Abstract
Hundreds of viruses cause central nervous system (CNS) disease, including meningoencephalitis and postinfectious encephalomyelitis, in humans. The cerebrospinal fluid (CSF) is abnormal in >90% of cases; however, routine CSF studies only rarely lead to identification of a specific etiologic agent. Diagnosis of viral infections of the CNS has been revolutionized by the advent of new molecular diagnostic technologies to amplify viral nucleic acid from CSF, including PCR, nucleic acid sequence-based amplification, and branched-DNA assay. PCR is ideally suited for identifying fastidious organisms that may be difficult or impossible to culture and has been widely applied for detection of both DNA and RNA viruses in CSF. The technique can be performed rapidly and inexpensively and has become an integral component of diagnostic medical practice in the United States and other developed countries. In addition to its use for identification of etiologic agents of CNS disease in the clinical setting, PCR has also been used to quantitate viral load and monitor duration and adequacy of antiviral drug therapy. PCR has also been applied in the research setting to help discriminate active versus postinfectious immune-mediate disease, identify determinants of drug resistance, and investigate the etiology of neurologic disease of uncertain cause. This review discusses general principles of PCR and reverse transcription-PCR, including qualitative, quantitative, and multiplex techniques, with comment on issues of sensitivity, specificity, and positive and negative predictive values. The application of molecular diagnostic methods for diagnosis of specific infectious entities is reviewed in detail, including viruses for which PCR is of proven efficacy and is widely available, viruses for which PCR is less widely available or for which PCR has unproven sensitivity and specificity, and nonviral entities which can mimic viral CNS disease.
INTRODUCTION
Over 100 viruses are known to cause acute viral encephalitis in humans (251). Viruses which infect the central nervous system (CNS) can selectively involve the spinal cord (myelitis), the brain stem (e.g., rhombencephalitis), the cerebellum (cerebellitis), or the cerebrum (encephalitis). Almost all acute viral infections of the CNS produce some degree of meningeal as well as parenchymal inflammation. The cardinal clinical and laboratory findings are largely similar regardless of the inciting agent and consist of fever, headache, and altered mental status, which are often accompanied by seizures and focal neurologic abnormalities. The cerebrospinal fluid (CSF) is abnormal in >90% of cases, typically consisting of a lymphocytic pleocytosis, mildly elevated protein, and normal glucose. In rare instances, such as West Nile virus (WNV) meningoencephalitis or cytomegalovirus (CMV) radiculomyelitis, polymorphonuclear cells rather than lymphocytes may be the predominant cell type, and this may provide a diagnostic clue. However, despite these variations, routine CSF studies only rarely lead to identification of a specific etiologic agent.
From a practical point of view, a clinician confronted with a patient with fever, headache, and altered mental status must initially distinguish encephalitis from noninfectious causes of brain dysfunction (encephalopathy). Having made this distinction, it is next necessary to distinguish cases in which brain injury is a direct consequence of viral infection from cases in which it occurs as a consequence of a postinfectious immune-mediated process (e.g., acute disseminated encephalomyelitis). Finally, the goal in cases of encephalitis is to identify a specific etiologic agent, with particular emphasis on diseases that require acute treatment, such as herpes simplex encephalitis (HSE) (17, 64, 190, 250, 252).
Diagnosis of viral infections of the CNS has been revolutionized by the advent of new molecular diagnostic technologies, such as the PCR to amplify viral nucleic acid from CSF (65, 230, 244, 261). Despite the use of newer techniques, including CSF PCR, up to 70% of cases of encephalitis remain of unknown etiology in modern surveys (100).
DISTINGUISHING ENCEPHALITIS FROM ENCEPHALOPATHY AND POSTINFECTIOUS ENCEPHALITIS
Encephalitis versus Encephalopathy
From a neuropathological perspective, the cardinal difference between encephalitis and encephalopathy is the presence of inflammation. Patients with encephalopathy have focal or generalized dysfunction of neurons and supporting cells without inflammation and as a result are far less likely to have either fever or headache. The inflammatory response engendered by infection also typically results in both peripheral leukocytosis and CSF pleocytosis, both of which are absent in encephalopathy. Direct viral infection often results in areas of focal CNS injury that are reflected in focal abnormalities on magnetic resonance imaging (MRI) or other neuroimaging studies and in focal discharges on electroencephalography (EEG). Conversely, the more diffuse nature of brain dysfunction in encephalopathy typically induces generalized slowing without focal features on EEG and nonfocal neuroimaging studies. Although encephalopathy usually results from noninfectious processes, it is important to recognize that some infectious agents induce CNS dysfunction in the absence of either direct invasion or induction of a postinfectious immune-mediated response. Viral culture and/or PCR is unlikely to be positive in cases of encephalopathy (64).
Encephalitis versus Postinfectious Encephalomyelitis
It has been recognized for over a century that certain types of vaccination or infection with certain organisms could be followed 1 to 3 weeks later by a multifocal inflammatory demyelinating process (acute disseminated encephalomyelitis [ADEM]) that could involve cranial nerves (e.g., optic nerve), the cerebrum, the brain stem, and the spinal cord (60). ADEM appears to result from an immune-mediated attack against an antigen or antigens present in brain myelin. Despite intensive investigations, the precipitating infection for ADEM remains unknown in at least one-third of cases. The most commonly identified antecedent causes of postinfectious encephalomyelitis in North America are respiratory tract infections, while measles is the most important antecedent agent in the developing world. Key features that help in differentiating ADEM from encephalitis include a history of vaccination or a prodromal illness in the weeks preceding neurologic signs and symptoms. In ADEM, demyelination involving the optic nerve or spinal cord can result in monocular visual loss or symptoms of spinal cord dysfunction, which are generally rare in acute encephalitis. Exceptions to this rule can occur with West Nile virus and enteroviral infections, in which a poliomyelitis-like acute flaccid paralysis may at times be the dominant clinical feature. MRI in ADEM typically shows multifocal lesions with a predilection for the white matter (see above), whereas acute encephalitis usually produces lesions that involve both gray and white matter. In classic encephalitis the brunt of injury typically involves the cerebrum. Although direct viral infection can be associated with both cerebellitis and brain stem encephalitis (BSE), the presence of cerebellar and brain stem lesions associated with cerebral involvement should suggest the possibility of ADEM. The CSF profiles are similar in ADEM and acute viral encephalitis, although oligoclonal bands are more common in ADEM than in acute encephalitis. A positive CSF PCR for viral nucleic acid or positive viral cultures from CSF are strongly suggestive of direct infection rather than postinfectious immune-mediated disease (64).
GENERAL PRINCIPLES OF MOLECULAR DIAGNOSTIC METHODS AS APPLIED TO THE DIAGNOSIS OF CNS INFECTION
CSF PCR
General comments.
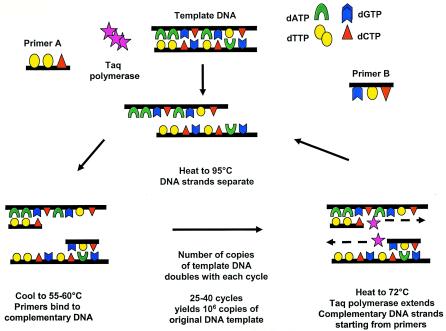
PCR can be applied to the diagnosis of any disease in which nucleic acids (e.g., DNA and RNA) or their expression as mRNA plays a role (65). PCR techniques allow for the in vitro synthesis of millions of copies of a specific gene segment of interest, allowing the rapid detection of as few as 1 to 10 copies of target DNA from the original sample (Fig. 1). PCR has been widely used for detection of both DNA and RNA viruses in CSF. The widespread availability of an in-laboratory-developed test in most hospitals or ready access to a reference laboratory has led to the incorporation of PCR testing of CSF and body fluids as an integral component of diagnostic medical practice in the United States and other developed countries. Although less commonly utilized in clinical practice, other nucleic acid amplification techniques have successfully been utilized for detection of viral nucleic acid in cerebrospinal fluid, including nucleic acid sequence-based amplification (NASBA) and branched-DNA assay (48). In addition to the aforementioned clinical utility of PCR in identifying etiologic agents of CNS disease in specific patients and in quantitation of viral load to monitor duration and adequacy of antiviral drug therapy in the setting of various viral infections (such as human immunodeficiency virus [HIV] and neonatal herpes simplex virus [HSV]), multiple other potential uses are being evaluated on a research basis. These include (i) identification of productive viral infection versus postinfectious immune-mediated disease, such as identifying the nature of relapses following HSV encephalitis; (ii) identification of determinants of drug resistance, such as sequencing of amplified genes including antiviral targets; and (iii) investigation of the etiologies of neurologic diseases of uncertain case, such as multiple sclerosis, Alzheimer's disease, and other neurodegenerative disorders. The discussion in this review focuses on the appropriate use of the PCR method and its application to the diagnosis of viral encephalitis (Tables 1 and 2).
