Abstract
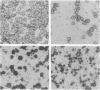
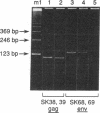
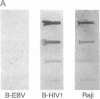
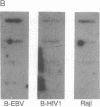
Aggressive B-cell lymphomas are occurring with increasing incidence among individuals infected with human immunodeficiency virus (HIV). Several lines of evidence implicate both Epstein-Barr virus (EBV) and c-myc activation in the pathogenesis of a major subset of these tumors. These observations prompted our investigation of interactions among EBV, c-myc, and HIV in primary B cells. We show that nonimmortalized peripheral B lymphocytes from EBV-seropositive, HIV-seronegative donors can be infected by HIV and that a subset of these lymphocytes become transformed. Malignant transformation was documented by several criteria. These cells displayed altered growth properties, propagating in 1% serum and cloning in soft agar, and formed invasive tumors of Burkitt lymphoma phenotype after subcutaneous injection into severe combined immunodeficiency mice. Such cells revealed marked enhancement of EBV DNA and RNA and of endogenous c-myc transcripts and protein. HIV-1 infection of already immortalized B-cell lines led to a similar upregulation of EBV and c-myc transcripts. These data indicate that HIV has properties of a transforming retrovirus, as it mediates two events linked to B-cell neoplasia: deregulation of c-myc and activation of EBV. They also raise the possibility of a role for HIV, apart from induction of immune suppression, in the pathogenesis of B-cell lymphoma in the acquired immune deficiency syndrome.
Full text
PDF

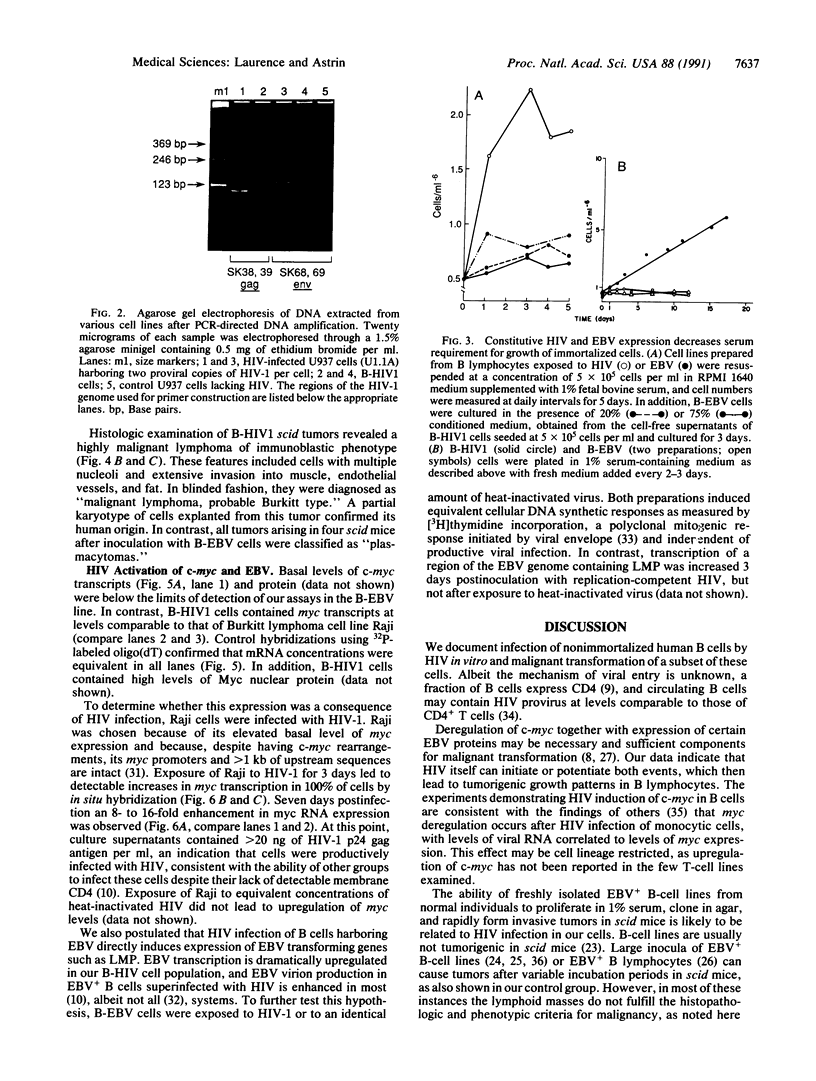
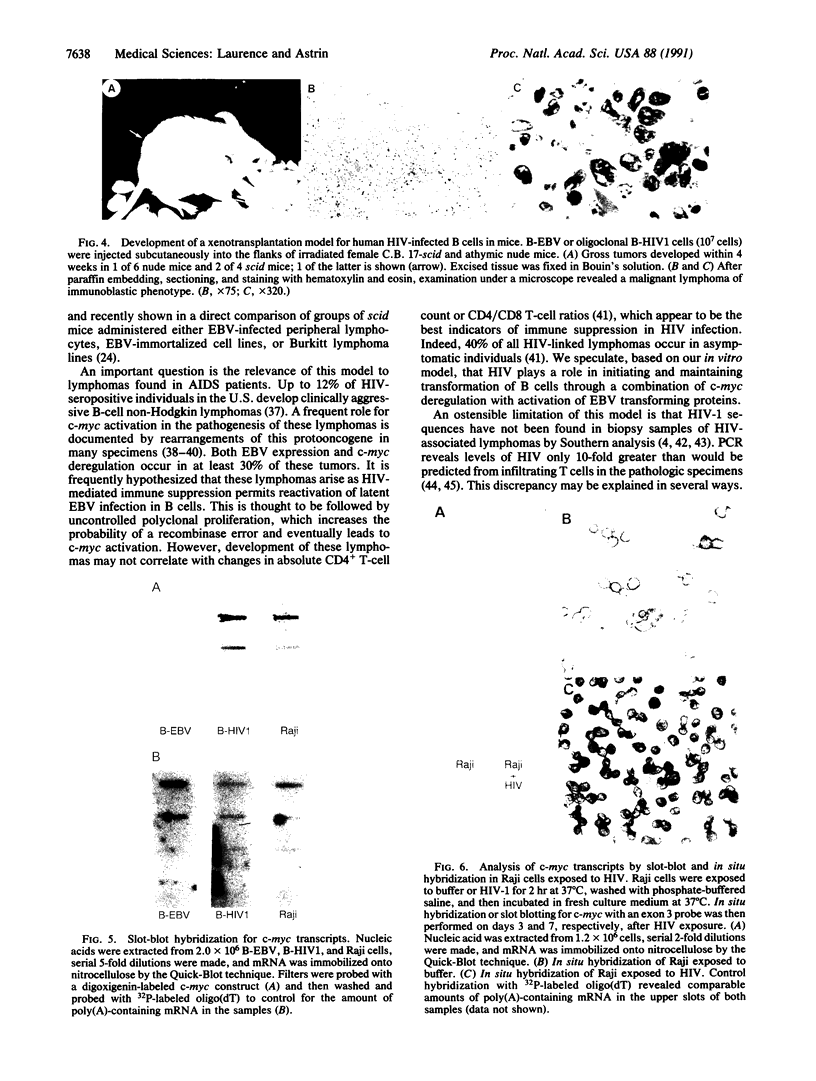

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckhardt R. N., Farady N., May M., Torres R. A., Strauchen J. A. Increased incidence of malignant lymphoma in AIDS: a comparison of risk groups and possible etiologic factors. Mt Sinai J Med. 1988 Oct;55(5):383–389. [PubMed] [Google Scholar]
- Bresser J., Hubbell H. R., Gillespie D. Biological activity of mRNA immobilized on nitrocellulose in NaI. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6523–6527. doi: 10.1073/pnas.80.21.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M. J., Pisa P., Fox R. I., Cooper N. R. Epstein-Barr virus induces aggressive lymphoproliferative disorders of human B cell origin in SCID/hu chimeric mice. J Clin Invest. 1990 Apr;85(4):1333–1337. doi: 10.1172/JCI114573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N., Kalyanaraman V. S., Saxinger C., Wong-Staal F., Ghrayeb J., Pahwa S. Localization of B-cell stimulatory activity of HIV-1 to the carboxyl terminus of gp41. AIDS Res Hum Retroviruses. 1990 Mar;6(3):299–305. doi: 10.1089/aid.1990.6.299. [DOI] [PubMed] [Google Scholar]
