Abstract
Chronic immune activation is one of the hallmarks of human immunodeficiency virus (HIV) infection. It is present also, with very similar characteristics, in very large human populations infested with helminthic infections. We have tried to review the studies addressing the changes in the immune profiles and responses of hosts infected with either one of these two chronic infections. Not surprisingly, several of the immune derangements and impairments seen in HIV infection, and considered by many to be the “specific” effects of HIV, can be found in helminth-infected but HIV-noninfected individuals and can thus be accounted for by the chronic immune activation itself. A less appreciated element in chronic immune activation is the immune suppression and anergy which it may generate. Both HIV and helminth infections represent this aspect in a very wide and illustrative way. Different degrees of anergy and immune hyporesponsiveness are present in these infections and probably have far-reaching effects on the ability of the host to cope with these and other infections. Furthermore, they may have important practical implications, especially with regard to protective vaccinations against AIDS, for populations chronically infected with helminths and therefore widely anergic. The current knowledge of the mechanisms responsible for the generation of anergy by chronic immune activation is thoroughly reviewed.
INTRODUCTION
AIDS is currently one of the biggest and most deadly worldwide epidemics of infectious diseases. Human immunodeficiency virus (HIV) infection has already caused approximately 25 million deaths; an estimated 42 million persons had been infected with HIV by the end of 2002, with at least 5 million new infections and 3.1 million deaths from AIDS occurring that same year (258). It is estimated that more than 100 million people would be carrying the virus in less than 10 years from then (257, 258). Sub-Saharan Africa is the region of the world most severely affected by HIV and AIDS; in that area, life expectancy has declined precipitously, in some countries by 50%, and infant death rates have doubled. The AIDS epidemic has intersected most notably with tuberculosis (TB) (22, 34, 60, 96, 108), and TB is the principal cause of death for persons with HIV-1 infection worldwide (29, 73).
Next to TB, the most common infections in the developing countries are helminthic infections. About one-quarter of the world's population are infested with one or more of the major soil-transmitted helminths, with the estimated number of infected people being over 1.5 billion (49, 68, 198). Helminths belong to two major groups of animals, the flatworms or Platyhelminthes (flukes and tapeworms) and the roundworms or Nematoda. The most serious helminth infections are acquired in poor tropical and subtropical areas (40, 51, 68, 179, 186, 274), but some also occur in the developed world (191, 213). Many potential helminthic infections are eliminated by host defenses; others become established and may persist for prolonged periods, even years. Although helminthic infections are often asymptomatic, severe pathology can occur (41, 69, 70, 75, 172, 190, 208). The most obvious forms of direct damage are those resulting from the blockage of internal organs or from the effects of pressure exerted by growing parasites. In addition, many helminths undergo extensive migrations through body tissues, which both damage tissues directly and initiate hypersensitivity reactions.
All helminths stimulate strong immune responses (8, 119, 162, 170, 242, 261, 269). Although these responses are useful for diagnosing infection, they frequently appear not to be protective. Moreover, damage also occurs indirectly as a result of the host defense mechanisms (124, 246). Immune-mediated inflammatory changes occur in the skin, lungs, liver, intestine, central nervous system, and eyes as worms migrate through these organs. Systemic changes such as eosinophilia, edema, and joint pain reflect local allergic responses to the parasites. The fact that many worms are extremely long-lived means that many inflammatory changes become irreversible, producing functional changes in tissues. All helminths release relatively large amounts of antigenic materials, and this voluminous production may divert immune responses or even locally exhaust the immune potential (see below).
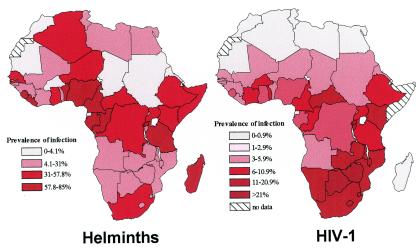
Since the prevalence and geographic distribution of helminthic infections and HIV-1, particularly in Africa (Fig. 1), are remarkably high, possible causal relationships between these infections may occur. The recent immigration of more than 50,000 Ethiopian Jews to Israel from areas with high prevalence of HIV infection and with a very high prevalence of helminthic infections has enabled us to address the effects of these pathogens on the host. Based on these studies, we have suggested that a major factor determining such interactions is the host response to the infections. Furthermore, we argued that immune activation of the host is the most critical determinant in the pathogenesis of HIV infection and that chronic immune activation of the host by the helminthic infections, so commonly found in the developing countries, may account for the more severe dissemination of AIDS in these countries (19-22). In this review we have tried to summarize the current knowledge of the effects of chronic immune activation in humans, specifically that caused by helminthic infections or HIV, on the host immune response, with special emphasis on the induction of hyporesponsiveness and anergy. The possible implications of these changes on the susceptibility of the host to HIV, on the natural course of the infection, and on the ability of the host to develop protective immunity to HIV following vaccination have also been addressed.
FIG. 1.
Distribution of helminths and HIV-1 in Africa. The prevalence of infection by helminths and HIV-1 is based on data obtained from references 43 and 274 for helminths and reference 257 for HIV.
HELMINTHIC INFECTIONS AND AIDS: MUTUAL EFFECTS AND VACCINATION
Since helminthic infection are so widely present in most developing countries and are present in populations where HIV-1 is highly endemic (Fig. 1), the interaction between these infections, in the same host and at the population level, is of great importance and has many potential practical implications. As mentioned above, and based on our main hypothesis, we have suggested that people infected with helminths will be immune activated and therefore more prone to become infected with HIV; furthermore, once these individuals have been infected with HIV, the infection will progress faster, and therefore eradication of worms from dually infected individuals will ameliorate HIV progression. We also suggested that since helminth infection is activating the immune system, it will cause an increase in the plasma HIV viral load (VL) and thereby will also affect HIV transmission. In addition, since the chronic helminthic infection skews the immune response profile toward a T-helper type 2 (TH2) profile and also leads to hyporesponsiveness and anergy, it will affect the ability of the host to generate potent and protective immune responses when vaccinated against HIV (19-21, 23, 31, 32). In the following section we review the studies addressing these issues, including our own work.
Effects of Helminths on Concurrent HIV Infection
We have looked specifically at the question whether helminthic infection would affect plasma HIV-1 VL in dually infected individuals and have found a significant correlation between egg excretion and HIV-1 VL (D. Wolday, S. Maayan, G. Miriam, and Z. Bentwich, Abstr. 7th Conf. Retrovirus Opportunistic Infect., abstr. 157, 2001). In other studies, notably of individuals dually infected with HIV-1 and Schistosoma, such correlations were not found (78, 144). Wolday et al. observed increased HIV VL in individuals dually infected with HIV-1 and Leishmania (Wolday et al., Abstr. 7th Conf. Retrovirus Opportunistic Infect., 2001); likewise, increased VL has been observed in malariaHIV and in TB-HIV dually infected patients (114, 143); (O. Jobe, K. McAdam, and N. Berry, Abstr. XII Int. Conf. AIDS, abstr. 60742, 1998). Regarding the possible effect of helminths on the course of HIV infection, namely, increasing its rate of progression, there are not enough studies that have addressed this question, and the proofs for that possibility are clearly insufficient. In studies carried out by us with HIV-1-infected (HIV+) Ethiopian immigrants in Israel, we have not seen any difference in the rate of progression between the Ethiopian immigrants and non-Ethiopian HIV+ people living in Israel (268), but that has been generally after eradication of worms in the Ethiopian immigrants. In a more recent study that we have carried out in Ethiopia, deworming of Ethiopians dually infected with HIV and helminths resulted in a moderate but significant decrease of HIV VL (Wolday et al., Abstr. 7th Conf. Retrovirus Opportunistic Infect., 2001). Such an effect may of course affect the rate of progression of the HIV infection. Does helminthic infection increase the susceptibility for HIV infection? We and others have shown that this happens in vitro (97, 232), but it has not yet been addressed in field studies. Another aspect of the same issue was to determine if helminthic infections enhanced TB infection, whether newly acquired or reactivated. In studies carried out by Beyers in areas of South Africa where TB is highly endemic, the following major observations were made: (i) most of the new active cases of TB were due to reactivation of a previous infection, (ii) a marked correlation was found between serum immunoglobulin E (IgE) levels and the incidence of TB, and (iii) total IgE and ascaris-specific IgE levels were both high in the TB patients and declined following successful treatment (3, 26). More recently, in studies carried out in Uganda by Elliot et al., a significant correlation was found between helminth infection and increased incidence of TB in HIV+ patients (77). Taken together, these studies support the notion that the helminth infections increase the susceptibility to active TB in HIV dually infected people, may increase susceptibility to HIV infection, and may also affect HIV progression and transmission, but larger studies are clearly needed to establish this concept.
Helminthic Infections, Deworming, and Vaccination
The possible role of the preexisting immune profile on the immune response has been addressed in a number of ways. A number of investigators have demonstrated impaired TH1 and specific cytotoxic T-lymphocyte (CTL) responses in immunized animals with a preexisting dominant TH2 profile as a result of schistosomal infections (2, 202, 204). We have also found a dominant TH2 response in Schistosoma-infected mice immunized with either plasmid DNA encoding β-galactosidase or HIV antigens (11, 12). The presence of helminthic infections in humans was found by us and also by others to be significantly associated with an impaired response to tuberculin purified protein derivative (PPD) in individuals either exposed to Mycobacterium tuberculosis or immunized previously with bacille Calmette-Guérin (BCG) (reference 76 and unpublished results). A generally weaker responsiveness to all or most vaccines in developing countries has been known for a quite a while. Thus, polio vaccination, although very successful on the whole in achieving a significant decrease in the incidence of new polio infections, achieved much lower levels of response in developing countries than in developed countries (122, 201). Likewise, and more importantly, the failure of universal BCG vaccination to decrease the rate and incidence of TB in developing countries is well known (31, 32, 76) and fits very well with the very high level of anergy to PPD found in such populations. The presence of anergy to PPD in regions where the incidence of TB is so high would seem to fit very well with the large number of studies indicating that helminths may lead to anergy and hyporesponsiveness (see below). Nevertheless, the causal relationship between helminthic infections and the decreased response to vaccination is not sufficiently established and requires larger and more detailed studies.
In recent years, the concept of deworming large populations has gained much support from the World Health Organization and other public health authorities around the world. This has been brought about by the growing awareness of the benefits conferred by such an approach on a number of health parameters, notably general morbidity, anemia, growth, and possibly learning abilities (15, 44, 45, 49, 105). The major thrust of these ongoing efforts is to deworm whole populations in several locations around the world, with much emphasis on younger ages. It has already proved feasible and not too costly. In all these efforts, however, the biomedical rationale has not been extended at all to the issues raised in this review, namely, to the possible effects deworming may have on the HIV epidemic and on the generation of protective immunity as a result of vaccination against these pathogens.
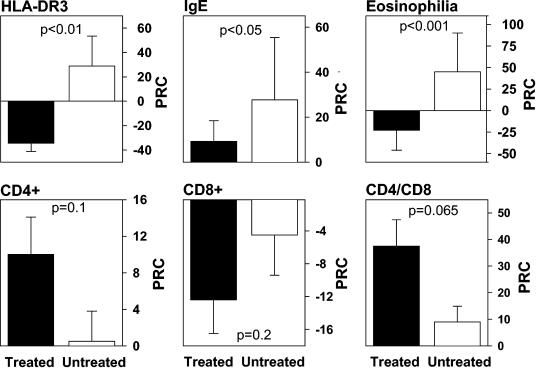
We have tried to look at the effect of deworming on the immune system in a number of ways. First, we showed that most of the immune system changes that were present in new Ethiopian immigrants on arrival to Israel (described extensively below) reverted completely or almost completely to our local Israeli normal levels in Ethiopian immigrants who had lived in Israel for several years and after eradication of the helminths (23, 24, 128). Second, to determine if indeed all the immune changes were the result of the helminthic infections and were not due to other factors, such as nutrition or hygiene, we carried out a prospective study of Ethiopian immigrants to Israel and compared the immune profiles of two groups: one that underwent deworming successfully shortly after arriving in Israel and one, that did not receive such treatment (by chance and not preplanned). Both groups lived in the same geographical locations and in a similar environment and were studied a short time after arriving and a year later. The results of that study, depicted in Fig. 2, clearly demonstrate that 6 to 12 months after deworming, a significant decrease in eosinophilia, blood IgE levels, and immune activation (HLA-DR on CD3+ cells), as well as a clear trend for normalization of blood T-cell subsets, is present. This clearly suggests that, indeed, the helminths by themselves are responsible for the immune changes that were found in new Ethiopian immigrants. The deworming in itself caused a normalization of the immune profile that was not found in the immigrants who continued to harbor the helminths. Regarding the skin reactivity and lymphoproliferative response to PPD, which were both significantly diminished in the Ethiopian immigrants with helminths, eradication of the worms brought a significant reversion to positive proliferative response while not affecting significantly the skin test responses.
FIG. 2.
Decreased immune activation following deworming. The effect of helminth eradication on the immune system was studied by following up several immunological markers in two subgroups of the Ethiopian immigrants to Israel. The treated group included 30 individuals infested with one or more helminthic parasites. Blood samples were taken from them before and 6 to 12 months after eradication of the parasites. The untreated group included 19 new Ethiopian immigrants to Israel, who lived in the same environment as the treated group but did not receive anthelminthic treatment and remained infested with helminths during the whole study period. The graphs show the percent relative changes (PRC) of immunological parameters between two consecutive blood tests (X1 and X2) taken 6 to 12 months apart. The PRC was calculated as follows: PRC = [(X2 − X1)/X1] × 100. Each bar represents the PRC mean ± standard error for each group. The significance of the difference between the PRC (P value), determined by the Mann-Whitney rank test, is shown.
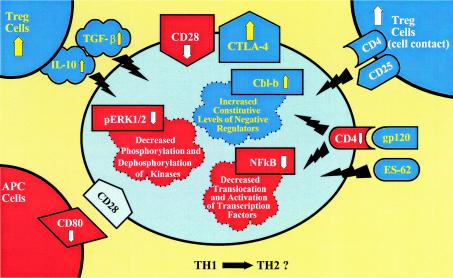
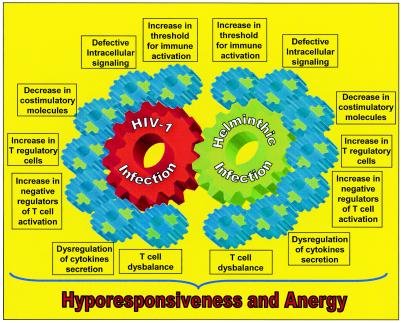
In the following sections we summarize published data and some of our unpublished observations that support the presence of immune dysfunction secondary to immune activation in both HIV-1 and helminthic infections and discuss how these diseases may influence each other. Figure 3 summarizes the various elements related to hyporesponsivness and anergy, which are addressed in the following sections.
FIG. 3.
T-cell hyporesponsiveness during chronic immune activation. The chart represents different features altered during chronic immune activation caused by persistent HIV-1 or helminthic infection. Representative factors found to be altered are depicted. Chronic immune activation causes (i) an increase in the level of CTLA-4, which downregulates T-cell responses by raising the threshold for effective T-cell activation (CTLA-4 engagement also leads to expression of the downregulatory cytokine TGF-β); (ii) downregulation of CD28 (effective stimulation of T cells via the TCR requires costimulatory signaling through the ligation of CD28 receptor with CD80 or CD86 ligands of APC; TCR stimulation in the absence of CD28-mediated costimulation induces a long-lasting hyporesponsive state); (iii) a reduction in CD80 in APC, which decreases the effective stimulation of T cells; (iv) an increase in constitutive levels of intracellular negative regulators, such as Cbl-b; (v) impaired cytoplasmatic signal transduction, such as decreased phosphorylation of ERK 1/2; (vi) decreased translocation to the nucleus and activation of transcription factors, such as NF-κB; and (vii) an increase in the number of Treg cells, which induce T-cell hyporesponsiveness directly by cell contact and through downregulatory cytokines such as IL-10 and TGF-β. The TH2 skewed immune profile associated with HIV-1 and helminthic infections results in upregulation of downregulatory cytokines and probably of Treg cells. In addition, soluble factors originating from HIV or helminthic parasites, such as gp120 and ES-62, interact with the T cells, augmenting cellular impairments and hyporesponsiveness.