FIG. 1.
General PCR schema.
TABLE 1.
Summary of PCR testing for viral encephalitis
| Organism | Sensitivity | Specificity | Comments | References |
|---|---|---|---|---|
| Viruses | ||||
| Adenovirus | Unknown | Unknown | 120, 219 | |
| Arboviruses | Unknown (not standardized) | Unknown | 107, 138, 153, 209 | |
| WNV | 60% | CSF serology is more sensitive | 30, 108, 140, 230, 249 | |
| BK virus | Unknown | Unknown | 20 | |
| Enterovirus | >95% | >95% | 6, 160, 178, 180, 187, 192, 193, 195, 200, 203-206, 239, 240, 246 | |
| Herpesviruses | ||||
| CMV | 82-100% in immunocompromised patients, ≥60% in congenital CMV infection | 86-100% | Quantitation available to monitor response to therapy and predict disease severity | 13, 21, 31, 45, 47, 48, 51-57, 98, 211, 215, 220, 221, 237, 254, 260 |
| EBV | 98.5% as tumor marker in HIV patients with CNS lymphoma | 100% | Predictive value in normal hosts is unclear; quantitation available for monitoring response to therapy and possibly assessing risk of CNS disease in HIV patients | 10-12, 26, 44, 50, 56, 63, 87, 102, 106, 110, 119, 141, 155, 170, 171, 183, 219-221, 223, 233, 248 |
| HHV-6 | >95% | Poor positive predictive value for disease (30% positivity in normal hosts) | 22, 34, 42, 73, 91, 109, 111, 128, 130-132, 145, 152, 222, 227, 228, 234, 259 | |
| HSV-1 and -2 | >95% | >95% | Quantitation available (see text for multiple uses) | 9, 16, 43, 52, 68-71, 83, 84, 102, 118, 123-127, 137, 139, 143, 156, 159, 163, 174-76, 182, 191, 212, 214, 229, 231, 232, 235, 236, 238, 243, 247, 253 |
| VZV | 80-95% in immunocompromised patients | >95% | 8, 59, 66, 75, 93-97, 112, 129, 134, 135 | |
| HIV | HIV RNA is present at all stages | >95% | Quantitation available, correlates with likelihood of neurologic disease, and is useful for monitoring response to antiretroviral therapy | 29, 32, 45-51, 53, 57, 77, 99, 151, 172, 184 |
| HTLV-1 and -2 | 90% for HAM/TSP | 90% | Quantitation available and may predict risk of neurologic disease | 149, 162, 173 |
| JC virus | 50-75% for PML | 98-100% | Quantitation available for assessing response to therapy and correlates with prognosis | 20, 63, 67, 72, 76, 81, 88, 89, 106, 133, 157, 202, 223, 225, 226, 245, 258 |
| Measles virus | Unknown | Unknown | Quantitation available to monitor load in SSPE patients in response to therapy | 105, 123, 136, 150, 242 |
| Rabies virus | 100% | 100% | 24, 61, 241 | |
| Nonviral entities in differential diagnosis | ||||
| Mycoplasma | Unknown | Sensitivity is difficult to assess in setting of clinical disease, since both active infection and autoimmune mechanisms of disease exist | 1, 23, 164, 210 | |
| M. tuberculosis | Variable (33-90%) | 88-100% | 18, 25, 40, 101, 169, 213, 230 | |
| B. burgdorferi (Lyme disease) | 17% for neuroborreliosis | Serologic diagnosis more sensitive | 33, 142, 143 | |
| Toxoplasma | Variable (50-75%) in HIV patients | Variable | 19, 58, 62, 116, 117 |
TABLE 2.
Summary of available diagnostic methods for viral meningitis and encephalitisa
| Virus | Effectivenessb of diagnosis by:
|
Other specimens | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR
|
Serologyc
|
Culture of specimens
|
|||||||
| Serum | CSF | Serum | CSF | Throat | Rectal | Blood | CSF | ||
| Adenovirus | − | + | +/− | +/− | ++ | + | − | + | Conjunctival swab, stool electron microscopy |
| Arboviruses | +d | +d | ++ | ++ | − | − | ++ | +/− | |
| WNV | +d | +d | ++ | ++ | − | − | − | − | Serology preferable |
| Enteroviruses | |||||||||
| Nonpoliovirus | + | ++ | + | − | + | ++ | +/− | ++ | |
| Poliovirus | + | ++ | +e | − | + | ++ | − | − | Urine |
| Herpesviruses | |||||||||
| CMV | + | ++ | + | ++ | + | − | + | Rare | Urine |
| EBV | + | + | ++ | ++ | +/− | − | +/− | +/− | |
| HHV-6 | +/− | +/− | +/− | − | − | − | +d | +d | |
| HSV-1, and HSV-2 | − | ++ | +/− | + | − | − | − | − | Skin vesicle, brain tissuef |
| VZV | ++ | + | +e | ++ | − | − | − | + | Skin vesiclef |
| HIV | ++ | + | + | − | − | − | +/− | − | |
| Influenza virus | − | − | +/− | − | ++f | − | − | − | |
| JCV | − | ++ | − | − | − | − | − | − | |
| Lymphocytic chorlomen- ingitis virus | − | + | ++ | ++ | +/− | − | + | ++ | Urine |
| Mumps virus | − | − | +e | + | ++ | − | +/− | ++ | Urine, saliva |
| Measles virus | − | +d | +e | + | + | − | + | − | Urine |
| Parainfluenza virus | − | − | +/− | − | ++ | − | − | − | |
| Parvovirus | ++ | − | + | − | − | − | − | − | |
| Rabies virus | − | + | ++ | ++ | − | − | − | + | Saliva, brain tissue, nuchal skin biopsy, corneal impressionsf |
Reprinted with permission from the American Academy of Neurology (65a).
++, extremely effective for diagnosis; +, effective for diagnosis; +/−, variable effectiveness; −, not useful.
Serologic indexing which compares CSF- to serum-specific antibody levels in reference to total CSF and serum albumin or total immunoglobulin may be required for definitive diagnosis. A fourfold rise in IgG from acute- and convalescent-phase specimens or a single positive IgM may also be diagnostic.
Test not widely available but can be sent to research labs.
Serology may be difficult to interpret for vaccinated patients or in a postviral setting. In these patients, the presence of IgM indicates active or reactivated viral disease rather than past immunity.
Direct fluorescent-antibody test (DFA) on tissues or secretions is more rapid and may be more sensitive than culture methods. The direct-fluorescent-antibody test has replaced Tzanck prep, which is less sensitive and specific.
CSF PCR for diagnosis of viral CNS infection.
One of the most successful applications of CSF PCR is the diagnosis of viral nervous system infections. PCR is ideally suited for identifying fastidious organisms that may be difficult or impossible to culture. The technique can be performed rapidly and inexpensively, with a turnaround time of 24 h or less rather than the standard minimum of 1 to 28 days required for culture. Unlike traditional culture methods that may yield negative results after a patient receives even small doses of antiviral drugs, CSF PCR retains its sensitivity after short courses of antiviral therapy or passive immunization. This allows the prompt initiation of empirical therapy when a patient first presents with suspected meningitis or encephalitis, without potentially compromising definitive diagnostic tests. PCR is also preferable to serologic testing, which often requires 2 to 4 weeks after acute infection for development of a diagnostic rise in antibody titers and is of limited value for viruses with high basal seroprevalence rates. In contrast to serologic testing, CSF PCR yields positive results during acute infection, when the amount of replicating virus is maximal. Finally, CSF PCR is substantially less invasive than brain biopsy, which was previously the “gold standard” for diagnosis of many CNS herpesvirus infections.