- Cremer K. J., Spring S. B., Gruber J. Role of human immunodeficiency virus type 1 and other viruses in malignancies associated with acquired immunodeficiency disease syndrome. J Natl Cancer Inst. 1990 Jun 20;82(12):1016–1024. doi: 10.1093/jnci/82.12.1016. [DOI] [PubMed] [Google Scholar]
- Dahl K., Martin K., Miller G. Differences among human immunodeficiency virus strains in their capacities to induce cytolysis or persistent infection of a lymphoblastoid cell line immortalized by Epstein-Barr virus. J Virol. 1987 May;61(5):1602–1608. doi: 10.1128/jvi.61.5.1602-1608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rossi A., Roncella S., Calabro M. L., D'Andrea E., Pasti M., Panozzo M., Mammano F., Ferrarini M., Chieco-Bianchi L. Infection of Epstein-Barr virus-transformed lymphoblastoid B cells by the human immunodeficiency virus: evidence for a persistent and productive infection leading to B cell phenotypic changes. Eur J Immunol. 1990 Sep;20(9):2041–2049. doi: 10.1002/eji.1830200924. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Biancalana S., Hudson D. Activity of synthetic peptides from the Tat protein of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7397–7401. doi: 10.1073/pnas.86.19.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetie M. A., Richardson J., Tucker T., Jones D., Uhr J. W., Vitetta E. S. Disseminated or localized growth of a human B-cell tumor (Daudi) in SCID mice. Int J Cancer. 1990 Mar 15;45(3):481–485. doi: 10.1002/ijc.2910450318. [DOI] [PubMed] [Google Scholar]
- Groopman J. E., Sullivan J. L., Mulder C., Ginsburg D., Orkin S. H., O'Hara C. J., Falchuk K., Wong-Staal F., Gallo R. C. Pathogenesis of B cell lymphoma in a patient with AIDS. Blood. 1986 Mar;67(3):612–615. [PubMed] [Google Scholar]
- Haluska F. G., Russo G., Kant J., Andreef M., Croce C. M. Molecular resemblance of an AIDS-associated lymphoma and endemic Burkitt lymphomas: implications for their pathogenesis. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8907–8911. doi: 10.1073/pnas.86.22.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. Z., Raab-Traub N., Miller G. Latent and replicating forms of Epstein-Barr virus DNA in lymphomas and lymphoproliferative diseases. J Infect Dis. 1989 Oct;160(4):589–598. doi: 10.1093/infdis/160.4.589. [DOI] [PubMed] [Google Scholar]