IMMUNE ACTIVATION AND DYSREGULATION CHARACTERIZE BOTH HELMINTHIC AND HIV-1 INFECTIONS
One of the main characteristics of HIV-1 infection is persistent systemic immune activation (9, 21, 103, 111, 143, 200). This immune activation and dysregulation is characterized by a specific pattern of cytokine production, expression of membrane activation molecules on the cells of the immune system, and changes in the levels of several immune parameters in blood. Infection by helminths also results in chronic immune activation, leading to similar immune dysregulation and immunological unresponsiveness of the host (19, 24, 33, 247). This section describes the common and distinguishing characteristics of the immune system during chronic HIV-1 or helminthic infections.
T-Cell Subset Profile
Abnormal T-cell subset profile during HIV-1 infection.
The hallmark of HIV-1 infection is the continuous attrition of CD4 T cells, both naive (CD45RA+) and memory (CD45RO+) cells, with the accompanying progressive immune deficiency (101, 154, 156, 180, 199). However, despite the great progress made in understanding the pathogenesis and mechanisms of HIV infection, it is still not clear whether the chain of events leading eventually to AIDS is brought about by the direct effects of the virus itself or, rather, by the indirect effects of its continuous presence on the responding immune system of the infected host. Although the cytopathic effects of HIV-1 on T cells in vitro have been well described (47, 93), the small number of truly infected T cells during the infection (7), the widespread changes in the immune responses and homeostasis well before the onset of AIDS, and the other features of immune imbalance associated with HIV infection (see below) all present complex situations that cannot be accounted by the “direct-killing” hypothesis (205, 206). As has been previously suggested by others and by us (9, 19, 21, 23, 25, 72, 84, 95, 102, 103, 110, 126, 152, 178, 239), indirect effects of the infection with HIV, primarily the wide and chronic immune activation of the host, probably account better for the main changes observed during HIV progression and AIDS. Indeed, we have found that immune activation, as determined by the membrane markers HLA-DR and CD38 and the intracellular nuclear antigen Ki-67, correlates better than HIV-1 plasma VL with CD4 T-cell decline during HIV-1 infection (150, 152, 268). In contrast, the correlations between HIV-1 plasma VL and CD4+ T-cell numbers or CD4/CD8 ratios were much weaker, and no correlation at all was found between the VL and CD8+ T-cell levels. The correlation between the percentage of Ki-67-expressing CD4+ cells and CD4+ T cells paralleled that of HLA-DR in CD3+ cells and reflected immune activation of the cells rather than increased cell proliferation. This conclusion is based on the fact that almost half of the Ki-67+ CD4+ cells coexpressed cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), which is considered a marker for activated cells arrested at the G1 stage of proliferation (42). Similar observations were also reported recently by Sousa et al. in studying both HIV-1- and HIV-2-infected patients (244). Furthermore, simian immunodeficiency virus (SIV) infection of mangabeys, which does not result in immune activation, also does not result in CD4 lymphopenia despite the presence of a chronic high-level viremia (240). This is in contrast to pathogenic SIV infection of rhesus macaques, which results in high immune activation and CD4 lymphopenia. Douek et al. (72) have recently suggested that in addition to the attrition of the resting memory and naive T-cell pools as a result of persistent immune activation during the chronic phase of HIV-1 infection, a rapid and massive depletion of CCR5+ CD4+ memory T cells, not related to immune activation, occurs during the acute phase of the infection. They suggest that the loss of these cells has a central impact on the subsequent course of the infection.
A key factor contributing to the immunodeficiency in HIV infection seems to be defective antigen presentation. T-cell responses such as proliferation, interleukin-2 (IL-2) secretion, and activation of cytolytic effector function from memory CD8+ precursor CTL require stimulation of T cells via the T-cell receptor (TCR) and costimulatory signaling through the ligation of CD28 receptor with the B7-1 (CD80) or B7-2 (CD86) ligands of antigen-presenting cells (APC) (13, 14, 123). By contrast, TCR stimulation in the absence of CD28-mediated costimulation not only results in little IL-2 production but also induces a long-lasting hyporesponsive state known as T-cell clonal anergy (38, 91, 146, 212). In healthy individuals, the CD28 molecule is present in about 95% of CD4+ T lymphocytes and in about 50% of CD8+ T cells (14, 123). During HIV-1 infection, a decrease in CD28+ expression occurs in both the CD4 and CD8 compartments (64, 150, 151, 156, 195, 278), and there is a decrease in the level of the costimulatory molecule CD80 in APC (54, 148). Furthermore, the CD40 ligand (CD40L, also called CD154 or TNFSF5), a crucial molecule for activating APC, which belongs to the tumor necrosis factor (TNF) superfamily (TNFSF) of ligands, is also decreased in expression during HIV infection (54, 140). Taken together, these results show that CD4+ T-cell memory populations, initially those expressing CD45RO, decrease in number not only because of their destruction but also because they fail to expand in response to antigenic stimulation (112).
Abnormal T-cell subset profile during helminthic infections.
Less well known and less appreciated is the dysbalance in peripheral lymphocyte populations observed in association with helminthic infections (24, 80, 153). These changes include: (i) a decrease in the number of CD4+ lymphocytes (79, 128, 243) and an increase in the number of CD8+ T lymphocytes (128, 243), and a reduction in CD4/CD8 ratios to below 1 (a similar reduction in the CD4+ cell numbers in the peripheral blood is also found in individuals infected with other parasites, such as the protozoan parasite Leishmania donovani [94]); (ii) a marked increase in the proportion of activated (HLA-DR+) CD4+ (80, 125, 128, 173, 268) and CD8+ (128, 173, 268) T cells; (iii) a significant increase in the numbers of memory (CD45RO+) CD4+ and CD8+ T cells (125, 128, 268), with a concomitant significant decrease in the proportion of naive (CD45RA+) CD4+ cells (125, 128, 268); and (iv) a major decrease in the number of CD8+ CD28+ T cells (128, 173, 176, 243, 268). All these changes in the peripheral blood lymphocytes subsets result in an impaired capacity to mount protective immune responses to the invading parasites, with a major impact on the host ability to respond immunologically (20, 31, 32, 74, 138, 243). Of great importance is the fact that most of these changes revert to normal levels, following eradication of the helminthic infections (23, 34, 128) (see also “Helminthic infections, deworming, and vaccination” above). Of interest, individuals constantly exposed to filarial infections have a greater expression of CD28 in both CD4+ and CD8+ T-cell subsets (247), suggesting a possible compensatory mechanism in some situations of chronic exposure to parasitic antigens (128, 268). Furthermore, expatriates with filarial infections, who do not harbor the parasite any more, do have lower CD8+ CD28+ levels than healthy individuals (244). Nevertheless, it may suggest differences between the effects of chronic HIV infection and different parasitic infections on the host immune system.
T-Helper (TH1/TH2) Profile
The role of TH1 and TH2 cells in controlling the immune response and in overcoming infections is well established. While cytokines produced from TH1 cells induce a cellular immune response, cytokines produced from TH2 cells induce a humoral immune response (1). These two cell types cross-regulate each other, and hence cytokines produced by one subset can suppress the production and/or activity of cytokines by the other subset (188, 189). More importantly, a stronger TH1 response may enable the host to better overcome certain types of infections, such as viral or fungal infections, while a stronger TH2 response has been found in parasitic diseases and may be more adequate in coping with them. The following sections, as well as Table 1, review what is currently known about the role played by the TH profile in both HIV and helminthic infections.
TABLE 1.
Role of TH1 and TH2 in protection against HIV-1 and helminthic infections
| TH1 and TH2 during HIV-1 infection | Main reference(s) |
|---|---|
| A correlation exists between TH1 profile and slow progression of HIV infection | 55, 222, 260 |
| Defective in vivo activation of HIV-1-specific CTL occurs in rapidly progressive infection | 99, 109, 156 |
| A switch from TH1 to TH2 profile is associated with fast disease progression | 59, 164, 223 |
| No switch from TH1 to TH2 cytokine profile was found during the progression of HIV disease | 100, 278 |
| HIV replicates preferentially in TH2 cells | 59, 164, 223 |
| TH1 cells clear primary viremia and maintain low viremia during the asymptomatic phase of the infection | 35, 130, 139, 241, 255, 256 |
| TH1 functions are correlated with better survival and slower disease progression | 57, 214, 220, 222 |
| Protection from HIV-1 infection is associated with TH1 responses | 16, 57, 58, 65, 89, 142, 160, 209, 210, 224, 225 |
| Cellular immunity induced by vaccination confers protection against SIV in primates | 39, 175, 215 |
| Progression to disease in SIV-infected macaques is associated with evasion of the CTL response | 81 |
| The low levels of IL-12 found in HIV+ individuals promote a TH2 immune response | 192 |
| Helminth infections are associated with a dominant TH2 immune profile | 24, 85, 118, 162, 166, 169, 196, 234, 276, 277 |
| T cells from chronically helminth-infected individuals do not produce TH1 cytokines following recall antigen | |
| stimulation | 33, 92, 227, 262 |
| TH1 cytokine levels are not always decreased during helminth infections | 61, 166, 203, 276 |
| The TH2-dominant immune profile in helminth infection drives the host response toward a TH2/TH3 type | 2, 19, 61, 118, 166, 167, 169, 234, 235, 271, 276 |
| Helminth-infected mice have a downregulated TH1 response | 11, 12, 263 |
TH1 and TH2 cells during HIV-1 infection.
Shearer, Clerici, and Romagnani were among the first investigators to point out the possible protective and beneficial role of a TH1 response on the course of HIV infection (55, 222). They showed a clear correlation between maintenance of a TH1 profile and slow progression of the infection whereas a switch of profile from TH1 to TH2 was associated with fast progression of the infection (59, 164, 223). Furthermore, CTL (TH1 cells) probably play a crucial role in controlling viremia, slowing disease progression, and perhaps preventing the establishment of infection (reviewed in reference 116). More specifically, (i) activated CTL and CD8+ T-cell-mediated noncytolytic inhibition of HIV are responsible for the initial clearance of primary viremia and probably for maintaining low viremia during the asymptomatic phase of the infection (35, 130, 139, 241, 255, 256); (ii) TH1 functions are correlated with better survival and slower progression (57, 214, 220, 222); (iii) TH0 cells or TH2 cloned cells show increased susceptibility for HIV infection and replication (164); (iv) progression may be correlated with a reduction of cellular immunity, together with higher permissiveness of TH0/TH2 cells to HIV infection (164). Protection from HIV infection may also be associated with an effective TH1 cellular defense. The best evidence is found in individuals who have been exposed to HIV yet remained HIV seronegative while having specific HIV cellular immunity (reviewed in references 16 and 141) and HIV-seronegative infants who were born to HIV-infected mothers and have HIV-specific CTL activity (65). The importance of cellular immunity in conferring protection from infection has also been shown in several studies of protective vaccination against SIV in primates (39, 175, 215). However, despite these important observations, several studies have not confirmed many of these findings (see, e.g., references 100 and 278). No significant dominance of a particular TH profile has been found to be associated with stages or courses of the infection. Elevated levels of TH2 cytokines have been found to be present even at early stages of the infection, while the levels and specificity of CTL activity have been correlated only poorly or not at all with better outcome of the infection (81, 99, 109, 156). Taken together, it is clear that until we have better correlates of immunity to viral suppression and/or control, we should consider these to be markers of immune activation and response with as yet unclear relevance to the natural course and prognosis of HIV infection.
TH1 and TH2 cells during helminthic infections.
One of the hallmarks of helminth infection is the dominant TH2 immune profile they elicit. We and others have been able to show that people infected with helminths have extreme blood eosinophilia, high serum IgE levels, and a TH2 cytokine profile with increased secretion of IL-4 and IL-5 in the absence of significant TH1 cytokine synthesis (24, 85, 118, 162, 166, 169, 196, 234, 276, 277). For instance, peripheral T cells obtained from humans who are chronically infected with the filarial parasite or Schistosoma fluke often fail to respond to parasite antigens (92, 227, 262), to recall antigen including PPD (33), or to anti-CD3 stimulation (Q. Leng et al., unpublished observations), in the form of proliferation or TH1-related cytokine production. This cytokine profile may vary, either during the same infection, such as a switch from TH1 to TH2 during Schistosoma infection, or in different helminthic infections, such as in filariasis (166, 276). Interestingly, the secretion of IL-2 and gamma interferon (IFN-γ) (TH1 cytokines) is not always decreased below normal levels during helminthic infections (61, 166, 203, 276). Be it as it may, the TH2 skewed immune profile associated with the helminthic infections influences the infected host immune response toward a TH2/TH3 (see “TGF-β and IL-10 in helminthic infections” below) type of response to other antigens (2, 19, 61, 118, 166, 167, 169, 234, 235, 271, 276). A TH2-like immune response with concomitant downregulation of TH1-associated immunity has also been shown in mice infected with Taenia (263).
Distinctive Features of HIV-1 and Helminthic Infections
As outlined above, there are several common features to HIV infection and chronic helminthic infections which we think are due to the fact that both are situations of chronic immune activation. There are, however, clear differences between these two conditions which need to be emphasized. The extreme T-cell depletion, and particularly the CD4 T-cell attrition and the severe immunodeficiency that comes with it and that is so typical of advanced HIV-1 infection, has never been observed or reported in chronic helminth infection. Furthermore, it is clear that helminth infections in different geographical locations are accompanied by different degrees of immune deficiency and T-cell impairment. Our observations of the Ethiopian immigrants have been confirmed by studies of other helminth-infected populations in India, the Caribbean, and, to a lesser extent, some parts of East Africa, but apparently not to the same extent in West Africa (127, 259). The interpretation of these differences is not so clear and probably reflects several additional factors, most of them unknown. On the one hand, HIV-1 is an intracellular pathogen with special tropism to the immune system, most notably to T cells, with the CD4 molecule that specifically marks T helper cells being its major cell membrane receptor. HIV-1 also has direct effects on the immune cells, at least in the acute phase of the infection, which have not been reported so far in any helminth infection. Helminths, on the other hand, are large extracellular parasites that generate a strong immune response to several antigens presented by them to the host. There may also be some antigenic similarities between helminth infections and HIV (132, 133; Z. Weisman, unpublished data), but these are certainly not well defined and probably do not constitute a major element that may account for either the similarities or the differences between the two types of infections. Lastly, it is clear that with respect to the TH1/TH2 effects, helminthic infections, unlike HIV-1, confer a dominant TH2 profile on the host, as summarized in Table 1.