CSF specimen handling and preparation.
Minimal amounts of specimen are required for CSF PCR testing; however, the sensitivity of PCR assays is related to the volume of specimen tested. Most assays can be run on a minimum of 30 μl of sample, but 100 to 200 μl may yield better results. The PCR is optimally run on freshly obtained CSF specimens; however, positive results have been obtained from archival specimens frozen for years. In general, the stability of RNA viruses in CSF samples is less that of DNA viruses. The decline in sensitivity over long-term storage has not been rigorously studied, but there is no appreciable loss in yield with brief storage at 4 or −20°C. If long-term archival storage is anticipated, specimens should be stored at −70°C and multiple freeze-thaw cycles should be avoided.
Prior to entry into the PCR, nucleic acids present in CSF must be made accessible. Although simple methods such as exposure to high temperature or repetitive freeze-thawing have been utilized to release nucleic acids, nucleic extraction and purification techniques, such as phenol-chloroform or spin column-based (Qiagen) techniques, have the advantage of providing pure nucleic acid for entry into the amplification reaction, along with removal of potential inhibitors of the PCR amplification reaction as well as substances that may degrade nucleic acid and reduce yield.
Detection of amplified products. (i) Qualitative PCR.
Traditionally, the amplified PCR product is electrophoresed on an agarose gel and is transferred to a nitrocellulose membrane. The membrane is hybridized with a specific probe complementary to the sequence of the desired PCR product and visualized with either radioactivity or chemiluminescence. More recently, colorimetric detection methods have been developed, entailing the use of biotinylated primers used within the amplification reaction and resulting in labeled amplicon PCR products. Unlabeled “capture” oligonucleotide probes (complementary to the internal sequence of the amplicon) are preapplied to 96-well plates, and the labeled PCR product is incubated with the plate. Avidin-horseradish peroxidase conjugate is added to detect bound amplicon. A colorimetric readout is utilized, which can be quantified by measuring absorbance at 450 nm in a microwell plate reader.
(ii) Quantitative PCR techniques.
An additional concern which may have an impact on the positive predictive value of a CSF PCR is that viruses which associate with peripheral blood mononuclear cells (PBMCs) (such as Epstein-Barr virus [EBV]) might be carried into CSF in a setting of inflammation, be detected by PCR (“bystander”), and lead to a spurious viral diagnosis. In actuality, this may be only a theoretical concern. In one study, only 11 of 2,222 specimens were positive for EBV by PCR, and in all cases the patients had illness consistent with EBV disease (CNS lymphoma, primary EBV infection, or bone marrow transplantation with fever) (180a). Quantitative PCR and reverse transcription-PCR (RT-PCR) have increasingly been applied to clinical samples. Several studies have suggested that nucleic acid copy number (viral load) may be a marker for the severity of disease or may help predict outcome, particularly in immunocompromised patients, although it remains to be determined whether this is true for all infections (2).
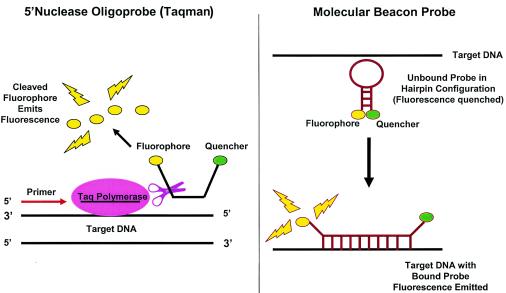
Qualitative PCR assays can be modified for quantification if an internal control is added at a known concentration or a standard curve is run in parallel with clinical specimens. However, real-time quantitative PCR techniques are more frequently utilized in clinical practice. In these assays, reagents that are capable of generating a fluorescent signal within the PCR amplification reaction can be monitored and quantified to reflect the starting amounts of nucleic acid template at each cycle of the PCR. Probe-based quantitative PCR methods rely on the use of a fluorescent reporter molecule that increases as product accumulates with each cycle of amplification (137) (Fig. 2). The reporter molecule probe is composed of a sequence-specific oligonucleotide with an integrated fluorophore and quencher, as in molecular beacon probes (e.g., Scorpion probes) or TaqMan probes. Molecular beacon probes are designed as a hairpin loop configuration with the fluorophore and quencher adjacent (nonfluorescent complex) in the unbound state (3, 80). Upon extension of the amplicon and probe hybridization, physical separation of the fluorophore and quencher occur and fluorescence is emitted. In the case of TaqMan probes, as primers anneal to the nucleic acid target and the PCR product is generated, the 5′-3′ nuclease activity of Taq polymerase cleaves the fluorescent probe, releasing the quencher from the fluorophore and leading to emission of fluorescence (3, 80, 137, 154).
FIG. 2.
Probes for probe-based quantitative PCR. In probe-based quantitative PCR, reagents which are capable of generating a fluorescent signal within the PCR can be monitored and quantified to reflect the starting amount of nucleic acid template at each cycle of the PCR. Probe-based quantitative PCR methods rely on the use of a fluorescent reporter molecule that increases as the PCR product accumulates, which is composed of a sequence-specific oligonucleotide with an integrated fluorophore and quencher, as in molecular beacon probes (Scorpion) and TaqMan probes. FRET-based PCR (not shown) makes use of dual sequence-specific oligonucleotide probes with either integrated 3′ donor or 5′ acceptor fluorophores; the PCR product is quantified as a function of measured energy transfer between adjacent donor and acceptor fluorophores.
More recently, fluorescence emission resonance transmission (FRET) technology has been utilized in probe-based real-time quantitative PCR (78, 243) For FRET-based PCR, an oligonucleotide probe with a donor fluorophore (i.e., fluorescein) linked to the 3′ end is excited by an external light source and emits light that is absorbed by a second oligonucleotide probe with an acceptor fluorophore (i.e., rhodamine) linked at the 5′ end. Since both fluorophore-labeled oligonucleotides are designed to hybridize to adjacent sections of the same DNA template, energy transfer between donor and acceptor, which is proportional to the amount of specific PCR product generated, can be monitored. Optimization of the separation between donor and acceptor is a critical aspect of FRET-based PCR: ground-state interference may occur between donor and acceptor if the separation is too little, whereas decreased energy transfer and sensitivity may occur if the separation is too great.
Regardless of the probe-based method used, real-time analysis at each step of amplicon production is monitored by assessing fluorescence intensity, which is directly proportional to the quantity of nucleic acid that was amplified from the original sample.
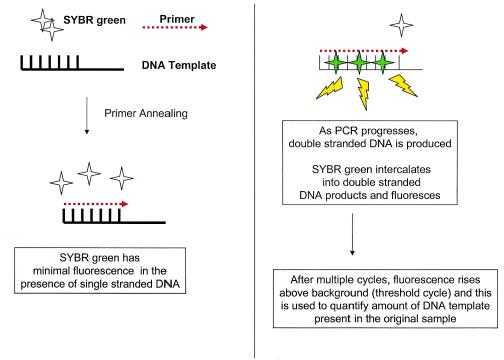
As an alternative to probe-based techniques, SYBR Green-based quantitative PCR utilizes a sensitive DNA binding dye that it binds to any double-stranded product generated during the PCR (Fig. 3). Thus, the same dye (and master mix) can be used in different quantitative PCR applications, in contrast to the case for probe methods that require synthesis of specific probes for each pathogen. Although intercalating dyes allow detection of nonspecific PCR products, the inclusion of a hot-start mechanism in the PCR reduces or eliminates mispriming events, which increases reaction specificity and helps ensure that only the desired product is measured (122, 144, 255). Postamplification melting curve analysis of PCR products, which detects sequence differences in target amplicons, is an additional safeguard to ensure the specificity of the PCR.
FIG. 3.