- Langdon W. Y., Harris A. W., Cory S., Adams J. M. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986 Oct 10;47(1):11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- Laurence J., Cooke H., Sikder S. K. Effect of tamoxifen on regulation of viral replication and human immunodeficiency virus (HIV) long terminal repeat-directed transcription in cells chronically infected with HIV-1. Blood. 1990 Feb 1;75(3):696–703. [PubMed] [Google Scholar]
- Laurence J., Friedman S. M., Chartash E. K., Crow M. K., Posnett D. N. Human immunodeficiency virus infection of helper T cell clones. Early proliferative defects despite intact antigen-specific recognition and interleukin 4 secretion. J Clin Invest. 1989 Jun;83(6):1843–1848. doi: 10.1172/JCI114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J., Kulkosky J., Dong B., Early E., Snyderman R., Cianciolo G. J. A soluble inhibitor of T lymphocyte function induced by HIV-1 infection of CD4+ T cells: characterization of a cellular protein and its relationship to p15E. Cell Immunol. 1990 Jul;128(2):337–352. doi: 10.1016/0008-8749(90)90031-l. [DOI] [PubMed] [Google Scholar]
- Lombardi L., Newcomb E. W., Dalla-Favera R. Pathogenesis of Burkitt lymphoma: expression of an activated c-myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987 Apr 24;49(2):161–170. doi: 10.1016/0092-8674(87)90556-3. [DOI] [PubMed] [Google Scholar]
- Meyer P. R., Yanagihara E. T., Parker J. W., Lukes R. J. A distinctive follicular hyperplasia in the acquired immune deficiency syndrome (AIDS) and the AIDS related complex. A pre-lymphomatous state for B cell lymphomas? Hematol Oncol. 1984 Oct-Dec;2(4):319–347. doi: 10.1002/hon.2900020403. [DOI] [PubMed] [Google Scholar]
- Mittal K., Neri A., Feiner H., Schinella R., Alfonso F. Lymphomatoid granulomatosis in the acquired immunodeficiency syndrome. Evidence of Epstein-Barr virus infection and B-cell clonal selection without myc rearrangement. Cancer. 1990 Mar 15;65(6):1345–1349. doi: 10.1002/1097-0142(19900315)65:6<1345::aid-cncr2820650616>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Monroe J. E., Calender A., Mulder C. Epstein-Barr virus-positive and -negative B-cell lines can be infected with human immunodeficiency virus types 1 and 2. J Virol. 1988 Sep;62(9):3497–3500. doi: 10.1128/jvi.62.9.3497-3500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A., Barriga F., Knowles D. M., Magrath I. T., Dalla-Favera R. Different regions of the immunoglobulin heavy-chain locus are involved in chromosomal translocations in distinct pathogenetic forms of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2748–2752. doi: 10.1073/pnas.85.8.