CHRONIC IMMUNE ACTIVATION RESULTS IN HYPORESPONSIVENESS AND ANERGY
A striking finding in studies of the immune response during chronic infections, particularly HIV and helminthic infections, is the high degree of low responsiveness to antigen and to immune stimulation. This has usually been ascribed to the immunodeficiency that accompanies HIV infection but was not sufficiently well recognized in other chronic infections such as helminthic infections. The use of TCR-transgenic mice has provided compelling evidence that anergy is an in vivo phenomenon, and not merely an in vitro artifact (146). We have previously suggested that the hyporesponsiveness and anergy that accompany HIV and helminthic infections may be caused partly by the chronic immune activation (18, 24, 33, 128). Such hyporesponsiveness and anergy could be tied to the effects of T-regulatory (Treg)/suppressor cells present in these situations. It is now over 5 years since the attention of the immunological community was drawn to the role, characterization, and function of Treg cells. This resurgence of interest in what historically were known as “suppressor T cells” (217, 231), has been brought about by the clear demonstration, first with animals and later with humans, that such cells do indeed exist and that their function is probably central to the control of several elements of the immune response. These cells constitute 5 to 10% of peripheral CD4+ T cells in naive mice and humans and suppress several potentially pathogenic responses in vivo, particularly T-cell responses directed to self-antigens. The first and most clear demonstration of their place and role was in the context of autoimmunity (228); this was followed by studies with both humans and animals, showing that such cells were involved in the control of the immune response to infections, neoplasia, and organ and bone marrow transplantation (30, 52, 88, 90, 236, 237, 265, 273). For example, during chronic infection by Leishmania major. Treg cells accumulate in the dermis, where they suppress, by both IL-10-dependent and IL-10-independent mechanisms, the ability of CD4+ effector T cells to eliminate the parasite from the site (17). By now it has become clear that Treg cells belong to a population of CD4 T cells that coexpress CD25 (the IL-2 receptor alpha-chain), constitutively express CTLA-4, often secrete IL-10, transforming growth factor β (TGF-β), IFN-α, and IL-5, and may have higher expression of a number of membrane markers (CD62L, CCR4, CCR8, and CD103) as well as of the cellular transcription factor FOXP3 (30, 52, 88, 90, 134, 228, 236, 237, 265, 273). The role of these cells in the context of the chronic immune activation that is present in HIV and helminthic infections has not been widely explored until recently. In the following sections, we summarize the studies of hyporesponsiveness and anergy in these infections and their possible link to Treg cells.
TGF-β and IL-10 during HIV-1 Infection
A complex and sequential pattern of loss of TH-cell function can occur years before the development of AIDS symptoms. Such suppression could be due to immunosuppressive factors that are either products of HIV, such as gp120 or its precursor gp160 and Tat (see below), or HIV-induced immunoregulatory cytokines, such as TGF-β and IL-10 (233); it could also be due to an increase in the number of Treg cells. Both IL-10 and TGF-β, which are also produced by TH3/regulatory cells (236, 237), show elevated levels during HIV-1 infection (4, 56, 59, 100, 161, 193, 221, 249). TGF-β plays an essential role in T-cell regulation, including its antiproliferative effects on T cells and acquisition of effector functions by naive T cells (reviewed in reference 98). However, overexpression of TGF-β can lead to the conversion of its protective functions to pathogenetic manifestations through its profound and broad inhibitory effects on different antiviral defense mechanisms. Thus, overproduction of TGF-β during HIV infection may contribute to noncytopathic mechanisms of immunodeficiency by suppressing cellular and humoral immune responses. One example is the decrease of B-lymphocyte proliferative responses of cells of HIV+ donors to Staphylococcus aureus Cowan 1 stimulation. This deficiency correlates closely with increased TGF-β secretion by peripheral blood mononuclear cells (PBMC) from HIV+ donors (131). Antibodies to TGF-β neutralize the inhibitory effect of HIV+ culture supernatants on normal B cells and increases low proliferative responses by HIV+ cells. Activated TGF-β from HIV+ PBMC is able to significantly reduce the induction of immunoglobulins, and this effect is also abrogated by anti-TGF-β (131). TGF-β can also suppress the production of IL-18, a cytokine which induces cellular immune responses (see “TH1 and TH2 cells during HIV-1 infection” above) in PBMC of HIV+ patients, and its levels in plasma are inversely correlated with levels of IL-18 in serum in HIV+ patients (4). HIV-1 gp120 and gp160 induce TGF-β secretion and TGF-β mRNA upregulation in PBMC (46, 117). Similarly, HIV-1 Tat was shown to increase the expression of TGF-β in human astrocytic glial cells (62). TGF-β promotes virus replication and spreading by multiple distinct mechanisms. It directly stimulates virus replication in infected monocytes and PBMC under certain in vitro conditions, and it stimulates the production of other cytokines that enhance virus replication (211).
The IL-10 concentration increases with HIV-1 disease progression (56, 59, 100, 221, 249) and is reduced in long-term nonprogressor HIV+ individuals (56, 249). Moreover, a dramatic increase in the plasma IL-10 level was shown to coincide with a rapid decrease in CD4 counts and progression to AIDS (245). Accordingly, highly active antiretroviral therapy (HAART) induced a significant, gradual decrease in IL-10 levels (249). Furthermore, the loss of cell-mediated immune responses found in HIV+ patients during successful HAART could be significantly improved in vitro by the addition of anti-IL-10 (250). HIV-1 gp120 has been also found to upregulate IL-10 in lymphocytes (46).
TGF-β and IL-10 during Helminthic Infections
Several studies support the notion that it is not the TH1-to-TH2 shift but, rather, other cytokines, primarily IL-10 and TGF-β, which mediate the antigen-specific hyporesponsiveness characteristic of chronic human or primate helminth infections (71, 113, 136, 183, 198, 218, 235). For instance, parasite antigen-specific cellular hyporesponsiveness in patients chronically infected with filarial helminths was associated with a lack of IL-4 production and significantly lower production of IL-5 by their PBMC compared to the same cells obtained from individuals with putative immunity. In contrast, the antigen-specific hyporesponsiveness could be reversed by the addition of anti-IL-10 and anti-TGF-β antibodies (71, 137, 165). In accordance, it has been shown that production of TGF-β is at least partially responsible for the failure to elicit protective immunity against Schistosoma mansoni by certain vaccination protocols (270). Additionally, increased expression of TGF-β produced by parasite antigen-specific peripheral T cells has been found in baboons repeatedly challenged with S. mansoni as well as in Wuchereria bancrofti-infected humans (86, 135, 183). Experimental infection of a nonhuman primate with the human parasite Loa loa resulted in a transient period of strong T-cell proliferation, cytokine production, and cytokine mRNA expression followed by an unresponsive state in which only IL-10 mRNA was expressed (153), supporting the notion that IL-10 plays a central role in downregulating and maintaining T-cell unresponsiveness. Injection of mice with oligosaccharides expressed on helminth parasites (lacto-N-neotetraose) conjugated to dextran caused an expansion in the number of suppressor macrophages, phenotypically defined as Gr1+ CD11b+ F4/80+, as early as 2 h after injection, which spontaneously produced low levels of proinflammatory cytokines but higher levels of IL-10 and TGF-β ex vivo, compared to peritoneal cells from mice injected with dextran only (252). Gr1+ cells adoptively suppressed naive CD4+ T-cell proliferation in vitro in response to anti-CD3/CD28 antibody stimulation, in an IFN-γ and nitric oxide-dependent mechanism that involves cell-cell contact (10, 252). We have found that helminth-infected individuals had two- to threefold higher plasma TGF-β concentrations than did helminth-uninfected subjects and that the TGF-β levels were correlated with HLA-DR expression on peripheral T cells (Q. Leng et al., submitted for publication), indicating that immune activation results in increased levels of down-regulatory cytokines such as TGF-β. The production of TGF-β and IL-10 was found to be significantly higher in helminth-infected females than in helminth-infected males (218), suggesting a gender-dependent immune regulation related to the chronicity of the infection, which may be caused by nonimmunological factors like sexual hormones.
It is possible that both parasite antigen-specific unresponsiveness characteristic of chronic helminth infections and the general cellular hyporesponsiveness, i.e., lower proliferation in response to anti-CD3 or mitogen stimulation, are caused by the increased levels of the inhibitory cytokine TGF-β. One possible way through which TGF-β downregulates T-cell responses is via upregulation of Cbl-b, an intracellular upstream negative regulator of T-cell activation (147, 159, 226). Cbl-b sets the threshold of signaling in T and B cells (226). We have found that stimulation of PBMC with TGF-β increases the intracellular pools of Cbl-b (Leng et al., submitted). This, together with the increased levels of CTLA-4 found in helminth-infected individuals (see “CTLA-4 upregulation during helminthic infections” below), raises the threshold for effective T-cell activation (177) and may explain the reduced proliferation following anti-CD3 stimulation, and the reduced phosphorylation of ERK-1/2, following phorbol myristate acetate and Ca2+-ionophore stimulation, of PBMC obtained from helminth-infected immune activated individuals (see “T-cell signal transduction impairments during helminthic infections” below) (also see Fig. 4). In addition, we have found that stimulation of PBMC with immobilized anti-CTLA-4 antibodies enhances Cbl-b expression. Enhancement of Cbl-b expression may result indirectly from the effect of TGF-β, since CTLA-4 engagement leads to TGF-β expression in T cells (53). Thus, the higher levels of CTLA-4 involved in the induction of TGF-β, together with the higher levels of TGF-β, are all associated with immune activation and may contribute significantly to the general and antigen-specific hyporesponsiveness characteristic of chronic helminthic infections (33, 34).
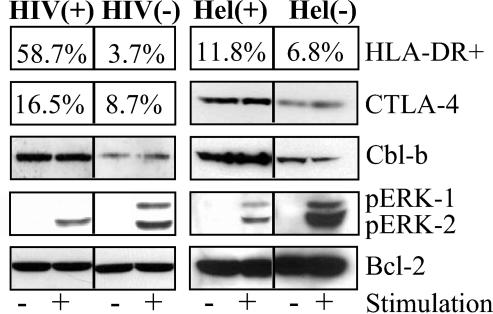
FIG. 4.
Increased levels of CTLA-4 and Cbl-b and attenuated phosphorylation of ERK-1/2 in immune-activated HIV-1 or helminth-infected individuals. PBMC were obtained from HIV-seronegative [HIV(−)], HIV+ [HIV(+)], Ethiopian immigrants to Israel (ETH) noninfected [Hel(−)], and ETH helminth-infected [Hel(+)] individuals. Lysates from the nonstimulated PBMC (−) or from PBMC stimulated with 0.5 μg of anti-CD3 monoclonal antibody for 5 min (+) were resolved on by sodium dodecyl sulfate-polyacylamide gel electrophoresis and immunoblotted with anti-BCL2, anti-phosphorylated p42/44 MAPK/ERK, anti-Cbl-b, or anti-CTLA-4 antibodies. In addition, the percent surface HLA-DR expression and intracellular pools of CTLA-4 were determined by flow cytometry.
Treg cells, as well as other T-cell populations and even nonlymphoid cells, such as epithelium in the process of healing, secrete TGF-β (98, 237). Other possible sources of TGF-β may be macrophages, since apoptotic cells trigger TGF-β production by macrophages (82) and since we have previously found increased lymphocyte apoptosis in Ethiopian immigrants heavily infected with helminths (128).
CTLA-4
While full activation of T cells requires costimulatory signaling through the ligation of the CD28 receptor to the B7-1 (CD80) or B7-2 (CD86) ligands of APC, CTL-associated antigen 4 (CTLA-4) engagement to B7 terminates ongoing responses and proliferation of activated helper T cells and results in apoptosis (254). Cross-linking of CTLA-4 reduces IL-2 production, arrests the cells in the G1 phase of the cell cycle (267), and downregulates T-cell responses by raising the threshold for effective T-cell activation (48, 207, 254, 267). Blockage of CTLA-4/B7 interactions prevents the induction of peripheral T-cell tolerance after vaccination with peptides under tolerogenic conditions (207), suggesting that CTLA-4 might be involved in the induction of anergy. CTLA-4 knockout mice have significantly higher levels of CD4+ T cells and CD4/CD8 ratios than do normal mice, from 6:1 to as high as 20:1 (48). Administration of monoclonal antibodies to CTLA-4 enhances CD4+ T-cell expansion in response to a variety of stimuli and is a potent antitumor and antiparasitic tool in experimental-animal models (145, 174, 238).
CTLA-4 upregulation during HIV-1 infection.
CTLA-4, upregulated during T-cell activation, may account for some of the main features of HIV infection and may therefore be a central player in HIV pathogenesis. We (150) and others (248) have found that the proportion of CTLA-4+ CD4+ cells is significantly higher in HIV+ individuals than in HIV-1-seronegative controls. Moreover, we found that (i) intracellular CTLA-4 levels in HIV+ individuals are inversely correlated to CD4+ levels and to the CD4/CD8 ratio; (ii) CTLA-4 levels are higher in HIV+ patients with advanced clinical symptoms or AIDS than in asymptomatic patients; (iii) the increase in CD4 counts in HAART-treated patients with undetectable VL is inversely correlated to the proportions of CTLA-4+ CD4+ cells; (iv) CTLA-4 expression and the ratio between the proportion of CTLA-4+ CD4+ cells and that of CD28+ CD4+ cells is correlated with disease stage and with immune activation; and (v) the capacity of PBMC from HIV-1-infected patients to respond to nonspecific or HIV-1-specific stimuli was inversely correlated to the levels of CTLA-4+ CD4+ cells (150).
In addition, we found a diminished expression of CD28 on CTLA-4+ cells and a clear association of CD28 expression with CD4 expression, raising the possibility that CTLA-4 indirectly downregulates CD4 expression by downregulating CD28 expression and maybe CD4 production as well (150). As described above, CTLA-4 plays a central role in the induction of T-cell anergy (207, 254, 267). The inverse correlation between CTLA-4 levels and the proliferative responses of PBMC from HIV+ patients stimulated with anti-CD3 antibody or HIV-1 antigens that we found (150) supports this notion. Furthermore, since we found that 30 to 40% of the CTLA-4+ CD4+ cells were also CD25+ cells and since CTLA-4 is a constitutive element of CD4+ CD25+ cells, an increase in the numbers of CTLA-4+ CD4+ cells would also mean an increase in the numbers of CD4+ CD25+ Treg cells.
Specific cytotoxic function of CD8+ cells during HIV infection, dependent on CD4 help, is essential for the ability of the host to contain HIV infection (16, 35, 58, 65, 89, 130, 139, 141, 142, 160, 209, 210, 225). CD8 T cytotoxicity against tumor cells in mice can be enhanced by blockade of CTLA-4 only in the presence of CD4 T cells, while CTL activity is lost in the absence of CD4 T cells (145), supporting the idea that functional CD4 T cells are essential for CD8 CTL activity. Thus, even a small increase in the number of dysfunctional CD4 cells, i.e., an increase in the proportion of CTLA-4+ CD4+ cells, may have dramatic effects on other compartments of the immune system, including the capacity of CD8 cells to specifically target HIV-infected cells.
In vivo, APC activate CD4+ T cells in part by signaling through the TCR and CD28. Cells stimulated in this manner are susceptible to HIV-1 infection. However, CD4+ T cells activated in vitro by anti-CD3/28-coated beads are resistant to infection by CC chemokine receptor 5 (CCR5)-dependent HIV-1 isolates. CTLA-4 engagement counteracts the CD28 antiviral effects, and the ratio of CTLA-4 to CD28 engagement determines the susceptibility to HIV-1 infection. Furthermore, unopposed CTLA-4 signaling provided by the CD28 blockade promotes vigorous HIV-1 replication, despite minimal T-cell proliferation (219). Since CTLA-4 binds to B7-1 or B7-2 with 20- to 100-fold higher affinity than CD28 does (157), and since by doing so it downregulates the immune response, the CD28/CTLA-4 ratio may be an important parameter for assessment of the immune response. The importance of this ratio in making cells more susceptible to HIV infection (219) is supported by our findings of increased CCR5 expression in CTLA-4+ cells (150). The increased CTLA-4/CD28 ratio that we have found in HIV+ individuals is due mainly to the increased expression of CTLA-4 in CD4+ cells and not to the reduction of CD28 expression on CD4+ cells.
The most likely reason for CTLA-4 upregulation in HIV infection is the immune activation caused by HIV antigens. This is supported by the following findings: (i) the proportion of CTLA-4+ cells in HIV infection is strongly correlated with other immune activation markers such as HLA-DR+ CD3+ cell levels; (ii) in early HIV infection, when the immune activation is low, CTLA-4 expression is low; (iii) in another chronic immune activation state, such as that caused by helminthic infections, we found similar increase in CTLA-4 expression together with CD4 diminution (33); and (iv) we have found a highly significant correlation between age and percentage of CTLA-4+ CD4+ cells (r = 0.6, P < 0.001) and between age and mean fluorescence intensities of CTLA-4 (i.e., number of molecules, r = 0.61, P < 0.001) in healthy individuals (149). The CTLA-4 levels were correlated with immune activation, determined by the levels of HLA-DR+ CD3+ cells (r = 0.55, P < 0.001). In contrast, we found a strong inverse correlation between age and numbers of CD28+ CD8+ T cells (r = −0.67, P < 0.001), leading us to postulate that immune senescence associated with age is caused in part by chronic immune activation with a related decrease in the number of CD28 costimulatory molecules and an increase in the number of inhibitory CTLA-4 molecules (149). Others have also reported an increase in immune activation (171, 216), increased expression of CTLA-4 on T cells (266), and decreased expression of CD28 on T cells (36, 83, 104) with age.