SYBR Green-based quantitative PCR. SYBR Green-based quantitative PCR utilizes a sensitive DNA binding dye that binds any double-stranded product generated by the PCR. Thus, the same dye can be used in different quantitative PCR approaches, but the specificity must be verified by other methods (see the text).
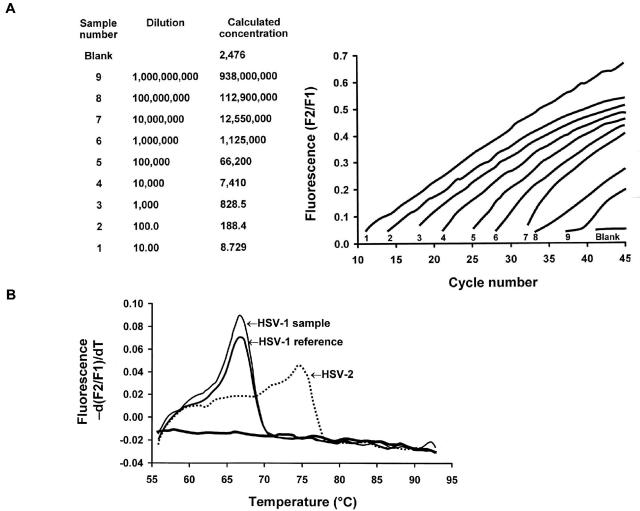
Regardless of whether probe-based or SYBR Green-based methods are utilized, a rapid thermal cycler platform (such as the Roche LightCycler and ABI Prism 7000) is frequently utilized to carry out and analyze the quantitative PCR (181). Amplification and quantification occur in tightly closed glass capillary tubes, making transfer of amplified products for detection unnecessary and thus decreasing risk of sample contamination substantially. A built-in microvolume fluorimeter rapidly quantifies amplified products every cycle, which shortens the time required for amplification to 30 to 45 min rather than 3 to 4 h. Data are immediately transferred to a computer, which allows monitoring of PCR kinetics in real-time (Fig. 4) (79). More sophisticated analyses such as melting curve analysis of all PCR products can allow distinction between specific viral strains within a single PCR with a single set of primers, as is seen in the case of HSV type 1 (HSV-1) and HSV-2 quantitative PCR.
FIG. 4.
Quantitative real-time PCR with melting curve analysis. (A) Detection of HSV DNA in a serially diluted suspension by LightCycler PCR with the FRET assay. The sequential numbers indicated at the base of each signal designation refer to the corresponding sample number and dilution (two left-hand columns). (B) Melting curve analysis of HSV-1 and HSV-2 genotypes determined by LightCycler PCR with the FRET assay. Reprinted from reference 79 with permission.
Sensitivity.
Although the PCR technique is inherently highly sensitive due to the millionfold amplification of the genome present in the tested sample, the exact sensitivity in particular clinical situations is not known for many organisms due to lack of a gold standard for comparison. For some organisms, sensitivity is low, leading to false-negative results. Factors which might contribute to low sensitivity (false negatives) include low viral load due to delay in obtaining the CSF specimen for testing or rapid clearance due to robust host neutralizing antibody responses. False-negative tests may also occur if endogenous polymerase inhibitors that interfere with the PCR are present in the CSF sample. Heme products from breakdown of erythrocytes may inhibit the PCR, but modest CSF xanthochromia, high protein levels, or high white blood cell counts do not have a negative impact on CSF PCR testing. In general, inhibitors such as endonucleases and exonucleases are much less commonly present in CSF than in other body fluids or tissues.
A variant of classical PCR termed nested PCR is utilized for analysis of specimens in which very few viral particles are presumed to be present (such as CSF), with the goal of substantially increasing the sensitivity and specificity of the PCR (224). In nested PCR, the first PCR is followed by an additional amplification with a second set of primers which are complementary to sequences internal to the sequence targeted by the first set of primers.
Replicating virus and viral nucleic acid do not persist indefinitely in infected patients, particularly in immunocompetent patients who mount an effective neutralizing antibody response. However, most CSF testing is performed in the clinical setting of suspected meningitis or meningoencephalomyelitis within 1 to 2 days following onset of neurologic symptoms, a time at which the yield from PCR testing is likely to be at its peak. Positive CSF PCR test results have been noted up to 4 weeks after onset of clinical symptoms, depending on the pathogen (244).
Specificity.
The specificity of the PCR must also be considered. The exquisite sensitivity of PCR is both its greatest virtue and greatest potential limitation. A positive CSF PCR result indicates the presence of viral nucleic acid. In general, a positive CSF PCR result indicates recent or ongoing active viral infection of the CNS by that particular pathogen, especially in immunocompetent individuals. Possible exceptions, in which a positive CSF PCR result does not indicate true CNS viral infection (false-positive result), include cases in which a breakdown of the blood-brain barrier (e.g., in severe bacterial meningitis) or contamination of the CSF with blood has occurred. In these cases, infectious nucleic acid may be carried into the CNS and detected as a contaminant, rather than reflecting local infection of the CNS itself. Some experts have also suggested that this scenario might also theoretically occur in the case of viral pathogens which preferentially infect leukocytes: viral genome present in circulating leukocytes may be carried into the CSF as bystanders during the host inflammatory response and detected by PCR (128, 244). Several other factors may contribute to the low specificity seen in some PCRs, such as nonspecific binding of primers to nontarget DNA, cross-reaction of primers with related viruses, and contamination of specimens. Methods to increase specificity (decrease false positives) of the PCR include stringent laboratory standards to avoid and detect contamination, such as inclusion of duplicate samples and positive and negative controls in each PCR run. A dedicated space for the PCR should be used, and positive pressure tips should be used for pipetting samples and reagents to limit the chance of carryover contamination. In addition, the specificity of all amplified PCR products should be confirmed by hybridizing the gel on which the product was electrophoresed and visualized with pathogen-specific probe.
Positive and negative predictive values.
The predictive value of any test depends not only on the specificity and sensitivity of the test but also on the prevalence of the pathogen or disease in the population in which it is being tested. Positive CSF PCR results may be more difficult to interpret for neurotropic pathogens which also cause non-CNS disease with a high baseline prevalence in the general population, since the frequency with which viral nucleic acid is detectable in CSF in the absence of clinical CNS disease is not well delineated. A good example of this is with regard to CSF PCR testing for human herpesvirus 6 (HHV-6) in immunocompetent hosts. CSF PCR testing for HHV-6 inherently has a low positive predictive value, since the viral genome has been detected in the CSF of healthy children in the absence of CNS disease (see “HHV-6” section below).
Multiplex and Consensus Primer PCRs
There has been great interest in the development of PCR assays that are capable of detecting multiple pathogens by PCR simultaneously in a single clinical CSF sample. Multiplex techniques utilize two or more primer pairs directed at pathogen-specific unique sequences within the same PCR. In order to preserve amplification efficiency, all primer pairs must be optimized for similar amplification conditions (48). Alternatively, consensus primer PCR makes use of a single primer set directed against sequences which are highly conserved among various viral species classified within a single viral family (for example, herpesviruses). In this case, a positive PCR result must be further characterized by hybridization with virus species-specific probes or analysis by restriction enzyme digestion.
Pan-herpesvirus assays.
Several groups has developed assays that contain primers for detection of HSV-1, HSV-2, varicella-zoster virus (VZV), CMV and EBV, and HHV-6 in various combinations (28, 35, 39, 86, 147, 158, 218). These assays may prove to be clinically useful in the diagnosis of HIV patients presenting with a CNS disease, since multiple herpesviruses are capable of causing neurologic symptoms in this subset of patients (177).
Herpesviruses with enteroviruses.
Since HSV and enterovirus (EV) are the two most commonly identifiable etiologies of sporadic encephalitis in immunocompetent individuals and since PCR testing for both diseases has become the gold standard for diagnosis (see “HSV” and “EV” sections below), there has been interest in the development of assays that can simultaneously detect these pathogens. Several groups have developed multiplex PCR assays that are capable of detecting HSV-1 and -2, EV, and VZV in a single CSF specimen (36, 37, 180).
