clin. Microbiol. 37:4127-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmid, G., E. Narcisi, D. Mosure, and E. Secor. 2001. Prevalence of metronidazole resistant Trichomonas vaginalis in a gynecology clinic. J. Reprod. Med. 46:545. [PubMed] [Google Scholar]
- 98.Schwebke, J., and E. I. Hook. 2003. High rates of Trichomonas vaginalis among men attending a sexually transmitted diseases clinic: implications for screening and urethritis management. J. Infect. Dis. 188:465-468. [DOI] [PubMed] [Google Scholar]
- 99.Schwebke, J., and L. Lawing. 2002. Improved detection by DNA amplification of Trichomonas vaginalis in males. J. Clin. Microbiol. 40:3681-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schwebke, J., M. Venglarik, and S. Morgan. 1999. Delayed versus immediate bedside inoculation of culture media for diagnosis of vaginal trichomonosis. J. Clin. Microbiol. 37:2369-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwebke, J. R., S. C. Morgan, and G. B. Pinson. 1997. Validity of self obtained vaginal specimens for diagnosis of trichomoniasis. J. Clin. Microbiol. 35:1618-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shaio, M. P. R. Lin, and J. Y. Liu. 1997. Colorimetric one-tube nested PCR for detection of Trichomonas vaginalis in vaginal discharge. J. Clin. Microbiol. 35:132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Silva-Filho, F., S. Kasai, M. Nomizu, L. B. Lopez, M. B. Melo-Braga, B. Rocha-Azevedo, D. B. Petropolis, and I. S. Horbach. 2002. How laminin-1 can be recognized by the protozoan parasite Tritrichomonas foetus: possible role played by the extracellular matrix glycoprotein in both cytoadhesion and cytotoxicity exerted by the parasite. Parasitol. Int. 51:305-307. [DOI] [PubMed] [Google Scholar]
- 104.Skirrow, S. Z., and R. H. BonDurant. 1988. Bovine trichomoniasis. Vet. Bull. 58:591-603. [DOI] [PubMed] [Google Scholar]
- 105.Sobel, J., V. Nagappan, and P. Nyirjesy. 1999. Metronidazole-resistant vaginal trichomoniasis: an emerging problem. N. Engl. J. Med. 341:292-293. [DOI] [PubMed] [Google Scholar]
- 106.Sobel, J., P. Nyirjesy, and W. Brown. 2001. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin. Infect. Dis. 33:1341-1346. [DOI] [PubMed] [Google Scholar]
- 107.Sorvillo, F., and P. Kerndt. 1998. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet 351:213-214. [DOI] [PubMed] [Google Scholar]
- 108.Thomason, J. L., S. M. Gelbart, J. F. Sobun, M. B. Schulien, and P. R. Hamilton. 1988. Comparison of four methods to detect Trichomonas vaginalis. J. Clin. Microbiol. 26:1869-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsai, C., H. W. Liu, and J. H. Tai. 2002. Characterization of an iron-responsive promoter in the protozoan pathogen Trichomonas vaginalis. J. Biol. Chem. 277:5153-5162. [DOI] [PubMed] [Google Scholar]
- 110.Van Andel, R., C. L. Franklin, M. C. St. Claire, L. K. Riley, C. L. Besch- Williford, and R. R. Hook. 1996. Lesions of experimental genital Tritrichomonas foetus infections in estrogenized BALB/c mice. Vet. Pathol. 33:407-411. [DOI] [PubMed] [Google Scholar]
- 111.Viscogliosi, E., V. P. Edgcomb, D. Gerbod, C. Noel, and P. Delgado-Viscogliosi. 1999. Molecular evolution inferred from small subunit rRNA sequences: what does it tell us about phylogenetic relationships and taxonomy of the parabasalids? Parasite 6:279-291. [DOI] [PubMed] [Google Scholar]
- 112.Voolman, T., and P. Boreham. 1993. Metronidazole resistant Trichomonas vaginalis in Brisbane. Med. J. Aust. 159:490. [DOI] [PubMed] [Google Scholar]
- 113.Wang, A., C. C. Wang, and J. F. Alderete. 1987. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J. Exp. Med. 166:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang, A., and C. C. Wang. 1986. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc. Natl. Acad. Sci. USA 83:7956-7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weber, B., T. M. Mapeka, M. A. Maahlo, and A. A. Hoosen. 2003. Double stranded RNA virus in South African Trichomonas vaginalis isolates. J. Clin. Pathol. 56:542-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weinstock, H., S. Berman, and W. Cates. 2004. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect. Sex. Reprod. Health 36:6-10. [DOI] [PubMed] [Google Scholar]
- 117.Wiese, W., S. R. Patel, S. C. Patel, C. A. Ohl, and C. A. Estrada. 2000. A meta-analysis of the Papanicolaou smear and wet mount for the diagnosis of vaginal trichomoniasis. Am. J. Med. 108:301-308. [DOI] [PubMed] [Google Scholar]
- 118.Wilcox, R. 1960. Epidemiological aspects of human trichomoniasis. Br. J. Vener. Dis. 36:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wølner-Hanssen, P., J. N. Krieger, C. E. Stevens, N. B. Kiviat, L. Koutsky, C. Critchlow, T. DeRouen, S. Hillier, and K. K. Holmes. 1989. Clinical manifestations of vaginal trichomoniasis. JAMA 261:571-576. [DOI] [PubMed] [Google Scholar]
- 120.Wood, B., and A. Monroe. 1975. Pharmacokinetics of tinidazole and metronidazole after single large oral doses. Br. J. Vener. Dis. 51:51-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wos, S., and R. M. Watt. 1986. Immunoglobulin isotypes of anti-Trichomonas vaginalis antibodies in patients with vaginal trichomoniasis. J. Clin. Microbiol. 24:790-795. [DOI] [PMC free article] [PubMed] [Google Scholar]