CTLA-4 upregulation during helminthic infections.
We have found significantly elevated CTLA-4 expression in CD4+ T cells in HIV-1-seronegative helminth-infected individuals (33, 34). The increased CTLA-4 expression was correlated with immune activation, as determined by the levels of HLA-DR+ CD3+ cells. Blocking of CTLA-4 enhanced the proliferative responses of PBMC to TB and HIV-1 antigens in nonresponsive PBMC obtained from highly immune-activated individuals (33, 34). Similar results were found by Steel and Nutman for individuals with long-standing filarial infections (247). These infected individual had significantly higher percentages of CD4+ CTLA-4+ and CD8+ CTLA-4+ cells than did uninfected individuals. Moreover, Steel and Nutman found a significant upregulation of CTLA-4 mRNA expression in PBMC obtained from uninfected adolescents exposed in utero to microfilarial antigen than that observed in cells from children born to uninfected mothers. In vitro blocking of CTLA-4 expression in PBMC from filaria-infected individuals induced a mean 44% increase in IL-5 production in response to microfilarial antigen, whereas there was a concurrent mean 42% decrease in IFN-γ production, suggesting that CTLA-4 may alter the TH1/TH2 balance in filaria-infected individuals. In both studies of intestinal helminth-infected individuals and of filaria-infected individuals, the highest intensity of CTLA-4 expression occurred in CD4+ CD25+ cells (247; and Leng et al., unpublished). Together, these data indicate that CTLA-4 plays a significant role in regulating the host response to helminths by contributing to the general anergy observed in these individuals.
T-Cell Signal Transduction Impairments during Chronic Immune Activation
Physiological activation of T lymphocytes requires costimulation through the TCR-CD3 complex and CD28 (158, 275). Following such costimulation, a cascade of phosphorylations and dephosphorylations of cytoplasmic kinases (e.g., Erk mitogen-activated protein kinase [ERK/MAPK] and p38 [5, 229]) and other proteins (e.g., IκBα [129]) occurs, leading to activation of transcription factors such as NF-κB, NFAT, and AP-1 (168, 184) and eventually to proliferation and/or protein expression. Incomplete T-cell activation, due to subtle alteration of the antigen or stimulation through the TCR in the absence of costimulation, results in induction of T-cell anergy (91, 123, 146, 212). T-cell anergy has been suggested to be an active negative state in which IL-2 production is inhibited both at the level of signal transduction and by cis-dominant repression at the level of the IL-2 promoter (212). Our observations of the impaired immune responsiveness during chronic immune activation associated with HIV and helminthic infections led us to explore T-cell signal transduction in these situations. As described in the following sections, signal transduction is indeed impaired in T cells obtained from patients with these diseases and is clearly correlated with the state and severity of the immune activation. The results of our studies and those by several other investigators that addressed these issues are summarized in the following sections.
T-cell signal transduction impairments during HIV-1 infection.
Decreased proliferation, secretion of IL-2 and other cytokines or chemokines, and upregulation of the receptor for IL-2 (IL-2R) in response to antigenic or mitogenic stimulation of HIV-noninfected T cells obtained from HIV+ individuals has been observed in many cases (150, 151, 156, 230, 251, 264). We found that this hyporesponsiveness is correlated with immune activation (151). Decreased responsiveness of CD4+ and CD8+ T cells from mice immunized with superantigens is also associated with anergy (see, e.g., reference 27). During early HIV-1 infection, the in vivo deletion of memory T cells can account for decreased responsiveness. Later in infection, when the balance between memory and naive T cells is normalized, both CD4+ and CD8+ cells are nonresponsive to recall antigen and low-dose anti-CD3 stimulation. This anergy is at the level of IL-2 gene expression, since early signal transduction events following CD2 and CD2 receptor occupancy are normal. Part of the reduced capacity of the T cells to respond to stimuli is probably due to decreased expression of CD28 and increased levels of CTLA-4, discussed in the previous sections. However, other mechanisms, such as impairments in signal transduction pathways in T cells of HIV+ individuals, may also explain the observed hyporesponsiveness (181). This postulation is based in part on the observation that stimulation by phytohemagglutinin or phorbol mycistate acetate and Ca2+-ionophore, which bypass the cell membrane and the TCR-CD3 complex; also result in impaired cellular responses (180) such as decreased ERK-1/2 phosphorylation, as we have found (151) in cells obtained from HIV+ subjects (Fig. 4). Decreased phosphorylation of p38 and ERK/MAPK have been observed also in anergic TH1 murine cells, even though the levels of these proteins remained unchanged (66, 67, 87). HIV-induced impairment of proliferation was linked to induction of the inhibitory protein kinase A (PKA) pathway by HIV proteins (115). Increases in cyclic AMP (cAMP)/PKA activity were shown to induce biochemical changes that impaired proliferation when the cells were stimulated with phytohemagglutinin. Agents, other than HIV proteins that increase cAMP/PKA activity (cholera toxoid and 8-bromo-cAMP) also decreased T-lymphocyte proliferation. Agents that reduced cAMP generation also neutralized the effect of HIV proteins and restored lymphocyte proliferation (115). These studies show that the HIV-induced augmentation of cAMP/PKA activity may be an important mechanism responsible for HIV-induced anergy of T lymphocytes. Additionally, PBMC of HIV+ individuals have increased constitutive levels of Cbl-b, an intracellular upstream negative regulator of T-cell activation (159), which we have found to be correlated with attenuated anti-CD3 reactivity (151) (Fig. 4). Similarly to CTLA-4, which downregulates T-cell responses by raising the threshold for effective T-cell activation (254), Cbl-b also sets the threshold for signaling in T and B cells (226). The increased threshold, caused by the higher constitutive levels of Cbl-b may explain the reduced phosphorylation of ERK-1 and ERK-2 (37), both of which are involved in IL-2 production (155), and the eventual lower proliferation following anti-CD3 stimulation. Exposure of lymphocytes to HIV or cholera toxoid leads to decreased membrane activity of the proliferation promoter protein kinase C (PKC) following stimulation (115). Furthermore, PKC activation in CD3+ T cells, following integrin stimulation, is impaired in HIV+ individuals, mostly among symptomatic patients and those with AIDS (194). Integrins play an important role in the induction of T-lymphocyte responses to antigenic challenge by providing a T-cell costimulatory signal, and they have been shown to rescue various cell types from undergoing apoptosis (182). However, in the majority of AIDS patients, integrin-mediated costimulation of TCR-induced T-cell proliferation and protection from aberrant cell death are absent; in asymptomatic HIV+ individuals they are intact (194).
The difference in the intracellular milieu of different proteins involved in signaling between infected and noninfected T cells obtained from HIV+ and HIV-seronegative individuals may be explained in part by the high levels of soluble gp120 found in HIV+ individuals (197). Ligation of soluble gp120 to CD4 receptors causes an increase in the intracellular calcium concentration, blockage of mitogen- or antigen-driven T-cell activation, induction of an altered cytokine production by activated PBMC subpopulations, impaired cytotoxicity and chemotactic response to antigens, interference with the activity of APC, induction of apoptosis, and upregulation of a number of cytokines, including IL-6, TNF, IL-1α, IL-1β, IL-10, and IL-8 and the costimulatory ligand CD40L (28, 46, 54). On the whole, these data indicate that HIV or its soluble products, such as gp120, can modify several PBMC functions in vivo, including impaired intracellular signaling. In vitro data support this possibility, as illustrated in the following examples. Binding of HIV envelope glycoprotein gp160/gp120 to CD4 molecules on CD4+ T cells, prior to TCR/CD3 activation, results in T-cell unresponsiveness (120, 230). Inhibition of IL-2R expression and proliferation induced by ligation of CD4 by gp120 is correlated with inhibition of expression and activation of Janus kinase (JAK3) (230). This kinase, which is associated with the gamma chain of IL-2R, is indispensable for normal T-cell function (163, 253). gp120-CD4 ligation strongly inhibits TCR-CD3-mediated phosphorylation and activation of lck and fyn (src-type protein tyrosine kinases), phosphorylation of CD3 zeta chain (187), and phosphorylation of Raf-1 and Erk2 and other unidentified proteins (120). Additionally, anti-CD3 monoclonal antibody activation of purified peripheral blood CD4+ T cells from healthy donors with prior exposure to HIV-1 gp160 results in marked inhibition of tyrosine phosphorylation of p59 Pyn phospholipase C-γ1, and ras activation (251).
T-cell signal transduction impairments during helminthic infections.
Similarly to the HIV+ individuals, helminth-infected individuals have impaired immune responses with decreased delayed-type skin hypersensitivity and impaired cell proliferation in response to recall antigens (see, e.g., references 33, 34, and 227). These defects may also be explained in part by impaired signal transduction; for example, inhibition of protein kinases prevents lymphocyte activation by S. mansoni antigens (6). We found a general defective or no early transmembrane signaling (phosphorylation and/or dephosphorylation of tyrosine kinases), deficient degradation of phosphorylated IκBα, and attenuated phosphorylation of MAPK kinases, such as ERK1/2 and p38, in chronically immune-activated helminth-infected individuals (33). Importantly, the signal transduction impairments were correlated with the immune activation state of the cells as determined by HLA-DR, CTLA-4 (33) and Cbl-b expression (Leng et al., submitted; Fig. 4). Cbl-b has recently been described as an intracellular upstream negative regulator of T-cell activation (159). Similar attenuated early transmembrane deficiencies were also found in anergic cells obtained from patients with primary intracranial tumors (185).
Similarly to HIV-1 infection, in which soluble gp120 may cause anergy, helminthic parasites secrete substances that render lymphocytes anergic. One example is ES-62, a phosphorylcholine-containing glycoprotein which is released by Acanthocheilonema viteae. Soluble ES-62 modulates activation of the tyrosine kinases Fyn, Lck, and ZAP-70, leading to selective disruption of TCR coupling to the phospholipase D, PKC, phosphoinositide-3-kinase, and ras/MAPK signaling cascades, eventually leading to suppression of the production of proinflammatory cytokines (106, 107). Another example is the release into the peripheral circulation of the L. loa female worm products in infected hosts. On their release, T-cell unresponsiveness occurs, apparently related to high IL-10 expression (153).
Role of T-Regulatory Cells in Hyporesponsiveness and Anergy
By now it is quite clear that chronic immune activations in HIV infection and in helminthic infection have several common features, among which are the hyporesponsiveness and anergy that accompany both of them. Most importantly, in both situations there are increased secretion and levels of immunosuppressive cytokines (IL-10 and TGF-β), an increase in the level of CTLA-4-positive CD4 cells, an increase in the proportion of CD4+ CD25+ T cells, and an increase in the levels of Cbl-b expression. These seemingly independent features, described in detail above for both HIV-1 infection and helminthic infections, could possibly be linked to one major player, that is, Treg cells/T suppressor cells. As cited above, such cells have all these common features: CD4+ CD25+, constitutive CTLA-4 expression, IL-10 and TGF-β secretion, and increased Cbl-b expression. Is it possible that the hyporesponsiveness seen in chronic immune activation is mainly the result of an increase in this population, which is part and parcel of the response to the chronic immune activation? We think this is indeed a very likely possibility, although obviously we are still short of sufficient hard data to fully support it. Based on the accumulated evidence cited above, such an interpretation would make much sense teleologically when one considers the dangers inherent in the continuous activation of the immune system caused by an infectious agent. As we now know, much of the immunopathology present in various infectious diseases is essentially the result of immune mechanisms directed against virus-infected host cells. The generation of Treg cells in such conditions allows the host to attenuate the dangerous outcomes of the immune response to the host itself. This is a very likely scenario in the case of both chronic HIV infection and chronic helminthic infection. However, this same suppression may jeopardize the ability of the host to contain the infection by mitigating the specific anti-infectious immunity. These issues are far from being resolved and will clearly be the focus of studies in the near future. However, our own studies, as well as those done by others, clearly show that chronic immune activation is accompanied by hyporesponsiveness and anergy and that this phenomenon may be partly or mainly caused by the generation of Treg cells that are upregulated during the chronic immune activation in both HIV and chronic helminthic infections. It is probably the balance between the specific protective immune response and its suppression that counts in the end and will determine the outcome to the host. In the case of helminthic infection, it may very well be that the persistence of the infection for several years is compatible with the life of the host, while in the case of HIV infection, the persistence may very well be undermining the ability of the host to live and overcome the infection. One attractive possibility, in the scene of HIV immunopathogenesis, is to link the state of LTNP HIV carriers, and also the absence of AIDS-like disease in HIV-infected chimpanzees (63) and in SIV-infected green monkeys (240), to the absence of immune activation in response to the virus. Such an absence of response could theoretically be the result of suppression by Treg cells. If this indeed proves to be the case, such hyporesponsiveness could become an appropriate objective for therapy of AIDS.
CONCLUDING REMARKS
This review has tried to summarize the evidence showing that hyporesponsiveness and anergy, which characterize both helminthic and HIV infections, are the result largely, of the chronic immune activation which accompanies these persistent infections. The main players in the complex interaction that takes place, and that contribute to anergy during chronic immune activation, are presented in Fig. 3 and can be summarized as follows: (a) a decrease in CD28 expression and increase in CTLA-4 expression in T cells is accompanied by reduction in the expression of CD28 ligands on APC (CD80), leading to ineffective stimulation of T cells; (ii) the increase in the proportion of Treg cells causes downregulation of T-cell responses either directly through cell-to-cell contact or indirectly by secreting inhibitory cytokines (such as IL-10 and TGF-β); (iii) there is an increase in the constitutive levels of the intracellular negative regulators of T cells, such as Cbl-b; (iv) exhaustion of the intracellular signaling machinery occurs, as indicated by a decreased capacity of kinases to phosphorylate and of phosphatases to dephosphorylate; and (v) NF-κB is downregulated, along with the transport of transcription factors to the nucleus. Thus, chronic immune activation triggers (i) an increased threshold for effective immune activation of T cells, (ii) defective intracellular signaling, (iii) a decrease in the number of costimulatory molecules, (iv) an increase in the number of Treg cell, (v) an increase in the amount of intracellular negative regulator of T-cell activation in T cells, (vi) dysregulation of cytokine secretion, and (vii) a T-cell imbalance, all leading to the hyporesponseviness and anergy characteristic of helminthic and HIV-1 infections (Fig. 5). As suggested by us previously, immune activation of the host is a critical determinant in the pathogenesis of infections, and chronic immune activation of the host by helminthic infections, so commonly found in developing countries, may play an important role in the dissemination of AIDS and TB in these countries. Moreover, effective vaccinations may fail in areas where helminthic parasites are endemic, due to the persistent immune activation and the accompanying immune hyporesponsiveness status of the population. We hope that this review, which compiles data demonstrating the direct contribution of chronic immune activation to anergy, will further strengthen this notion and enhance the understanding that eradication of persistent parasitic infections may be a prerequisite for effective protective vaccination in areas where parasitic infections are endemic.
FIG. 5.