Others.
Multiplex and/or consensus PCR has also been developed for detecting multiple polyomaviruses (JC virus [JCV], BK virus, and simian virus 40) (82) in immunocompromised patients, for codetection of EBV and Toxoplasma in AIDS patients (183), and for detection of various agents of arboviral infection for purposes of both clinical diagnosis and mosquito surveillance (107, 207).
CNS Tissue PCR
Tissue PCR remains a research technique that requires further study prior to its potential use as a clinical diagnostic method. The significance of detection of several types of viral genomes, such as HSV, in CNS tissues is uncertain. Further studies are needed to determine whether the entire viral genome is present and, if so, whether this represents latent virus or virus potentially capable of reactivating. Positive PCR results may instead represent the presence of random viral sequences or fragments of virus in neurons or other CNS cells (128).
SPECIFIC ENTITIES FOR WHICH MOLECULAR DIAGNOSTIC METHODS MAY BE CONSIDERED
Viruses for Which PCR Is of Proven Efficacy in Diagnosis and Is Widely Available
Herpes simplex virus (HSV). (i) General comments.
HSE is the most common cause of acute sporadic (nonepidemic) focal encephalitis in both the United States and the Western world. Ninety percent of adult cases of HSE result from infection with HSV-1, with the remainder due to HSV-2 (15). Fewer than 10% of cases of HSE in immunocompetent adults are due to HSV-2. However, a number of cases of adult HSV-2 meningoencephalitis have been reported in patients with AIDS (15, 90, 114, 115, 146, 190). The search for a rapid, sensitive, and specific test for the noninvasive diagnosis of HSE has been one of the most urgent problems in clinical neurovirology. PCR amplification of HSV DNA from CSF has finally filled this void.
The widespread use of PCR amplification of HSV DNA from CSF has led to modification of our understanding of the signs and symptoms of HSE and has dramatically broadened the recognized spectrum of HSV CNS infections (70, 71, 83). The clinical features of PCR-defined HSE are generally similar to those previously defined for biopsy-proven disease, although the overall severity of illness identified in PCR-proven cases appears to be considerably milder than that in biopsy-proven series. This difference is also reflected in the fact that in PCR-based series approximately 15 to 20% of patients are identified as having mild or atypical forms of HSE that would almost certainly have previously gone unrecognized.
(ii) Sensitivity, specificity, and predictive value.
In cases of HSE, virus is cultured from CSF in fewer than 5% of cases. However, in the overwhelming majority of cases, HSV-specific DNA is present and can be detected by PCR (139, 174, 175). Although previously considered the optimal diagnostic approach for patients suspected of having HSE (214), brain biopsy is now only rarely employed for diagnosis of HSE, since amplification of HSV DNA from CSF by PCR has provided a noninvasive test with speed, specificity, and sensitivity equivalent to that of brain biopsy but without the associated risk of complications. In the most comprehensive study to date, CSF PCR was positive in 53 of 54 patients (98%) with biopsy-proven HSE and was negative in 94% of biopsy-negative cases. This results in a sensitivity of 98%, a specificity of 94%, a positive predictive value of 95%, and a negative predictive value of 98% (139). A recent review of the use of PCR for diagnosis of HSE that combined results achieved in several studies estimated that the sensitivity of CSF PCR for the diagnosis of HSE was 96% and that the specificity was 99% (229). These values can be used in Bayesian decision analysis to determine the effects of both positive and negative CSF PCR results on the likelihood of HSE (Table 3) (229).
TABLE 3.
Predictive use of HSV CSF PCR for diagnosis of HSEa
| Pretest probability (%) of HSE | Posttest probability (%)
|
|
|---|---|---|
| CSF PCR positive | CSF PCR negative | |
| 5 (low) | 83.5 | 0.2 |
| 35 (medium) | 98 | 2 |
| 60 (high) | 99 | 6 |
Reprinted with permission from Excerpta Medica (229).
It is critical to recognize that in a patient with a high probability of HSE based on clinical and laboratory findings, a negative CSF PCR does not rule out HSE; it merely reduces its likelihood. Information concerning the sensitivity and specificity of HSV DNA amplification from CSF in pediatric and neonatal HSE is limited (9, 231, 236). In one study (232), CSF PCR for HSV DNA correctly diagnosed HSE in three of four neonates with HSV infection and CNS involvement and in all four “proven” cases of HSE in infants and children. No HSV DNA was detected in 105 control children or in 4 neonates with HSV infection that did not involve the CNS. Six infants and children with non-HSV encephalitis were also PCR negative for HSV DNA. Overall, HSV PCR in neonates had a sensitivity of 75%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 98%.
(a) False negatives. The potential of generating false-negative PCR results is obviously of concern, as this may result in failure to treat or to complete treatment in patients who would benefit from therapy. In a study by Lakeman and colleagues (139), 164 CSF specimens were tested for PCR-inhibitory activity by spiking them with 200 copies of HSV DNA and then testing the capacity of PCR to detect this added DNA. Two of the 164 specimens (1%) contained inhibitory activity. In another similar study, only 1 of 64 CSF specimens (1%) was found to have PCR-inhibitory activity (159). In the study by Lakeman and colleagues, one of the PCR-inhibitory CSF specimens contained hemolyzed erythrocytes, and the other was quite xanthochromic. It has been suggested that porphyrin compounds derived from the degradation of heme in erythrocytes may account for some false-negative PCR results (16). This suggests that caution should be used in interpreting negative PCR results in bloody CSF specimens. Rare CSF specimens contain inhibitory activity for the Taq polymerase, even in the absence of hemolyzed erythrocytes or their breakdown products (16, 68). Determination of intrathecal synthesis of HSV-specific antibody (118) may complement PCR, especially in patients who have already been ill for a week or more at the time that the first CSF specimen becomes available for diagnostic testing (52). Antibody tests are insensitive early after onset of illness but become positive in the majority of patients 2 weeks after onset of illness and later (14). For this reason, especially for patients with symptoms that have lasted a week or more, both CSF PCR and CSF or serum antibody studies should be performed.
(b) False positives. The incidence of false-positive CSF PCR results for HSV meningitis and encephalitis seems to be exceedingly low when the studies are performed in experienced laboratories using accepted standards and methods to avoid inadvertent contamination. Isolated examples of laboratories with high numbers of false-positive tests have been traced to improper procedures resulting in sample-to-sample contamination (84), but fortunately this problem can be eliminated by appropriate attention to approved laboratory practices. As noted, in the series by Lakeman and colleagues 3 of 47 brain biopsy-negative patients were found to be CSF PCR positive for HSV DNA. If all of these patients are considered false positives, this represents an incidence of about 6%. This is likely to be a substantial overestimate, as it is likely that most, if not all, of these cases were actually examples of false-negative brain biopsy results (139). In another study, none of 83 patients with “other neurologic disorders” and none of 30 patients with non-HSV-related meningitis or encephalitis had positive HSV CSF PCR results (159). It is important to recognize that these studies assume that PCR testing was done in an appropriate clinical setting (e.g., for a patient with suspected acute meningitis or encephalitis).
(iii) Kinetics of PCR positivity.
It is vital in evaluating the diagnostic utility of PCR for HSE to understand when viral DNA initially appears in the CSF, how long it remains detectable, and what effects antiviral therapy have on CSF HSV DNA levels. Initial studies of CSF PCR in HSE suggested that results became positive early after infection. However, recent studies have found that CSF PCR may be negative when performed during the first 72 h. In one recent study, 3 of 11 patients were found to have an initially negative CSF PCR performed at 1 to 3 days after onset of symptoms, which subsequently became positive when repeated 4 to 7 days later (247). This result is significantly higher than the rate of ∼5% seen in earlier studies (102). Until further data are available, it would seem prudent to interpret negative CSF PCRs with caution when they are obtained within 72 h of symptom onset. Patients for whom no alternate diagnosis is identified should have their HSV CSF PCR repeated between days 4 and 7. Acyclovir therapy should not be discontinued on the basis of a negative CSF PCR obtained early (<72 h) after onset of symptoms for patients in whom the clinical suspicion of HSE remains high. Despite these concerns, retrospective studies of patients with negative HSV PCR testing suggest that fewer than 0.4% of these cases may have been false negatives (examples of HSE missed by PCR) (176).