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M., Taguchi Y., Nakamine H., Pirruccello S. J., Davis J. R., Beisel K. W., Kleveland K. L., Sanger W. G., Fordyce R. R., Purtilo D. T. Characterization of Epstein-Barr virus-induced lymphoproliferation derived from human peripheral blood mononuclear cells transferred to severe combined immunodeficient mice. Am J Pathol. 1990 Sep;137(3):517–522. [PMC free article] [PubMed] [Google Scholar]
- Pauza C. D., Galindo J., Richman D. D. Human immunodeficiency virus infection of monoblastoid cells: cellular differentiation determines the pattern of virus replication. J Virol. 1988 Oct;62(10):3558–3564. doi: 10.1128/jvi.62.10.3558-3564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Arlin Z. A., Wieczorek R., Luciw P., Dina D., Basilico C., Dalla-Favera R. Multiple monoclonal B cell expansions and c-myc oncogene rearrangements in acquired immune deficiency syndrome-related lymphoproliferative disorders. Implications for lymphomagenesis. J Exp Med. 1986 Dec 1;164(6):2049–2060. doi: 10.1084/jem.164.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack A., Hartl G., Zimber U., Freese U. K., Laux G., Takaki K., Hohn B., Gissmann L., Bornkamm G. W. A complete set of overlapping cosmid clones of M-ABA virus derived from nasopharyngeal carcinoma and its similarity to other Epstein-Barr virus isolates. Gene. 1984 Mar;27(3):279–288. doi: 10.1016/0378-1119(84)90072-6. [DOI] [PubMed] [Google Scholar]
- Poznansky M. C., Walker B., Haseltine W. A., Sodroski J., Langhoff E. A rapid method for quantitating the frequency of peripheral blood cells containing HIV-1 DNA. J Acquir Immune Defic Syndr. 1991;4(4):368–373. [PubMed] [Google Scholar]
- Rabbitts T. H., Hamlyn P. H., Baer R. Altered nucleotide sequences of a translocated c-myc gene in Burkitt lymphoma. Nature. 1983 Dec 22;306(5945):760–765. doi: 10.1038/306760a0. [DOI] [PubMed] [Google Scholar]
- Ramsay G. M., Moscovici G., Moscovici C., Bishop J. M. Neoplastic transformation and tumorigenesis by the human protooncogene MYC. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2102–2106. doi: 10.1073/pnas.87.6.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Ben-Bassat I., Berkowicz M., Martinowitz U., Brok-Simoni F., Neumann Y., Vansover A., Gotlieb-Stematsky T., Ramot B. Molecular analysis of Burkitt's leukemia in two hemophilic brothers with AIDS. Blood. 1987 Dec;70(6):1713–1717. [PubMed] [Google Scholar]