Possible interference of helminthic and/or HIV-1 infections on the capacity of the host to mount immune responses to exogenous antigens or agents. Infection with helminthic parasites and HIV-1 induce (i) an increase in the threshold for effective immune stimulation (e.g., upregulation in CTLA-4), (ii) a defective intracellular signaling (e.g., phosphorylation and dephosphorylation of kinases), (iii) a decrease in the number of costimulatory molecules necessary for effective antigen presentation (e.g., CD28), (iv) an increase in the number of T-regulatory/inhibitory cells, (v) an increase in the levels of negative regulators of T-cell activation (e.g., Cbl-b), (vi) an increase in the levels of downregulatory cytokines (e.g., TGF-β), and (vii) an imbalance in the proportion of T cells (e.g., a decrease in the relative number of CD4+ cells). All of these changes contribute to hyporesponsiveness and anergy of the immune system. Coinfection with helminths and HIV-1 exacerbates this process and may also result in accelerated progression to AIDS.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Actor, J. K., M. Shirai, M. C. Kullberg, R. M. Buller, A. Sher, and J. A. Berzofsky. 1993. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc. Natl. Acad. Sci. USA 90:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams, J. F., E. H. Scholvinck, R. P. Gie, P. C. Potter, N. Beyers, and A. D. Beyers. 1999. Decline in total serum IgE after treatment for tuberculosis. Lancet 353:2030-2033. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad, R., S. T. Sindhu, E. Toma, R. Morisset, and A. Ahmad. 2002. Elevated levels of circulating interleukin-18 in human immunodeficiency virus-infected individuals: role of peripheral blood mononuclear cells and implications for AIDS pathogenesis. J. Virol. 76:12448-12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberola-Ila, J., and G. Hernandez-Hoyos. 2003. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 191:79-96. [DOI] [PubMed] [Google Scholar]
- 6.Almeida, C. A., M. A. Romano-Silva, and A. M. Goes. 1998. Inhibition of protein kinases prevents lymphocyte activation by Schistosoma mansoni antigens and reduces in vitro [correction of in vivo] granuloma reaction. Immunol. Lett. 62:137-143. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, R. W., M. S. Ascher, and H. W. Sheppard. 1998. Direct HIV cytopathicity cannot account for CD4 decline in AIDS in the presence of homeostasis: a worst-case dynamic analysis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:245-252. [DOI] [PubMed] [Google Scholar]
- 8.Andreassen, J. 1997. Interactions between intestinal tapeworms and their hosts: present knowledge and problems. Parassitologia 39:259-267. [PubMed] [Google Scholar]
- 9.Ascher, M. S., and H. W. Sheppard. 1988. AIDS as immune system activation: a model for pathogenesis. Clin. Exp. Immunol. 73:165-167. [PMC free article] [PubMed] [Google Scholar]
- 10.Atochina, O., T. Daly-Engel, D. Piskorska, E. McGuire, and D. A. Harn. 2001. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1+ macrophages that suppress naive CD4+ T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J. Immunol. 167:4293-4302. [DOI] [PubMed] [Google Scholar]
- 11.Ayash-Rashkovsky, M., Z. Weisman, J. Diveley, R. B. Moss, Z. Bentwich, and G. Borkow. 2002. Generation of Th1 immune responses to inactivated, gp120-depleted HIV-1 in mice with a dominant Th2 biased immune profile via immunostimulatory oligonucleotides—relevance to AIDS vaccines in developing countries. Vaccine 20:2684-2692. [DOI] [PubMed] [Google Scholar]
- 12.Ayash-Rashkovsky, M., Z. Weisman, S. Zlotnikov, E. Raz, Z. Bentwich, and G. Borkow. 2001. Induction of antigen-specific Th1-biased immune responses by plasmid DNA in Schistosoma-infected mice with a preexistent dominant Th2 immune profile. Biochem. Biophys. Res. Commun. 282:1169-1176. [DOI] [PubMed] [Google Scholar]
- 13.Azuma, M., and L. L. Lanier. 1995. The role of CD28 costimulation in the generation of cytotoxic T lymphocytes. Curr. Top. Microbiol. Immunol. 198:59-74. [DOI] [PubMed] [Google Scholar]
- 14.Azuma, M., J. H. Phillips, and L. L. Lanier. 1993. CD28-T lymphocytes. Antigenic and functional properties. J. Immunol. 150:1147-1159. [PubMed] [Google Scholar]
- 15.Beasley, N. M., A. M. Tomkins, A. Hall, C. M. Kihamia, W. Lorri, B. Nduma, W. Issae, C. Nokes, and D. A. Bundy. 1999. The impact of population level deworming on the haemoglobin levels of schoolchildren in Tanga, Tanzania. Trop. Med. Int. Health 4:744-750. [DOI] [PubMed] [Google Scholar]
- 16.Beattie, T., S. Rowland-Jones, and R. Kaul. 2002. HIV-1 and AIDS: what are protective immune responses? J. HIV Ther. 7:35-39. [PubMed] [Google Scholar]
- 17.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 18.Bentwich Z, Z. Weisman, Z. Grossman, Galai N, and Kalinkovich A. 1997. Pathogenesis of AIDS in Africa: lessons from the Ethiopian immigrants in Israel. Immunologist 5:21-26. [Google Scholar]
- 19.Bentwich, Z., A. Kalinkovich, and Z. Weisman. 1995. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol. Today 16:187-191. [DOI] [PubMed] [Google Scholar]
- 20.Bentwich, Z., A. Kalinkovich, Z. Weisman, G. Borkow, N. Beyers, and A. D. Beyers. 1999. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol. Today 20:485-487. [DOI] [PubMed] [Google Scholar]
- 21.Bentwich, Z., A. Kalinkovich, Z. Weisman, and Z. Grossman. 1998. Immune activation in the context of HIV infection. Clin. Exp. Immunol. 111:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bentwich, Z., G. Maartens, D. Torten, A. A. Lal, and R. B. Lal. 2000. Concurrent infections and HIV pathogenesis. AIDS 14:2071-2081. [DOI] [PubMed] [Google Scholar]
- 23.Bentwich, Z., Z. Weisman, Z. Grossman, N. Galai, and A. Kalinkovich. 1997. Pathogenesis of AIDS in Africa—lessons from the Ethiopian immigrants in Israel. Immunologist 5:211-226. [Google Scholar]
- 24.Bentwich, Z., Z. Weisman, C. Moroz, S. Bar-Yehuda, and A. Kalinkovich. 1996. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections? Clin. Exp. Immunol. 103:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyers, A. D., A. van Rie, J. Adams, G. Fenhalls, R. Gie, and N. Beyers. 1998. Signals that regulate the host response to Mycobacterium tuberculosis. Novartis Found. Symp. 217:145-157. [PubMed] [Google Scholar]
- 27.Bhandoola, A., E. A. Cho, K. Yui, H. U. Saragovi, M. I. Greene, and H. Quill. 1993. Reduced CD3-mediated protein tyrosine phosphorylation in anergic CD4+ and CD8+ T cells. J. Immunol. 151:2355-2367. [PubMed] [Google Scholar]
- 28.Biard-Piechaczyk, M., V. Robert-Hebmann, V. Richard, J. Roland, R. A. Hipskind, and C. Devaux. 2000. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120). Virology 268:329-344. [DOI] [PubMed] [Google Scholar]
- 29.Bleed, D., C. Dye, and M. C. Raviglione. 2000. Dynamics and control of the global tuberculosis epidemic. Curr. Opin. Pulm. Med. 6:174-179. [DOI] [PubMed] [Google Scholar]
- 30.Bluestone, J. A., and A. K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol 3:253-257. [DOI] [PubMed] [Google Scholar]
- 31.Borkow, G., and Z. Bentwich. 2000. Eradication of helminthic infections may be essential for successful vaccination against HIV and tuberculosis. Bull. W. H. O. 78:1368-1369. [PMC free article] [PubMed] [Google Scholar]
- 32.Borkow, G., and Z. Bentwich. 2002. Host background immunity and human immunodeficiency virus protective vaccines: a major consideration for vaccine efficacy in Africa and in developing countries. Clin. Diagn. Lab. Immunol. 9:505-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borkow, G., Q. Leng, Z. Weisman, M. Stein, N. Galai, A. Kalinkovich, and Z. Bentwich. 2000. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J. Clin. Investig. 106:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borkow, G., Z. Weisman, Q. Leng, M. Stein, A. Kalinkovich, D. Wolday, and Z. Bentwich. 2001. Helminths, human immunodeficiency virus and tuberculosis. Scand. J. Infect. Dis. 33:568-571. [DOI] [PubMed] [Google Scholar]
- 35.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 36.Boucher, N., T. Dufeu-Duchesne, E. Vicaut, D. Farge, R. B. Effros, and F. Schachter. 1998. CD28 expression in T cell aging and human longevity. Exp. Gerontol. 33:267-282. [DOI] [PubMed] [Google Scholar]
- 37.Boussiotis, V. A., G. J. Freeman, A. Berezovskaya, D. L. Barber, and L. M. Nadler. 1997. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rapl. Science 278:124-128. [DOI] [PubMed] [Google Scholar]
- 38.Boussiotis, V. A., G. J. Freeman, J. G. Gribben, and L. M. Nadler. 1996. The role of B7-1/B7-2:CD28/CLTA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol. Rev. 153:5-26. [DOI] [PubMed] [Google Scholar]
- 39.Boyer, J. D., B. Wang, K. E. Ugen, M. Agadjanyan, A. Javadian, P. Frost, K. Dang, R. A. Carrano, R. Ciccarelli, L. Coney, W. V. Williams, and D. B. Weiner. 1996. In vivo protective anti-HIV immune responses in non-human primates through DNA immunization. J. Med. Primatol. 25:242-250. [DOI] [PubMed] [Google Scholar]
- 40.Brooker, S., M. Rowlands, L. Haller, L. Savioli, and D. A. Bundy. 2000. Towards an atlas of human helminth infection in sub-Saharan Africa: the use of geographical information systems (GIS). Parasitol. Today 16:303-307. [DOI] [PubMed] [Google Scholar]
- 41.Brown, H. A., and F. A. Neva. 1983. Basic clinical parasitology. Appleton-Century-Crofts, East Norwalk, Conn.
- 42.Brunner, M. C., C. A. Chambers, F. K. Chan, J. Hanke, A. Winoto, and J. P. Allison. 1999. CTLA-4-mediated inhibition of early events of T cell proliferation. J. Immunol. 162:5813-5820. [PubMed] [Google Scholar]
- 43.Bundy, D., A. Sher, and E. Michael. 2000. Good worms or bad worms: do worm infections affect the epidemiological patterns of other diseases? Parasitol. Today 16:273-274. [DOI] [PubMed] [Google Scholar]
- 44.Bundy, D. A., and N. R. de Silva. 1998. Can we deworm this wormy world? Br. Med. Bull. 54:421-432. [DOI] [PubMed] [Google Scholar]
- 45.Bundy, D. A., and G. F. Medley. 1992. Immuno-epidemiology of human geohelminthiasis: ecological and immunological determinants of worm burden. Parasitology. 104(Supp1):S105-S119. [DOI] [PubMed] [Google Scholar]
- 46.Capobianchi, M. R. 1996. Induction of lymphomonocyte activation by HIV-1 glycoprotein gp120. Possible role in AIDS pathogenesis. J. Biol. Regul. Homeostatic Agents 10:83-91. [PubMed] [Google Scholar]
- 47.Casella, C. R., and T. H. Finkel. 1997. Mechanisms of lymphocyte killing by HIV. Curr. Opin. Hematol. 4:24-31. [DOI] [PubMed] [Google Scholar]
- 48.Chambers, C. A., T. J. Sullivan, and J. P. Allison. 1997. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 7:885-895. [DOI] [PubMed] [Google Scholar]
- 49.Chan, M. S. 1997. The global burden of intestinal nematode infections—fifty years on. Parasitol. Today 13:438-443. [DOI] [PubMed] [Google Scholar]
- 50.Reference deleted.
- 51.Chan, M. S., G. F. Medley, D. Jamison, and D. A. Bundy. 1994. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology 109:373-387. [DOI] [PubMed] [Google Scholar]
- 52.Chatenoud, L., B. Salomon, and J. A. Bluestone. 2001. Suppressor T cells—they're back and critical for regulation of autoimmunity! Immunol. Rev. 182:149-163. [DOI] [PubMed] [Google Scholar]
- 53.Chen, W., W. Jin, and S. M. Wahl. 1998. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4+ T cells. J. Exp. Med. 188:1849-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chirmule, N., T. W. McCloskey, R. Hu, V. S. Kalyanaraman, and S. Pahwa. 1995. HIV gp120 inhibits T cell activation by interfering with expression of costimulatory molecules CD40 ligand and CD80 (B71). J. Immunol. 155:917-924. [PubMed] [Google Scholar]
- 55.Clerici, M., and G. M. Shearer. 2001. The TH1-TH2 hypothesis of HIV infection: new insights. Immunol. Today 12:575-581. [DOI] [PubMed] [Google Scholar]
- 56.Clerici, M., C. Balotta, L. Meroni, E. Ferrario, C. Riva, D. Trabattoni, A. Ridolfo, M. Villa, G. M. Shearer, M. Moroni, and M. Galli. 1996. Type 1 cytokine production and low prevalence of viral isolation correlate with long-term nonprogression in HIV infection. AIDS Res. Hum. Retroviruses 12:1053-1061. [DOI] [PubMed] [Google Scholar]
- 57.Clerici, M., J. V. Giorgi, C. C. Chou, V. K. Gudeman, J. A. Zack, P. Gupta, H. N. Ho, P. G. Nishanian, J. A. Berzofsky, and G. M. Shearer. 1992. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J. Infect. Dis. 165:1012-1019. [DOI] [PubMed] [Google Scholar]
- 58.Clerici, M., J. M. Levin, H. A. Kessler, A. Harris, J. A. Berzofsky, A. L. Landay, and G. M. Shearer. 1994. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA 271:42-46. [PubMed] [Google Scholar]
- 59.Clerici, M., and G. M. Shearer. 1993. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol. Today 14:107-111. [DOI] [PubMed] [Google Scholar]
- 60.Coovadia, H. M., P. Jeena, and D. Wilkinson. 1998. Childhood human immunodeficiency virus and tuberculosis co-infections: reconciling conflicting data. Int. J. Tuberc. Lung Dis. 2:844-851. [PubMed] [Google Scholar]
- 61.Correa-Oliveira, R., L. C. Malaquias, P. L. Falcao, I. R. Viana, L. M. Bahia-Oliveira, A. M. Silveira, L. A. Fraga, A. Prata, R. L. Coffman, J. R. Lambertucci, J. R. Cunha-Melo, O. A. Martins-Filho, R. A. Wilson, and G. Gazzinelli. 1998. Cytokines as determinants of resistance and pathology in human Schistosoma mansoni infection. Braz. J. Med. Biol. Res 31:171-177. [DOI] [PubMed] [Google Scholar]
- 62.Cupp, C., J. P. Taylor, K. Khalili, and S. Amini. 1993. Evidence for stimulation of the transforming growth factor beta 1 promoter by HIV-1 Tat in cells derived from CNS. Oncogene 8:2231-2236. [PubMed] [Google Scholar]
- 63.Dalgleish, A. G., and K. J. O'Byrne. 2002. Chronic immune activation and inflammation in the pathogenesis of AIDS and cancer. Adv. Cancer Res. 84:231-276. [DOI] [PubMed] [Google Scholar]
- 64.Dalod, M., M. Sinet, J. C. Deschemin, S. Fiorentino, A. Venet, and J. G. Guillet. 1999. Altered ex vivo balance between CD28+ and CD28− cells within HIV-specific CD8+ T cells of HIV-seropositive patients. Eur. J. Immunol. 29:38-44. [DOI] [PubMed] [Google Scholar]
- 65.De Maria, A., C. Cirillo, and L. Moretta. 1994. Occurrence of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T cell activity in apparently uninfected children born to HIV-1-infected mothers. J. Infect. Dis. 170:1296-1299. [DOI] [PubMed] [Google Scholar]
- 66.DeSilva, D. R., W. S. Feeser, E. J. Tancula, and P. A. Scherle. 1996. Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J. Exp. Med. 183:2017-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeSilva, D. R., E. A. Jones, W. S. Feeser, E. J. Manos, and P. A. Scherle. 1997. The p38 mitogen-activated protein kinase pathway in activated and anergic Th1 cells. Cell. Immunol. 180:116-123. [DOI] [PubMed] [Google Scholar]
- 68.de Silva, N. R., M. S. Chan, and D. A. Bundy. 1997. Morbidity and mortality due to ascariasis: re-estimation and sensitivity analysis of global numbers at risk. Trop. Med. Int. Health 2:519-528. [DOI] [PubMed] [Google Scholar]
- 69.Despommier, D. D., R. W. Gwadz, and P. J. Hotez. 1995. Parasitic diseases. Springer-Verlag, New York, N.Y.