(iv) Effect of antiviral therapy on PCR positivity.
Lakeman and colleagues (139) found no effect of antiviral therapy lasting 1 week or less on detection of HSV DNA in CSF. However, only 47% of specimens from patients who received 8 to 14 days of antiviral therapy and only 21% of specimens from those receiving more than 2 weeks of therapy remained positive for HSV DNA. For the majority of patients included in this study, antiviral therapy consisted of vidarabine rather than acyclovir, so caution should be used in extrapolating these results to patients treated with acyclovir. However, in another study in which approximately two-thirds of the patients were treated with acyclovir (the remaining one-third having received vidarabine), HSV was shown to persist for at least 5 days after the start of antiviral therapy (212). Interestingly, it has been suggested that the amount of HSV DNA in the CSF actually increases during the first 5 to 6 days of acyclovir therapy and then subsequently declines (102, 174). Abortive replication of HSV in the presence of acyclovir may generate large quantities of small viral DNA fragments that facilitate PCR amplification (102, 174). In the majority of treated cases of HSE there is a sharp decline in the number of PCR-positive cases after 2 weeks, and HSV DNA is virtually never detected after 30 days (16, 212). As a result of these findings, it was recently suggested that CSF PCR should be performed upon completion of 10 to 14 days of acyclovir therapy and that patients who still have detectable HSV DNA in their CSF should receive an additional course of therapy (52).
(v) Quantitative HSV PCR.
Few studies evaluating the potential utility of quantitative HSV PCR in determining prognosis of HSV encephalitis currently exist (69, 182, 253). In one study (69), patients with >100 copies of HSV DNA per μl of CSF (high copy number) had poorer outcomes than those with lower levels. In general, larger amounts of HSV DNA were found in CSF of patients with a longer duration of symptoms. For example, patients with high copy numbers had a mean of 6.6 ± 3 (standard deviation) days of symptoms, compared to 3.9 ± 3 days for those with low copy numbers. Patients with higher DNA copy numbers also tended to be older (mean age of 45 ± 19 years, compared to 25 ± 19 years). A higher copy number also reflected disease severity, with all patients having high copy numbers having a reduced level of consciousness, compared to only 43% of those with low copy numbers. All patients with high copy numbers also had CT scan abnormalities, compared to only 14% of patients with low copy numbers. As might be expected from these data, patients with high copy numbers also had a significantly worse prognosis than those with low copy numbers. Mortality was 11% in those with high copy numbers, and 63% of the survivors had moderate or severe impairment at a 3-month follow-up. By contrast, all patients with low copy numbers survived, and all had normal function at a 3-month follow-up. However, in a separate study (182), initial HSV DNA levels were not related to severity of clinical symptoms or predictive of outcome.
Quantitation of HSV viral load in the setting of neonatal HSV infection has been studied and may be useful for assessing prognosis, as well as provide additional information for clinical management (127, 182). Patients with disseminated infection had higher viral loads in their sera, whereas patients with CNS infection exhibited higher CSF viral loads. Serum viral loads were significantly higher in patients who subsequently died, and HSV-2-infected patients had higher CSF viral loads than HSV-1-infected patients, along with more CNS involvement and neurologic impairment.
(vi) Role of HSV PCR in special clinical situations.
(a) Management of recurrent disease. A small number of adult patients with confirmed HSE will have a recurrence of symptoms following antiviral therapy (126, 238). The incidence of recurrent disease has been <10% in most reports of adult cases, but it may be significantly higher in children (126) and particularly in neonates (125) (see below for further discussion of neonatal HSV). It may be difficult to distinguish between postinfectious demyelination and ongoing infection as causes of recurrent symptoms. The absence of HSV DNA detectable by CSF PCR and the presence of white matter lesions on MRI suggest an immune-mediated postinfectious encephalopathy. A persisting positive CSF PCR or reisolation of virus on brain biopsy should prompt retreatment. Acyclovir-resistant isolates of HSV may arise as a result of mutations in the genes encoding either the viral thymidine kinase or DNA polymerase.
(b) HSV brain stem encephalitis. HSV is probably the most important cause of sporadic viral brain stem encephalitis (BSE) (186, 208). The clinical presentation of HSV BSE is indicative of multifocal involvement of the brain stem from midbrain to medulla. Several cases have been reported in patients with AIDS (104, 161). HSV CSF cultures are positive in a minority of cases (191), but evidence of increased intrathecal synthesis of HSV-specific antibody is supportive of the diagnosis. CSF PCR has been positive in isolated cases of both acute and recurrent brain stem encephalitis (43, 156, 163). CSF PCR has been used to amplify HSV-1-specific DNA from the CSF of a patient with recurrent episodes of BSE (235). These reports suggest that the diagnosis, as well as the clinical and epidemiological spectra of HSV BSE, may become more clearly delineated with the more widespread use of CSF PCR for antemortem diagnosis.
(c) Neonatal disease. The application of PCR to neonatal HSV disease has expanded our understanding of the extent to which CNS involvement occurs in each of the three presentations of neonatal HSV disease. In a retrospective study of 77 neonates with HSV (125), HSV DNA was detected in the CSF of 93% of infants with disseminated infection and 76% of infants with CNS disease, but also in 24% of infants with disease presumed to be limited to skin, eye, and mouth (SEM disease). This finding has led to the appreciation that infants diagnosed with SEM disease may also be at increased risk for long-term neurologic sequelae, particularly with three or more SEM recurrences in the first year following diagnosis. CSF PCR was also analyzed as a prognostic marker in that study: 95% of infants with positive PCR following completion of 10 days of antiviral therapy experienced significant morbidity and mortality. Quantitative PCR techniques are currently being studied to provide more detailed information about the kinetics of neonatal HSV disease and impact of viral load on outcome. In a subsequent prospective study of 26 neonates with SEM HSV disease, the efficacy of suppressive acyclovir against recurrences of SEM disease was evaluated for 6 months following resolution of the initial neonatal HSV diagnosis. Eighty-one percent of the infants remained recurrence free, in comparison to 54% of historical controls who did not receive suppressive therapy, and one infant with SEM recurrence had detectable HSV DNA in CSF by PCR (124).
Enteroviruses (EV). (i) General comments.
Enteroviruses are RNA viruses (plus sense) of the picornavirus family. Sixty-four unique serotypes have been identified, which have been subgrouped as poliovirus, coxsackievirus, echovirus, or enteroviruses 68 to 71. Enteroviral infection occurs predominantly as a summer and fall illness (July to October), with a higher incidence in children than in adults (198, 203-205). Although EV infection may present as a nonspecific febrile illness with or without rash, the virus is neurotropic and CNS infection may occur. The spectrum of CNS disease is broad, including benign aseptic meningitis, which leads to 75,000 cases and 50,000 hospitalizations per year in the United States and accounts for 80 to 92% of cases where an etiologic agent is identified (189). Fifty percent of infants and children with febrile illness due to EV have concomitant meningeal involvement. Enteroviral encephalitis occurs as either a focal or a diffuse process and is the third most commonly identified cause of all encephalitis, after herpes simplex virus and arboviruses (194). EV may also cause a severe sepsis syndrome in newborns, consisting of meningoencephalitis, myocarditis, and hepatitis, leading to severe morbidity and mortality in that population (5). Chronic meningoencephalitis may occur in agammaglobulinemic patients (246).
(ii) Sensitivity and specificity of EV RT-PCR compared to culture.
The 5′ nontranslated of the EV genome is useful for detection by RT-PCR, since it is a highly conserved sequence among all enterovirus serotypes. This region contains elements essential for replication of the viral genome as well as translation of its protein-coding region. Enteroviral RT-PCR allows rapid detection (<24 h) of EV in clinical samples, in contrast to the 4 to 8 days required for viral culture (192, 193). Rapid distinction of polioviruses from other enteroviruses is possible by utilizing restriction analysis of PCR products. Colorimetric, nonquantitative methods are highly accurate and provide results within 4 h (200). Single-tube, quantitative detection of the EV genome by using real-time EV RT-PCR has been demonstrated in a linear range spanning 5 log units, with a sensitivity of 96 to 100% and a specificity of 96% compared to viral culture and with results available in 4 h (160, 178, 239, 240).