- Rothberg P. G., Erisman M. D., Diehl R. E., Rovigatti U. G., Astrin S. M. Structure and expression of the oncogene c-myc in fresh tumor material from patients with hematopoietic malignancies. Mol Cell Biol. 1984 Jun;4(6):1096–1103. doi: 10.1128/mcb.4.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Higgins S. E., Folks T., Fauci A. S. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science. 1986 Sep 5;233(4768):1084–1086. doi: 10.1126/science.3016902. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Shibata D., Brynes R. K., Nathwani B., Kwok S., Sninsky J., Arnheim N. Human immunodeficiency viral DNA is readily found in lymph node biopsies from seropositive individuals. Analysis of fixed tissue using the polymerase chain reaction. Am J Pathol. 1989 Oct;135(4):697–702. [PMC free article] [PubMed] [Google Scholar]
- Skowronski J. Expression of a human immunodeficiency virus type 1 long terminal repeat/simian virus 40 early region fusion gene in transgenic mice. J Virol. 1991 Feb;65(2):754–762. doi: 10.1128/jvi.65.2.754-762.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subar M., Neri A., Inghirami G., Knowles D. M., Dalla-Favera R. Frequent c-myc oncogene activation and infrequent presence of Epstein-Barr virus genome in AIDS-associated lymphoma. Blood. 1988 Aug;72(2):667–671. [PubMed] [Google Scholar]
- Swendeman S., Thorley-Lawson D. A. The activation antigen BLAST-2, when shed, is an autocrine BCGF for normal and transformed B cells. EMBO J. 1987 Jun;6(6):1637–1642. doi: 10.1002/j.1460-2075.1987.tb02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V., Britton S., Ehrnst A., Lenkei R., Strannegård O. Persistent productive HIV infection in EBV-transformed B lymphocytes. J Med Virol. 1989 Jan;27(1):19–24. doi: 10.1002/jmv.1890270105. [DOI] [PubMed] [Google Scholar]
- Vuist W. M., v Buitenen F., de Rie M. A., Hekman A., Rümke P., Melief C. J. Potentiation by interleukin 2 of Burkitt's lymphoma therapy with anti-pan B (anti-CD19) monoclonal antibodies in a mouse xenotransplantation model. Cancer Res. 1989 Jul 15;49(14):3783–3788. [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang F., Tsang S. F., Kurilla M. G., Cohen J. I., Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990 Jul;64(7):3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. N., Weiss R. A. HIV infection: the cellular picture. Sci Am. 1988 Oct;259(4):100–109. doi: 10.1038/scientificamerican1088-100. [DOI] [PubMed] [Google Scholar]
- Yokoi T., Miyawaki T., Yachie A., Kato K., Kasahara Y., Taniguchi N. Epstein-Barr virus-immortalized B cells produce IL-6 as an autocrine growth factor. Immunology. 1990 May;70(1):100–105. [PMC free article] [PubMed] [Google Scholar]