- 70.Despommier, D. D., and J. W. Karapelou. 1988. Parasite life cycles. Springer-Verlag, New York, N.Y.
- 71.Doetze, A., J. Satoguina, G. Burchard, T. Rau, C. Loliger, B. Fleischer, and A. Hoerauf. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int. Immunol. 12:623-630. [DOI] [PubMed] [Google Scholar]
- 72.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 73.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 74.Ekkens, M. J., Z. Liu, Q. Liu, A. Foster, J. Whitmire, J. Pesce, A. H. Sharpe, J. F. Urban, and W. C. Gause. 2002. Memory Th2 effector cells can develop in the absence of B7-1/B7-2, CD28 interactions, and effector Th cells after priming with an intestinal nematode parasite. J. Immunol. 168:6344-6351. [DOI] [PubMed] [Google Scholar]
- 75.El Garem, A. A. 1998. Schistosomiasis. Digestion 59:589-605. [DOI] [PubMed] [Google Scholar]
- 76.Elias, D., D. Wolday, H. Akuffo, B. Petros, U. Bronner, and S. Britton. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guérin (BCG) vaccination. Clin. Exp. Immunol. 123:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elliott, A. M., J. Kyosiimire, M. A. Quigley, J. Nakiyingi, C. Watera, M. Brown, S. Joseph, N. French, C. F. Gilks, and J. A. G. Withworth. 2003. Eosinophilia and progression to active tuberculosis in HIV-1-infected Ugandans. Trans. R. Soc. Trop. Med. Hyg. 97:477-480. [DOI] [PubMed] [Google Scholar]
- 78.Elliott, A. M., P. A. Mawa, S. Joseph, P. B. Namujju, J. S. Nakiyingi, C. Watera, D. W. Dunne, and J. A. G. Withworth. 2003. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans. R. Soc. Trop. Med. Hyg. 97:103-108. [DOI] [PubMed] [Google Scholar]
- 79.Else, K. J., and R. K. Grencis. 1991. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology 72:508-513. [PMC free article] [PubMed] [Google Scholar]
- 80.Elson, L. H., S. Shaw, R. A. Van Lier, and T. B. Nutman. 1994. T cell subpopulation phenotypes in filarial infections: CD27 negativity defines a population greatly enriched for Th2 cells. Int. Immunol. 6:1003-1009. [DOI] [PubMed] [Google Scholar]
- 81.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 82.Fadok, V. A., D. L. Bratton, D. M. Rose, A. Pearson, R. A. Ezekewitz, and P. M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405:85-90. [DOI] [PubMed] [Google Scholar]
- 83.Fagnoni, F. F., R. Vescovini, G. Passeri, G. Bologna, M. Pedrazzoni, G. Lavagetto, A. Casti, C. Franceschi, M. Passeri, and P. Sansoni. 2000. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood 95:2860-2868. [PubMed] [Google Scholar]
- 84.Fahey, J. L., J. M. Taylor, B. Manna, P. Nishanian, N. Aziz, J. V. Giorgi, and R. Detels. 1998. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS 12:1581-1590. [DOI] [PubMed] [Google Scholar]
- 85.Falcao, P. L., L. C. Malaquias, O. A. Martins-Filho, A. M. Silveira, V. M. Passos, A. Prata, G. Gazzinelli, R. L. Coffman, and R. Correa-Oliveira. 1998. Human schistosomiasis mansoni: IL-10 modulates the in vitro granuloma formation. Parasite Immunol. 20:447-454. [DOI] [PubMed] [Google Scholar]
- 86.Farah, I. O., P. W. Mola, T. M. Kariuki, M. Nyindo, R. E. Blanton, and C. L. King. 2000. Repeated exposure induces periportal fibrosis in Schistosoma mansoni-infected baboons: role of TGF-beta and IL-4. J. Immunol. 164:5337-5343. [DOI] [PubMed] [Google Scholar]
- 87.Fields, P. E., T. F. Gajewski, and F. W. Fitch. 1996. Blocked Ras activation in anergic CD4+ T cells. Science 271:1276-1278. [DOI] [PubMed] [Google Scholar]
- 88.Fontenot, J. D., M. A. Gavin, and A. Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330-336. [DOI] [PubMed] [Google Scholar]
- 89.Fowke, K. R., N. J. Nagelkerke, J. Kimani, J. N. Simonsen, A. O. Anzala, J. J. Bwayo, K. S. MacDonald, E. N. Ngugi, and F. A. Plummer. 1996. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet 348:1347-1351. [DOI] [PubMed] [Google Scholar]
- 90.Francois, B. J. 2003. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 3:189-198. [DOI] [PubMed] [Google Scholar]
- 91.Frauwirth, K. A., M. L. Alegre, and C. B. Thompson. 2000. Induction of T cell anergy in the absence of CTLA-4/B7 interaction. J. Immunol. 164:2987-2993. [DOI] [PubMed] [Google Scholar]
- 92.Gallin, M., K. Edmonds, J. J. Ellner, K. D. Erttmann, A. T. White, H. S. Newland, H. R. Taylor, and B. M. Greene. 1988. Cell-mediated immune responses in human infection with Onchocerca volvulus. J. Immunol. 140:1999-2007. [PubMed] [Google Scholar]
- 93.Gandhi, R. T., B. K. Chen, S. E. Straus, J. K. Dale, M. J. Lenardo, and D. Baltimore. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J. Exp. Med. 187:1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghosh, M. K., A. K. Ghosh, M. Addy, A. Nandy, and A. C. Ghose. 1996. Subpopulations of T lymphocytes in the peripheral blood and lymph nodes of Indian kala-azar patients. Med. Microbiol. Immunol. 185:183-187. [DOI] [PubMed] [Google Scholar]
- 95.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859-870. [DOI] [PubMed] [Google Scholar]
- 96.Goletti, D., D. Weissman, R. W. Jackson, N. M. Graham, D. Vlahov, R. S. Klein, S. S. Munsiff, L. Ortona, R. Cauda, and A. S. Fauci. 1996. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J. Immunol. 157:1271-1278. [PubMed] [Google Scholar]
- 97.Gopinath, R., M. Ostrowski, S. J. Justement, A. S. Fauci, and T. B. Nutman. 2000. Filarial infections increase susceptibility to human immunodeficiency virus infection in peripheral blood mononuclear cells in vitro. J. Infect. Dis. 182:1804-1808. [DOI] [PubMed] [Google Scholar]
- 98.Gorelik, L., and R. A. Flavell. 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2:46-53. [DOI] [PubMed] [Google Scholar]
- 99.Goulder, P. J., and B. D. Walker. 1999. The great escape—AIDS viruses and immune control. Nat. Med. 5:1233-1235. [DOI] [PubMed] [Google Scholar]
- 100.Graziosi, C., G. Pantaleo, K. R. Gantt, J. P. Fortin, J. F. Demarest, O. J. Cohen, R. P. Sekaly, and A. S. Fauci. 1994. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science 265:248-252. [DOI] [PubMed] [Google Scholar]
- 101.Graziosi, C., H. Soudeyns, G. P. Rizzardi, P. A. Bart, A. Chapuis, and G. Pantaleo. 1998. Immunopathogenesis of HIV infection. AIDS Res. Hum. Retroviruses 14(Suppl. 2):S135-S142. [PubMed] [Google Scholar]
- 102.Grossman, Z., Z. Bentwich, and R. B. Herberman. 1993. From HIV infection to AIDS: are the manifestations of effective immune resistance misinterpreted? Clin. Immunol. Immunopathol. 69:123-135. [DOI] [PubMed] [Google Scholar]
- 103.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 104.Grubeck-Loebenstein, B. 1997. Changes in the aging immune system. Biologicals 25:205-208. [DOI] [PubMed] [Google Scholar]
- 105.Gupta, M., K. L. Arora, S. Mithal, and B. N. Tandon. 1977. Effect of periodic deworming on nutritional status of Ascaris-infested preschool children receiving supplementary food. Lancet 2:108-110. [DOI] [PubMed] [Google Scholar]
- 106.Harnett, M. M., M. R. Deehan, D. M. Williams, and W. Harnett. 1998. Induction of signalling anergy via the T-cell receptor in cultured Jurkat T cells by pre-exposure to a filarial nematode secreted product. Parasite Immunol. 20:551-563. [DOI] [PubMed] [Google Scholar]
- 107.Harnett, W. and M. M. Harnett. 2001. Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes. Biochim. Biophys. Acta 1539:7-15. [DOI] [PubMed] [Google Scholar]
- 108.Havlir, D. V. and P. F. Barnes. 1999. Tuberculosis in patients with human immunodeficiency virus infection. N. Engl. J. Med. 340:367-373. [DOI] [PubMed] [Google Scholar]
- 109.Hay, C. M., D. J. Ruhl, N. O. Basgoz, C. C. Wilson, J. M. Billingsley, M. P. DePasquale, R. T. D'Aquila, S. M. Wolinsky, J. M. Crawford, D. C. Montefiori, and B. D. Walker. 1999. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J. Virol. 73:5509-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 17:1881-1888. [DOI] [PubMed] [Google Scholar]
- 111.Hazenberg, M. D., J. W. Stuart, S. A. Otto, J. C. Borleffs, C. A. Boucher, R. J. de Boer, F. Miedema, and D. Hamann. 2000. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood 95:249-255. [PubMed] [Google Scholar]
- 112.Helbert, M. R., J. L'age-Stehr, and N. A. Mitchison. 1993. Antigen presentation, loss of immunological memory and AIDS. Immunol. Today 14:340-344. [DOI] [PubMed] [Google Scholar]
- 113.Hoerauf, A., and N. Brattig. 2002. Resistance and susceptibility in human onchocerciasis—beyond Th1 vs. Th2. Trends Parasitol. 18:25-31. [DOI] [PubMed] [Google Scholar]
- 114.Hoffman, I. F., C. S. Jere, T. E. Taylor, P. Munthali, J. R. Dyer, J. J. Wirima, S. J. Rogerson, N. Kumwenda, J. J. Eron, S. A. Fiscus, H. Chakraborty, T. E. Taha, M. S. Cohen, and M. E. Molyneux. 1999. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS 13:487-494. [DOI] [PubMed] [Google Scholar]
- 115.Hofmann, B., P. Nishanian, T. Nguyen, P. Insixiengmay, and J. L. Fahey. 1993. Human immunodeficiency virus proteins induce the inhibitory cAMP/protein kinase A pathway in normal lymphocytes. Proc. Natl. Acad. Sci. USA 90:6676-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hogan, C. M., and S. M. Hammer. 2001. Host determinants in HIV infection and disease. 1. Cellular and humoral immune responses. Ann. Intern. Med. 134:761-776. [DOI] [PubMed] [Google Scholar]
- 117.Hu, R., N. Oyaizu, S. Than, V. S. Kalyanaraman, X. P. Wang, and S. Pahwa. 1996. HIV-1 gp160 induces transforming growth factor-beta production in human PBMC. Clin. Immunol. Immunopathol. 80:283-289. [DOI] [PubMed] [Google Scholar]
- 118.Infante-Duarte, C., and T. Kamradt. 1999. Th1/Th2 balance in infection. Springer Semin. Immunopathol. 21:317-338. [DOI] [PubMed] [Google Scholar]
- 119.Ishikawa, N., P. K. Goyal, Y. R. Mahida, K. F. Li, and D. Wakelin. 1998. Early cytokine responses during intestinal parasitic infections. Immunology 93:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jabado, N., A. Pallier, F. Le Deist, F. Bernard, A. Fischer, and C. Hivroz. 1997. CD4 ligands inhibit the formation of multifunctional transduction complexes involved in T cell activation. J. Immunol. 158:94-103. [PubMed] [Google Scholar]
- 121.Reference deleted.