Although EV RT-PCR is readily available at most academic institutions, NASBA is an additional molecular method that may be considered as a suitable alternative for sensitive amplification and detection of enterovirus sequences in a range of clinical specimens, including CSF (85). NASBA is an isothermal, in vitro transcription-based amplification method which does not require specialized equipment (such as a thermal cycler) and has been developed into convenient kits for use in clinical laboratories in which RT-PCR methodology is not available.
There are multiple reasons why nonmolecular methods of enteroviral detection are suboptimal (160, 178, 194, 239, 240). Viral culture is insensitive (65 to 75%), likely due to the presence of neutralizing antibody, the relatively low viral load at the time of diagnosis, and the fact that several EV serotypes are simply not cultivable. Prolonged time is required for isolation of EV from CSF by tissue culture, often up to 8 days. Culture is labor-intensive, requiring cultivation on multiple cell lines. Culture of throat swabs or stool specimens provides only circumstantial evidence of etiology, not causality, in the setting of CNS disease, since prolonged excretion from these sites (4 weeks from the oropharynx and 16 weeks from the gastrointestinal tract) may occur following EV infection. Serologic approaches are hampered by the diversity of EV serotypes. Because of these problems, there is often difficulty in distinguishing EV disease from bacterial meningitis or sepsis. This leads to excessive use of empirical intravenous antimicrobial interventional procedures and tests and prolonged hospitali-zation.
The sensitivity of EV RT-PCR greatly exceeds that of culture techniques, and it has replaced culture as the gold standard for diagnosis of EV infection of the CNS. Enterovirus PCR has high sensitivity and specificity, which are both estimated at >95%, and has been validated in multiple independent studies (160, 178, 187, 195, 200, 239, 240). The sensitivity of CSF PCR exceeds that of serum PCR (approximately 81 to 92% sensitivity) and urine PCR (62 to 77% sensitivity). Several strains of EV which are not cultivable are detectable by EV RT-PCR. This is one explanation for the observations in one study that several clinical samples with negative results by culture showed detectable EV genome by RT-PCR (206). Despite the excellent sensitivity of the CSF EV RT-PCR, results may be negative in the setting of EV71 CNS infection (unpublished observation).
EV PCR is a sensitive diagnostic modality for EV CNS disease in the pediatric population. Up to 50% of children less than 1 month of age with clinical illness compatible with EV infection but without CSF pleocytosis have been noted to have detectable EV genome in their CSF by RT-PCR. EV PCR of serum and urine is also highly useful in the diagnosis of neonatal enteroviral disease. In one study, a colorimetric (PCR) assay was demonstrated to be more sensitive than viral culture in identifying viral infection in initial serum and urine specimens from 16 enterovirus-infected newborn infants, and it remained more sensitive throughout their illnesses, with a combined sensitivity of serum and urine PCR of 88% and a specificity of 100% (6).
(iii) Impact on hospitalization and patient management.
Enterovirus PCR has had a profound impact on the management of patients with aseptic meningitis (217). In a large study in 1998, a retrospective chart review was performed for 276 inpatients for whom CSF PCR for enterovirus had been performed (179). Diagnoses in these patients included aseptic meningitis, convulsions, fever, and unspecified illness. The impact of EV PCR on clinical parameters, including length of stay, time to discharge following the PCR result, use of medications, and ancillary tests, was evaluated. In this study, 187 of 276 patients (70%) had the EV PCR result available prior to hospital discharge; 95 of these patients were positive, and 92 were negative. When the 95 patients with positive test results were compared to the 92 patients with negative test results, significant reductions (P < 0.001 to 0.005) were found in length of hospital stay (42.4 versus 71.5 h), time from PCR test to hospital discharge (5.2 versus 27.4 h), number of ancillary tests performed (head CT or MRI, 10 versus 71.2%; EEG, 1 versus 18%), and days of intravenous antimicrobial exposure (2 versus 3.5 days). Several groups have attempted to estimate potential cost reductions related to management of patients with enteroviral meningitis with use of RT-PCR (148, 165, 168, 185). Marshall's group (148) found a 59% reduction in overall hospital costs in one study of infants <6 months of age with CNS pleocytosis. Robinson's group (185) found a mean cost savings of >$2,000 per patient in a study of pediatric aseptic meningitis. An important proviso is that these cost reductions are only possible with rapid turnaround of results to the clinician, which is feasible only when in-laboratory-developed testing is available.
While the availability of EV RT-PCR in cases of aseptic meningitis has had an impact on hospital costs and reduced unnecessary use of antimicrobials and imaging studies, EV PCR has also allowed the rapid identification of cases of more severe EV CNS infection and neonatal sepsis, allowing for initiation of specific antiviral therapy. Pleconaril, a drug which integrates into a hydrophobic pocket on the surface of the EV capsid and inhibits viral receptor binding and uncoating, has undergone extensive clinical testing and appears to be both safe and effective (4, 188, 196, 199). In a recent study of life-threatening enteroviral infections, 12 of 16 patients with chronic enteroviral meningoencephalitis, 2 of 3 patients with paralytic poliomyelitis (two vaccine associated and one wild type) and 2 of 3 patients with encephalitis and/or transverse myelitis showed clinical improvement following treatment. In most cases in which data were available, clinical improvement was associated with virological responses, including sterilization of positive cultures or conversion of positive PCRs to negative (197, 201). Although currently limited predominantly to research studies, Pleconaril is available on an open-label compassionate-use basis and should be considered for patients with potentially life threatening EV infections, including severe encephalitis, flaccid paralysis, neonatal sepsis, and chronic meningoencephalitis (197, 216).
JC virus (JCV).
JC virus is the causative agent of progressive multifocal leukoencephalopathy (PML), a demyelinating CNS infection primarily affecting patients with AIDS. The utilization of CSF PCR to detect JCV DNA has become an important minimally invasive diagnostic test for this disease, which previously required brain biopsy for definitive diagnosis (63, 202). CSF PCR for JCV has a sensitivity of 50 to 75% and a specificity of 100% for the diagnosis of PML as demonstrated in prospective analyses of HIV-infected patients presenting with focal neurologic signs and symptoms (106, 245). Performing PCR on pellets of centrifuged CSF may be even more sensitive than analysis of CSF supernatants (133). JCV viral loads in urine and blood are not predictive of clinical CNS infection due to JCV, as viruria is found as frequently in HIV-positive individuals as in uninfected control subjects, and JCV viremia correlates only with the level of immunosuppression and not with the presence of PML (133).
A potential correlation between the JCV viral load in CSF and the PML prognosis has also been investigated by using quantitative PCR techniques (76, 81, 88, 89, 133, 225, 226, 258). In a recent study, CSF samples from 12 patients with PML were noted to have wide variations in JCV load (>6 log units), with values of >4.7 log units associated with shorter patient survival time. No correlation was found between CSF viral load values and the global volume of damaged brain tissue, however (89). Another group studying patients with PML found differences between CSF and tissue JCV DNA concentrations of approximately 4 orders of magnitude and highly variable JCV DNA viral loads in CSF samples taken at comparable states of disease (72). CSF JCV loads were not related to absolute CD4 cell counts or plasma HIV viral loads in at least one study (226).
Other groups have used quantitative PCR to investigate the effect of highly active antiretroviral therapy (HAART) (72, 88), as well as cidofovir treatment (226), on JC virus load in CSF and to determine the clinical outcomes of patients with AIDS-associated PML. A recent multicenter analysis of 57 such patients revealed that among HAART-treated patients with baseline JCV levels of <4.7 log units, reaching undetectable levels after therapy predicted longer survival (67). Similar findings have been reported by other groups (67, 157).
Epstein-Barr virus (EBV). (i) Immunocompetent hosts.