- 122.John, T. J., and P. Jayabal. 1972. Oral polio vaccination of children in the tropics. 1. The poor seroconversion rates and the absence of viral interference. Am. J. Epidemiol. 96:263-269. [DOI] [PubMed] [Google Scholar]
- 123.June, C. H., J. A. Bluestone, L. M. Nadler, and C. B. Thompson. 1994. The B7 and CD28 receptor families. Immunol. Today 15:321-331. [DOI] [PubMed] [Google Scholar]
- 124.Jusot, J. F., P. Simarro, and A. De Muynck. 1996. Historical aspects of the risk factors of Schistosoma intercalatum schistosomiasis. Sante 6:165-172. (in French.) [PubMed]
- 125.Kalinkovich, A., G. Borkow, Z. Weisman, A. Tsimanis, M. Stein, and Z. Bentwich. 2001. Increased CCR5 and CXCR4 expression in Ethiopians living in israel: environmental and constitutive factors. Clin. Immunol. 100:107-117. [DOI] [PubMed] [Google Scholar]
- 126.Kalinkovich, A., S. Maayan, Z. Weisman, N. Harpaz, and Z. Bentwich. 1994. Immune activation, a co-factor for HIV transmission in Thailand? Lancet 343:1506-1507. [DOI] [PubMed] [Google Scholar]
- 127.Kalinkovich, A., Z. Weisman, R. Burstein, and Z. Bentwich. 1998. Standard values of T-lymphocyte subsets in Africa. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:183-185. [DOI] [PubMed] [Google Scholar]
- 128.Kalinkovich, A., Z. Weisman, Z. Greenberg, J. Nahmias, S. Eitan, M. Stein, and Z. Bentwich. 1998. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin. Exp. Immunol. 114:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karin, M., and Y. Ben Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 130.Kaufmann, G. R., C. Duncombe, J. Zaunders, P. Cunningham, and D. Cooper. 1998. Primary HIV-1 infection: a review of clinical manifestations, immunologic and virologic changes. AIDS Patient Care STDs 12:759-767. [DOI] [PubMed] [Google Scholar]
- 131.Kekow, J., W. Wachsman, J. A. McCutchan, W. L. Gross, M. Zachariah, D. A. Carson, and M. Lotz. 1991. Transforming growth factor-beta and suppression of humoral immune responses in HIV infection. J. Clin. Investig. 87:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khalife, J., J. M. Grzych, R. Pierce, J. C. Ameisen, A. M. Schacht, H. Gras-Masse, A. Tartar, J. P. Lecocq, and A. Capron. 1990. Immunological crossreactivity between the human immunodeficiency virus type 1 virion infectivity factor and a 170-kD surface antigen of Schistosoma mansoni. J. Exp. Med. 172:1001-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khalife, J., R. J. Pierce, C. Godin, and A. Capron. 1994. Molecular characterization of two Schistosoma mansoni proteins sharing common motifs with the vif protein of HIV-1. Parasitology 108:533-542. [DOI] [PubMed] [Google Scholar]
- 134.Khattri, R., T. Cox, S. A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337-342. [DOI] [PubMed] [Google Scholar]
- 135.King, C. L., M. Connelly, M. P. Alpers, M. Bockarie, and J. W. Kazura. 2001. Transmission intensity determines lymphocyte responsiveness and cytokine bias in human lymphatic filariasis. J. Immunol. 166:7427-7436. [DOI] [PubMed] [Google Scholar]
- 136.King, C. L., S. Mahanty, V. Kumaraswami, J. S. Abrams, J. Regunathan, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1993. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Investig. 92:1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.King, C. L., A. Medhat, I. Malhotra, M. Nafeh, A. Helmy, J. Khaudary, S. Ibrahim, M. El Sherbiny, S. Zaky, R. J. Stupi, K. Brustoski, M. Shehata, and M. T. Shata. 1996. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J. Immunol. 156:4715-4721. [PubMed] [Google Scholar]
- 138.King, C. L., J. Xianli, C. H. June, R. Abe, and K. P. Lee. 1996. CD28-deficient mice generate an impaired Th2 response to Schistosoma mansoni infection. Eur. J. Immunol. 26:2448-2455. [DOI] [PubMed] [Google Scholar]
- 139.Klein, M. R., C. A. van Baalen, A. M. Holwerda, G. Kerkhof, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kornbluth, R. S. 2002. An expanding role for CD40L and other tumor necrosis factor superfamily ligands in HIV infection. J. Hematother. Stem Cell Res. 11:787-801. [DOI] [PubMed] [Google Scholar]
- 141.Kulkarni, P. S., S. T. Butera, and A. C. Duerr. 2003. Resistance to HIV-1 infection: lessons learned from studies of highly exposed persistently seronegative (HEPS) individuals. AIDS Rev. 5:87-103. [PubMed] [Google Scholar]
- 142.Langlade-Demoyen, P., N. Ngo-Giang-Huong, F. Ferchal, and E. Oksenhendler. 1994. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV-infected patients. J. Clin. Investig. 93:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lawn, S. D., S. T. Butera, and T. M. Folks. 2001. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 14:753-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lawn, S. D., D. M. Karanja, P. Mwinzia, J. Andove, D. G. Colley, T. M. Folks, and W. E. Secor. 2000. The effect of treatment of schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS 14:2437-2443. [DOI] [PubMed] [Google Scholar]
- 145.Leach, D. R., M. F. Krummel, and J. P. Allison. 1996. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734-1736. [DOI] [PubMed] [Google Scholar]
- 146.Lechler, R., J. G. Chai, F. Marelli-Berg, and G. Lombardi. 2001. T-cell anergy and peripheral T-cell tolerance. Philos. Trans. R. Soc. Lond. Ser. B 356:625-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee, K. M., E. Chuang, M. Griffin, R. Khattri, D. K. Hong, W. Zhang, D. Straus, L. E. Samelson, C. B. Thompson, and J. A. Bluestone. 1998. Molecular basis of T cell inactivation by CTLA-4. Science 282:2263-2266. [DOI] [PubMed] [Google Scholar]
- 148.Legendre, C., M. Raphael, G. Gras, E. A. Lefevre, J. Feuillard, D. Dormont, and Y. Richard. 1998. CD80 expression is decreased in hyperplastic lymph nodes of HIV+ patients. Int. Immunol. 10:1847-1851. [DOI] [PubMed] [Google Scholar]
- 149.Leng, Q., Z. Bentwich, and G. Borkow. 2002. CTLA-4 upregulation during aging. Mech. Ageing Dev. 123:1419-1421. [DOI] [PubMed] [Google Scholar]
- 150.Leng, Q., Z. Bentwich, E. Magen, A. Kalinkovich, and G. Borkow. 2002. CTLA-4 upregulation during HIV infection: association with anergy and possible target for therapeutic intervention. AIDS 16:519-529. [DOI] [PubMed] [Google Scholar]
- 151.Leng, Q., G. Borkow, and Z. Bentwich. 2002. Attenuated signaling associated with immune activation in HIV-1-infected individuals. Biochem. Biophys. Res. Commun. 298:464-467. [DOI] [PubMed] [Google Scholar]
- 152.Leng, Q., G. Borkow, Z. Weisman, M. Stein, A. Kalinkovich, and Z. Bentwich. 2001. Immune activation correlates better than HIV plasma viral load with CD4 T-cell decline during HIV infection. J. Acquir. Immune Defic. Syndr. 27:389-397. [DOI] [PubMed] [Google Scholar]
- 153.Leroy, E., S. Baize, G. Wahl, T. G. Egwang, and A. J. Georges. 1997. Experimental infection of a nonhuman primate with Loa loa induces transient strong immune activation followed by peripheral unresponsiveness of helper T cells. Infect. Immun. 65:1876-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Levy, J. A. 1993. Pathogenesis of human immunodeficiency virus infection. Microbiol. Rev. 57:183-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li, W., C. D. Whaley, A. Mondino, and D. L. Mueller. 1996. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science 271:1272-1276. [DOI] [PubMed] [Google Scholar]
- 156.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98:1667-1677. [DOI] [PubMed] [Google Scholar]
- 157.Linsley, P. S., J. L. Greene, W. Brady, J. Bajorath, J. A. Ledbetter, and R. Peach. 1994. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1:793-801. [DOI] [PubMed] [Google Scholar]
- 158.Linsley, P. S., and J. A. Ledbetter. 1993. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 11:191-212. [DOI] [PubMed] [Google Scholar]
- 159.Liu, Y. C., and H. Gu. 2002. Cbl and Cbl-b in T-cell regulation. Trends Immunol. 23:140-143. [DOI] [PubMed] [Google Scholar]
- 160.Looney, D. J. 1994. Immune responses to human immunodeficiency virus type 1 in exposed but uninfected individuals: protection or chance? J. Clin. Investig. 93:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lotz, M., and P. Seth. 1993. TGF beta and HIV infection. Ann. N. Y. Acad. Sci. 685:501-511. [DOI] [PubMed] [Google Scholar]
- 162.Loukas, A., and P. Prociv. 2001. Immune responses in hookworm infections. Clin. Microbiol. Rev. 14:689-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lyszkiewicz, M., and E. Pajtasz-Piasecka. 2002. Contribution of interleukin 2 and interleukin 12 in signal transduction during activation of the immune system. Postepy Hig. Med. Dosw. 56:707-731. [PubMed] [Google Scholar]
- 164.Maggi, E., M. Mazzetti, A. Ravina, F. Annunziato, M. de Carli, M. P. Piccinni, R. Manetti, M. Carbonari, A. M. Pesce, and G. Del Prete. 1994. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science 265:244-248. [DOI] [PubMed] [Google Scholar]
- 165.Mahanty, S., S. N. Mollis, M. Ravichandran, J. S. Abrams, V. Kumaraswami, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1996. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J. Infect. Dis. 173:769-773. [DOI] [PubMed] [Google Scholar]
- 166.Maizels, R. M., D. A. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 167.Maizels, R. M., and M. J. Holland. 1998. Parasite immunology: pathways for expelling intestinal helminths. Curr. Biol. 8:R711-R714. [DOI] [PubMed] [Google Scholar]
- 168.Makarov, S. S. 2000. NF-κB as a therapeutic target in chronic inflammation: recent advances. Mol. Med. Today 6:441-448. [DOI] [PubMed] [Google Scholar]
- 169.Malaquias, L. C., P. L. Falcao, A. M. Silveira, G. Gazzinelli, A. Prata, R. L. Coffman, V. Pizziolo, C. P. Souza, D. G. Colley, and R. Correa-Oliveira. 1997. Cytokine regulation of human immune response to Schistosoma mansoni: analysis of the role of IL-4, IL-5 and IL-10 on peripheral blood mononuclear cell responses. Scand. J. Immunol. 46:393-398. [DOI] [PubMed] [Google Scholar]
- 170.Malhotra, I., J. Ouma, A. Wamachi, J. Kioko, P. Mungai, A. Omollo, L. Elson, D. Kocch, J. W. Kazura, and C. L. King. 1997. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J. Clin. Investig. 99:1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Malinowski, K., and F. T. Rapaport. 1995. Effects of aging upon the expression of differentiation and class II MHC antigens on the surface of T lymphocytes from normal human subjects. Cell. Immunol. 162:68-73. [DOI] [PubMed] [Google Scholar]
- 172.Manson-Bahr, P. E. C., and D. R. Bell. 1987. Manson's tropical diseases. Ballière Tindall, London, United Kingdom.
- 173.Martins-Filho, O. A., J. R. Cunha-Melo, J. R. Lambertucci, A. M. Silveira, D. G. Colley, G. Gazzinelli, and R. Correa-Oliveira. 1999. Clinical forms of human Schistosoma mansoni infection are associated with differential activation of T-cell subsets and costimulatory molecules. Dig. Dis. Sci. 44:570-577. [DOI] [PubMed] [Google Scholar]
- 174.McCoy, K., M. Camberis, and G. L. Gros. 1997. Protective immunity to nematode infection is induced by CTLA-4 blockade. J. Exp. Med. 186:183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 176.Messele, T., M. Abdulkadir, A. L. Fontanet, B. Petros, D. Hamann, M. Koot, M. T. Roos, P. T. Schellekens, F. Miedema, and T. F. Rinke de Wit. 1999. Reduced naive and increased activated CD4 and CD8 cells in healthy adult Ethiopians compared with their Dutch counterparts. Clin. Exp. Immunol. 115:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Metz, D. P., D. L. Farber, T. Taylor, and K. Bottomly. 1998. Differential role of CTLA-4 in regulation of resting memory versus naive CD4 T cell activation. J. Immunol. 161:5855-5861. [PubMed] [Google Scholar]
- 178.Meyaard, L., and F. Miedema. 1995. Programmed death of T cells in HIV infection: result of immune activation? Curr. Top. Microbiol. Immunol. 200:213-221. [DOI] [PubMed] [Google Scholar]
- 179.Michael, E., D. A. Bundy, and B. T. Grenfell. 1996. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology 112:409-428. [DOI] [PubMed] [Google Scholar]
- 180.Miedema, F. 1992. Immunological abnormalities in the natural history of HIV infection: mechanisms and clinical relevance. Immunodefic. Rev. 3:173-193. [PubMed] [Google Scholar]
- 181.Milman, G., and M. P. D'Souza. 1994. HIV-mediated defects in immune regulation. AIDS Res. Hum. Retroviruses 10:421-430. [DOI] [PubMed] [Google Scholar]
- 182.Miyamoto, Y. J., B. F. Andruss, J. S. Mitchell, M. J. Billard, and B. W. McIntyre. 2003. Diverse roles of integrins in human T lymphocyte biology. Immunol. Res. 27:71-84. [DOI] [PubMed] [Google Scholar]
- 183.Mola, P. W., I. O. Farah, T. M. Kariuki, M. Nyindo, R. E. Blanton, and C. L. King. 1999. Cytokine control of the granulomatous response in Schistosoma mansoni-infected baboons: role of exposure and treatment. Infect. Immun. 67:6565-6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mondino, A., C. D. Whaley, D. R. DeSilva, W. Li, M. K. Jenkins, and D. L. Mueller. 1996. Defective transcription of the IL-2 gene is associated with impaired expression of c-Fos, FosB, and JunB in anergic T helper 1 cells. J. Immunol. 157:2048-2057. [PubMed] [Google Scholar]
- 185.Morford, L. A., L. H. Elliott, S. L. Carlson, W. H. Brooks, and T. L. Roszman. 1997. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J. Immunol. 159:4415-4425. [PubMed] [Google Scholar]
- 186.Morgan, J. A., R. J. Dejong, S. D. Snyder, G. M. Mkoji, and E. S. Loker. 2001. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology 123(Suppl.):S211-S228. [DOI] [PubMed] [Google Scholar]
- 187.Morio, T., T. Chatila, and R. S. Geha. 1997. HIV glycoprotein gp120 inhibits TCR-CD3-mediated activation of fyn and lck. Int. Immunol. 9:53-64. [DOI] [PubMed] [Google Scholar]
- 188.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 189.Mosmann, T. R., S. Sad, L. Krishnan, T. G. Wegmann, L. J. Guilbert, and M. Belosevic. 1995. Differentiation of subsets of CD4+ and CD8+ T cells. Ciba Found. Symp. 195:42-50. [DOI] [PubMed] [Google Scholar]
- 190.Muller, R., and J. R. Baker. 1990. Medical parasitology. JB Lippincott Co., Philadelphia, Pa.
- 191.Muro, A., C. Genchi, M. Cordero, and F. Simon. 1999. Human dirofilariasis in the European Union. Parasitol. Today 15:386-389. [DOI] [PubMed] [Google Scholar]
- 192.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 193.Navikas, V., J. Link, B. Wahren, C. Persson, and H. Link. 1994. Increased levels of interferon-gamma (IFN-γ), IL-4 and transforming growth factor-beta (TGF-β) mRNA expressing blood mononuclear cells in human HIV infection. Clin. Exp. Immunol. 96:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ng, T. T., S. B. Kanner, M. J. Humphries, R. G. Wickremasinghe, K. E. Nye, J. Anderson, S. H. Khoo, and W. J. Morrow. 1997. The integrin-triggered rescue of T lymphocyte apoptosis is blocked in HIV-1-infected individuals. J. Immunol. 158:2984-2999. [PubMed] [Google Scholar]
- 195.Niehues, T., J. Ndagijimana, G. Horneff, and V. Wahn. 1998. CD28 expression in pediatric human immunodeficiency virus infection. Pediatr. Res. 44:265-268. [DOI] [PubMed] [Google Scholar]
- 196.Nutman, T. B., and V. Kumaraswami. 2001. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 23:389-399. [DOI] [PubMed] [Google Scholar]
- 197.Oh, S. K., W. W. Cruikshank, J. Raina, G. C. Blanchard, W. H. Adler, J. Walker, and H. Kornfeld. 1992. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J. Acquir. Immune Defic. Syndr. 5:251-256. [PubMed] [Google Scholar]
- 198.Olsen, A., L. Mubila, and A. L. Willingham III. 2001. Human helminth infections—future research foci. Trends Parasitol. 17:303-305. [DOI] [PubMed] [Google Scholar]
- 199.Pantaleo, G., and A. S. Fauci. 1996. Immunopathogenesis of HIV infection. Annu. Rev. Microbiol. 50:825-854. [DOI] [PubMed] [Google Scholar]
- 200.Pantaleo, G., C. Graziosi, and A. S. Fauci. 1993. The role of lymphoid organs in the immunopathogenesis of HIV infection. AIDS 7(Suppl. 1):S19-S23. [PubMed] [Google Scholar]
- 201.Patriarca, P. A., P. F. Wright, and T. J. John. 1991. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev. Infect. Dis. 13:926-939. [DOI] [PubMed] [Google Scholar]
- 202.Pearce, E. J., P. Caspar, J. M. Grzych, F. A. Lewis, and A. Sher. 1991. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 173:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pearce, E. J., A. La Flamme, E. Sabin, and L. R. Brunet. 1998. The initiation and function of Th2 responses during infection with Schistosoma mansoni. Adv. Exp. Med. Biol. 452:67-73. [DOI] [PubMed] [Google Scholar]
- 204.Pearlman, E., J. W. Kazura, F. E. Hazlett, and W. H. Boom. 1993. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J. Immunol. 151:4857-4864. [PubMed] [Google Scholar]
- 205.Perelson, A. S., P. Essunger, and D. D. Ho. 1997. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS 11(Suppl. A):S17-S24. [PubMed] [Google Scholar]
- 206.Perelson, A. S., A. U. Neumann, M. Markowitz, J. M. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582-1586. [DOI] [PubMed] [Google Scholar]
- 207.Perez, V. L., L. van Parijs, A. Biuckians, X. X. Zheng, T. B. Strom, and A. K. Abbas. 1997. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity 6:411-417. [DOI] [PubMed] [Google Scholar]
- 208.Peters, W., and H. M. Gilles. 1989. A colour atlas of tropical medicine and parasitology. Wolfe Medical Publications, London, United Kingdom.
- 209.Pinto, L. A., J. Sullivan, J. A. Berzofsky, M. Clerici, H. A. Kessler, A. L. Landay, and G. M. Shearer. 1995. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J. Clin. Investig. 96:867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Plummer, F. A., T. B. Ball, J. Kimani, and K. R. Fowke. 1999. Resistance to HIV-1 infection among highly exposed sex workers in Nairobi: what mediates protection and why does it develop? Immunol. Lett. 66:27-34. [DOI] [PubMed] [Google Scholar]
- 211.Poli, G., and A. S. Fauci. 1992. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res. Hum. Retroviruses 8:191-197. [DOI] [PubMed] [Google Scholar]
- 212.Powell, J. D., J. A. Ragheb, S. Kitagawa-Sakakida, and R. H. Schwartz. 1998. Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunol. Rev. 165:287-300. [DOI] [PubMed] [Google Scholar]
- 213.Prociv, P. 2001. Gastrointestinal worm infections. The prevalence and treatment in Australia. Aust. Fam. Physician 30:755-761. [PubMed] [Google Scholar]
- 214.Propato, A., E. Schiaffella, E. Vicenzi, V. Francavilla, L. Baloni, M. Paroli, L. Finocchi, N. Tanigaki, S. Ghezzi, R. Ferrara, R. Chesnut, B. Livingston, A. Sette, R. Paganelli, F. Aiuti, G. Poli, and V. Barnaba. 2001. Spreading of HIV-specific CD8+ T-cell repertoire in long-term nonprogressors and its role in the control of viral load and disease activity. Hum. Immunol. 62:561-576. [DOI] [PubMed] [Google Scholar]
- 215.Putkonen, P., B. Makitalo, D. Bottiger, G. Biberfeld, and R. Thorstensson. 1997. Protection of human immunodeficiency virus type 2-exposed seronegative macaques from mucosal simian immunodeficiency virus transmission. J. Virol. 71:4981-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rea, I. M., S. E. McNerlan, and H. D. Alexander. 1999. CD69, CD25, and HLA-DR activation antigen expression on CD3+ lymphocytes and relationship to serum TNF-α, IFN-γ, and sIL-2R levels in aging. Exp. Gerontol. 34:79-93. [DOI] [PubMed] [Google Scholar]
- 217.Reinherz, E. L., and S. F. Schlossman. 1980. Current concepts in immunology: regulation of the immune response—inducer and suppressor T-lymphocyte subsets in human beings. N. Engl. J. Med. 303:370-373. [DOI] [PubMed] [Google Scholar]
- 218.Remoue, F., D. Van To, A. M. Schacht, M. Picquet, O. Garraud, J. Vercruysse, A. Ly, A. Capron, and G. Riveau. 2001. Gender-dependent specific immune response during chronic human Schistosomiasis haematobia. Clin. Exp. Immunol. 124:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Riley, J. L., K. Schlienger, P. J. Blair, B. Carreno, N. Craighead, D. Kim, R. G. Carroll, and C. H. June. 2000. Modulation of susceptibility to HIV-1 infection by the cytotoxic T lymphocyte antigen 4 costimulatory molecule. J. Exp. Med. 191:1987-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rinaldo, C., X. L. Huang, Z. F. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, and M. Cottrill. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69:5838-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rizzardini, G., S. Piconi, S. Ruzzante, M. L. Fusi, M. Lukwiya, S. Declich, M. Tamburini, M. L. Villa, M. Fabiani, F. Milazzo, and M. Clerici. 1996. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. AIDS 10:1535-1542. [DOI] [PubMed] [Google Scholar]
- 222.Romagnani, S., and E. Maggi. 1994. Th1 versus Th2 responses in AIDS. Curr. Opin. Immunol. 6:616-622. [DOI] [PubMed] [Google Scholar]
- 223.Romagnani, S., E. Maggi, and G. Del Prete. 1994. HIV can induce a TH1 to TH0 shift, and preferentially replicates in CD4+ T-cell clones producing TH2-type cytokines. Res. Immunol. 145:611-617. [DOI] [PubMed] [Google Scholar]
- 224.Rowland-Jones, S. L., T. Dong, K. R. Fowke, J. Kimani, P. Krausa, H. Newell, T. Blanchard, K. Ariyoshi, J. Oyugi, E. Ngugi, J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 1998. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Investig. 102:1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rowland-Jones, S. L., S. Pinheiro, R. Kaul, P. Hansasuta, G. Gillespie, T. Dong, F. A. Plummer, J. B. Bwayo, S. Fidler, J. Weber, A. McMichael, and V. Appay. 2001. How important is the ‘quality’ of the cytotoxic T lymphocyte (CTL) response in protection against HIV infection? Immunol. Lett. 79:15-20. [DOI] [PubMed] [Google Scholar]
- 226.Rudd, C. E., and H. Schneider. 2000. Lymphocyte signaling: Cbl sets the threshold for autoimmunity. Curr. Biol. 10:R344-R347. [DOI] [PubMed] [Google Scholar]
- 227.Sabin, E. A., M. I. Araujo, E. M. Carvalho, and E. J. Pearce. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 173:269-272. [DOI] [PubMed] [Google Scholar]
- 228.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18-32. [DOI] [PubMed] [Google Scholar]
- 229.Seger, R., and E. G. Krebs. 1995. The MAPK signaling cascade. FASEB J. 9:726-735. [PubMed] [Google Scholar]
- 230.Selliah, N., and T. H. Finkel. 1998. Cutting edge: JAK3 activation and rescue of T cells from HIV gp120-induced unresponsiveness. J. Immunol. 160:5697-5701. [PubMed] [Google Scholar]
- 231.Sercarz, E., and U. Krzych. 1991. The distinctive specificity of antigen-specific suppressor T cells. Immunol. Today 12:111-118. [DOI] [PubMed] [Google Scholar]
- 232.Shapira-Nahor, O., A. Kalinkovich, Z. Weisman, Z. Greenberg, J. Nahmias, M. Shapiro, A. Panet, and Z. Bentwich. 1998. Increased susceptibility to HIV-1 infection of peripheral blood mononuclear cells from chronically immune-activated individuals. AIDS 12:1731-1733. [PubMed] [Google Scholar]
- 233.Shearer, G. M., and M. Clerici. 1993. Abnormalities of immune regulation in human immunodeficiency virus infection. Pediatr. Res. 33:S71-S74. [DOI] [PubMed] [Google Scholar]
- 234.Sher, A., D. Fiorentino, P. Caspar, E. Pearce, and T. Mosmann. 1991. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J. Immunol. 147:2713-2716. [PubMed] [Google Scholar]
- 235.Sher, A., R. T. Gazzinelli, I. P. Oswald, M. Clerici, M. Kullberg, E. J. Pearce, J. A. Berzofsky, T. R. Mosmann, S. L. James, and H. C. Morse III. 1992. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol. Rev. 127:183-204. [DOI] [PubMed] [Google Scholar]
- 236.Shevach, E. M. 2001. Certified professionals: CD4+CD25+ suppressor T cells. J. Exp. Med. 193:F41-F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Shevach, E. M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389-400. [DOI] [PubMed] [Google Scholar]
- 238.Shrikant, P., A. Khoruts, and M. F. Mescher. 1999. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 11:483-493. [DOI] [PubMed] [Google Scholar]
- 239.Silvestri, G., and M. B. Feinberg. 2003. Turnover of lymphocytes and conceptual paradigms in HIV infection. J. Clin. Investig. 112:821-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 241.Singleton, L., M. Turner, R. Haskal, S. Etkind, M. Tricarico, and E. Nardell. 1997. Long-term hospitalization for tuberculosis control. Experience with a medical-psychosocial inpatient unit. JAMA 278:838-842. [PubMed] [Google Scholar]
- 242.Soboslay, P. T., S. M. Geiger, N. Weiss, M. Banla, C. G. Luder, C. M. Dreweck, E. Batchassi, B. A. Boatin, A. Stadler, and H. Schulz-Key. 1997. The diverse expression of immunity in humans at distinct states of Onchocerca volvulus infection. Immunology 90:592-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Soltys, J., P. K. Goyal, and D. Wakelin. 1999. Cellular immune responses in mice infected with the intestinal nematode Trichuris muris. Exp. Parasitol. 92:40-47. [DOI] [PubMed] [Google Scholar]
- 244.Sousa, A. E., J. Carneiro, M. Meier-Schellersheim, Z. Grossman, and R. M. Victorino. 2002. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J. Immunol. 169:3400-3406. [DOI] [PubMed] [Google Scholar]
- 245.Srikanth, P., R. C. Castillo, G. Sridharan, T. J. John, A. Zachariah, D. Mathai, and D. H. Schwartz. 2000. Increase in plasma IL-10 levels and rapid loss of CD4+ T cells among HIV-infected individuals in south India. Int. J STD AIDS 11:49-51. [DOI] [PubMed] [Google Scholar]
- 246.Stadecker, M. J., and H. J. Hernandez. 1998. The immune response and immunopathology in infection with Schistosoma mansoni: a key role of major egg antigen Sm-p40. Parasite Immunol. 20:217-221. [DOI] [PubMed] [Google Scholar]
- 247.Steel, C., and T. B. Nutman. 2003. CTLA-4 in filarial infections: implications for a role in diminished T cell reactivity. J. Immunol. 170:1930-1938. [DOI] [PubMed] [Google Scholar]
- 248.Steiner, K., I. Waase, T. Rau, M. Dietrich, B. Fleischer, and B. M. Broker. 1999. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin. Exp. Immunol. 115:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stylianou, E., P. Aukrust, D. Kvale, F. Muller, and S. S. Froland. 1999. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression—down-regulatory effect of potent anti-retroviral therapy. Clin. Exp. Immunol. 116:115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Stylianou, E., P. Aukrust, I. Nordoy, F. Muller, and S. S. Froland. 2000. Enhancement of lymphocyte proliferation induced by interleukin-12 and anti-interleukin-10 in HIV-infected patients during highly active antiretroviral therapy. APMIS 108:601-607. [DOI] [PubMed] [Google Scholar]
- 251.Tamma, S. M., N. Chirmule, T. W. McCloskey, N. Oyaizu, V. S. Kalyanaraman, and S. Pahwa. 1997. Signals transduced through the CD4 molecule interfere with TCR/CD3-mediated ras activation leading to T cell anergy/apoptosis. Clin. Immunol. Immunopathol. 85:195-201. [DOI] [PubMed] [Google Scholar]
- 252.Terrazas, L. I., K. L. Walsh, D. Piskorska, E. McGuire, and D. A. Harn, Jr. 2001. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1+ cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4+ cells: a potential mechanism for immune polarization in helminth infections. J. Immunol. 167:5294-5303. [DOI] [PubMed] [Google Scholar]
- 253.Thomis, D. C., and L. J. Berg. 1997. The role of Jak3 in lymphoid development, activation, and signaling. Curr. Opin. Immunol. 9:541-547. [DOI] [PubMed] [Google Scholar]
- 254.Thompson, C. B., and J. P. Allison. 1997. The emerging role of CTLA-4 as an immune attenuator. Immunity 7:445-450. [DOI] [PubMed] [Google Scholar]
- 255.Tomaras, G. D., and M. L. Greenberg. 2001. CD8 + T cell mediated noncytolytic inhibition of human immunodeficiency virus type 1. Front. Biosci. 6:D575-D598. [DOI] [PubMed] [Google Scholar]
- 256.Torres, M., T. Herrera, H. Villareal, E. A. Rich, and E. Sada. 1998. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect. Immun. 66:176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.UNAIDS. 2001. Report on global HIV-AIDS epidemic. United Nations, Geneva Switzerland.
- 258.UNAIDS. 2002. AIDS epidemic update. United Nations, Geneva, Switzerland.
- 259.Urassa, W. K., E. M. Mbena, A. B. Swai, H. Gaines, F. S. Mhalu, and G. Biberfeld. 2003. Lymphocyte subset enumeration in HIV seronegative and HIV-1 seropositive adults in Dar es Salaam, Tanzania: determination of reference values in males and females and comparison of two flow cytometric methods. J. Immunol. Methods 277:65-74. [DOI] [PubMed] [Google Scholar]
- 260.Valdez, H., and M. M. Lederman. 1997. Cytokines and cytokine therapies in HIV infection. AIDS Clin. Rev. 1997-1998:187-228. [PubMed] [Google Scholar]
- 261.van Dam, G. J., F. F. Stelma, B. Gryseels, S. T. Falcao Ferreira, I. Talla, M. Niang, J. P. Rotmans, and A. M. Deelder. 1996. Antibody response patterns against Schistosoma mansoni in a recently exposed community in Senegal. J. Infect. Dis. 173:1232-1241. [DOI] [PubMed] [Google Scholar]
- 262.van Den Biggelaar, A. H., J. L. Grogan, Y. Filie, R. Jordens, P. G. Kremsner, F. Koning, and M. Yazdanbakhsh. 2000. Chronic schistosomiasis: dendritic cells generated from patients can overcome antigen-specific T cell hyporesponsiveness. J. Infect. Dis. 182:260-265. [DOI] [PubMed] [Google Scholar]
- 263.Villa, O. F., and R. E. Kuhn. 1996. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology 112:561-570. [DOI] [PubMed] [Google Scholar]
- 264.Vingerhoets, J., M. Dohlsten, G. Penne, R. Colebunders, D. Sansom, E. Bosmans, L. Kestens, and G. Vanham. 1998. Superantigen activation of CD4+ and CD8+ T cells from HIV-infected subjects: role of costimulatory molecules and antigen-presenting cells (APC). Clin. Exp. Immunol. 111:12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.von Herrath, M. G., and L. C. Harrison. 2003. Antigen-induced regulatory T cells in autoimmunity. Nat. Rev. Immunol. 3:223-232. [DOI] [PubMed] [Google Scholar]
- 266.Wakikawa, A., M. Utsuyama, and K. Hirokawa. 1997. Altered expression of various receptors on T cells in young and old mice after mitogenic stimulation: a flow cytometric analysis. Mech. Ageing Dev. 94:113-122. [DOI] [PubMed] [Google Scholar]
- 267.Walunas, T. L., C. Y. Bakker, and J. A. Bluestone. 1996. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 183:2541-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Weisman, Z., A. Kalinkovich, G. Borkow, M. Stein, Z. Greenberg, and Z. Bentwich. 1999. Infection by different HIV-1 subtypes (B and C) results in a similar immune activation profile despite distinct immune backgrounds. J. Acquir. Immune Defic. Syndr. 21:157-163. [PubMed] [Google Scholar]
- 269.White, A. C., Jr., P. Robinson, and R. Kuhn. 1997. Taenia solium cysticercosis: host-parasite interactions and the immune response. Chem. Immunol. 66:209-230. [PubMed] [Google Scholar]
- 270.Williams, M. E., P. Caspar, I. Oswald, H. K. Sharma, O. Pankewycz, A. Sher, and S. L. James. 1995. Vaccination routes that fail to elicit protective immunity against Schistosoma mansoni induce the production of TGF-β, which down-regulates macrophage antiparasitic activity. J. Immunol. 154:4693-4700. [PubMed] [Google Scholar]
- 271.Wolday, D., N. Berhe, H. Akuffo, and S. Britton. 1999. Leishmania-HIV interaction: immunopathogenic mechanisms. Parasitol. Today 15:182-187. [DOI] [PubMed] [Google Scholar]
- 272.Reference deleted.
- 273.Wood, K. J., and S. Sakaguchi. 2003. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 3:199-210. [DOI] [PubMed] [Google Scholar]
- 274.World Health Organization. 2003. WHO Mapping Initiative. Africa geohelminths and Schistosoma. World Health Organization, Geneva, Switzerland.
- 275.Wulfing, C., and M. M. Davis. 1998. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 282:2266-2269. [DOI] [PubMed] [Google Scholar]
- 276.Yazdanbakhsh, M. 1999. Common features of T cell reactivity in persistent helminth infections: lymphatic filariasis and schistosomiasis. Immunol. Lett. 65:109-115. [DOI] [PubMed] [Google Scholar]
- 277.Yazdanbakhsh, M., A. van den Biggelaar, and R. M. Maizels. 2001. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 22:372-377. [DOI] [PubMed] [Google Scholar]
- 278.Zanussi, S., C. Simonelli, M. D'Andrea, C. Caffau, M. Clerici, U. Tirelli, and P. DePaoli. 1996. CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin. Exp. Immunol. 105:220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]