EBV has been implicated as an etiologic agent of benign aseptic meningitis, encephalitis, cerebellar ataxia, myelitis, and ADEM in immunocompetent hosts. Meningoencephalitis can complicate infectious mononucleosis or can occur as the sole manifestation of EBV infection. There are no distinctive clinical features of EBV meningoencephalitis. Patients are often children or young adults. CSF PCR has been extremely useful in diagnosis of EBV CNS infections (87, 110, 119, 141, 219-221, 233). One study detected EBV DNA in the CSF of 7.4% of immunocompetent individuals with CNS disease of unknown etiology, using nested PCR (171). Surprisingly, given that seropositivity approaches 90% in older adults, CSF PCR is not positive in patients with latent EBV infection, and false positives rarely occur in EBV-seropositive individuals who develop non-EBV meningoencephalitis. Quantitative PCR studies are performed at some research laboratories, and determination of the EBV DNA copy number may help further distinguish true-positive active infections from rare cases with false-positive results from latent virus or rare patients with dual-positive CSF PCRs (220).
(ii) Immunocompromised hosts.
EBV infection is associated with AIDS-related CNS lymphomas in HIV-infected patients, including primary CNS lymphoma (PCNSL) and AIDS-related non-Hodgkin lymphoma with CNS involvement (CNS-NHL). Patients with PCNSL and CNS-NHL often have positive EBV CSF PCR results, and the test appears to be a sensitive indicator of the presence of a tumor, with nearly 100% sensitivity and 98.5% specificity (44, 50, 56, 63, 170, 223). Using quantitative techniques, high CSF EBV DNA levels have been found in these settings, and viral load may be clinically useful, with high plasma EBV DNA levels also potentially predictive of CNS involvement (26, 106, 155). The availability of EBV PCR, in conjunction with JCV PCR (see “JCV” section above) in AIDS patients presenting with an intracranial mass has been a major advance in the management of these patients. PCR, in combination with clinical and neuroradiologic characteristics, provides another noninvasive tool to help differentiate CNS lymphomas from brain masses due to Toxoplasma infection or PML (JCV infection) without requiring brain biopsy in many cases (10).
In a recent study, real-time PCR was performed to quantify EBV DNA in CSF and plasma from 42 patients with AIDS-related NHL, with and without CNS involvement, as well as 20 patients with PCNSL and 16 HIV-infected controls with other CNS disorders (11, 26). EBV DNA was detected in the CSF from 8 of 12 (67%) patients with CNS-NHL and 16 of 20 (80%) patients with PCNSL, in contrast to only 7 of 22 (32%) patients with systemic NHL and 2 of 16 (13%) of the controls. EBV DNA levels were significantly higher in the CSF from patients with PCNSL or CNS-NHL compared to patients with systemic NHL or controls. No difference in plasma viral load was found between patient groups.
Quantitative EBV PCR has also recently been utilized to stratify the risk of EBV-associated CNS disease, including CNS lymphoma, encephalitis, and postinfectious neurologic complications. CNS lymphoma patients had high EBV loads (4.8 ± 0.2 log10 DNA copies/ml) and low CSF leukocyte counts, whereas encephalitis patients had high EBV loads (4.2 ± 0.3 log10 DNA copies/ml) and high leukocyte counts and postinfectious patients showed low EBV loads (3.0 ± 0.3 log10 DNA copies/ml) and high leukocyte counts. Additionally, lytic-cycle EBV mRNA, a marker of viral replication, was shown to present only in patients with CNS lymphoma and encephalitis (248).
PCR has also been utilized to monitor the response to therapy in HIV-infected patients with AIDS-related primary CNS lymphoma. Reductions in CSF EBV DNA burden following chemotherapy, as monitored by semiquantitative nested PCR, has been significantly associated with prolonged survival in these patients., suggesting that EBV DNA monitoring might be helpful in predicting response to chemotherapy (11, 12).
In contrast to the association of EBV with CNS lymphomas in HIV patients, other authors have indicated uncertainty about the role of EBV in other neurologic illnesses in these patients. One study of HIV-infected patients with CNS disorders distinct from and unassociated with primary CNS lymphoma revealed detectable EBV DNA in the CSF of 10 of 79 patients. However, only 1 sample in 10 had EBV as the sole pathogen detected, whereas 6 samples in 10 were also positive for other microbial agents of recognized neurologic pathogenicity. None of six tested samples had a detectable intrathecal EBV antibody response, despite the presence of the EBV genome. The authors hypothesized that the presence of EBV DNA in the CSF of a large fraction of these patients could be an incidental event associated with EBV reactivation in host PBMCs, rather than indicating EBV-associated CNS disease (170).
Cytomegalovirus (CMV).
Although 80% of immunocompetent individuals are seropositive for CMV by adulthood, CMV meningoencephalitis is largely a disease of congenitally infected newborns and immunocompromised individuals, including organ transplant recipients and those with advanced HIV infection. In immunocompromised individuals, infection results predominantly from reactivation of latent virus. Nervous system complications include diffuse ventriculoencephalitis, focal periventricular disease, polyradiculomyelitis peripheral neuropathies, and retinitis (51). CSF PCR has proven to be an extremely useful technique for detecting CMV CNS infections in immunodeficient hosts, with a excellent sensitivity (82 to 100%) and specificity (86 to 100%) in HIV-infected patients (21, 30, 51, 55, 256, 257). CSF PCR appears to be a specific indicator of CNS infection, including retinitis (31, 237). The use of DNA PCR to detect CMV in CSF has greatly enhanced the ability to diagnose and differentiate CMV from other AIDS-related CNS diseases (98, 220, 221).
Quantitative CMV PCR is available in a number of laboratories, and CMV DNA copy number may provide a better index of disease severity (13, 211, 254). The CMV viral load in peripheral blood leukocytes has also been monitored as a method to predict which immunosuppressed patients might develop end-organ disease. The clinical utility of the test in the setting of congenital CMV infection is also being investigated, particularly with respect to correlating neurologic outcome with CSF viral load and efficacy of antiviral therapy (103, 121).
CMV PCR of CSF has also been utilized to identify the presence of antiviral-resistant strains from patient isolates (215, 256). Mutations within the UL97 region, which encodes a human CMV protein kinase, have been found to confer ganciclovir resistance and can be detected in plasma and CSF by direct sequencing of PCR products.
Other molecular techniques have been applied to the diagnosis of CNS CMV infection, including detection of CMV transcripts (human CMV mRNA). Transcription of late human CMV genes occurs only after viral replication, and therefore analysis for late gene transcripts should distinguish between active and latent infection. The pp67 late-gene transcript assay has been studied and compared to PCR and culture techniques for diagnosis of CNS CMV infection (21, 98, 260), and superior sensitivity has been suggested in some studies (98).
As with EBV PCR, CMV-seropositive individuals with latent infection do not have detectable DNA based on PCR testing. In one small study, 9% of immunocompetent CMV-seropositive individuals (1 out of 11) developed a positive CMV CSF PCR during bacterial meningitis, suggesting that other CNS infections can cause false-positive CMV PCRs, although the frequency with which this occurs in the population as a whole is unclear (221).
Viruses for Which PCR Has Potential Efficacy in Diagnosis but Is Less Widely Available
Other human herpesvirus infections. (i) Varicella-zoster virus
(a) Primary infection. Direct infection of the CNS by VZV during chicken pox is fortunately rare but can produce meningoencephalitis. Pathological studies indicate that many cases of encephalitis are in fact due to either large- or small-vessel vasculopathy resulting from viral infection of vascular endothelium (94, 129). More frequently CNS involvement occurs as a result of ADEM, a postinfectious, immune-mediated, predominantly demyelinating process. Both the direct and immune-mediated CNS effects of varicella need to be distinguished from Reye's syndrome (74, 75).
VZV is rarely cultured from CSF, but a positive CSF PCR for VZV DNA has been found in ∼60% of meningoencephalitis cases and, if present, distinguishes this from ADEM. Lower rates of CSF PCR positivity (∼25%) have been reported in later analyses which included all CNS symptoms associated with VZV, not limited to meningoencephalitis, regardless of whether symptoms occurred in the setting of primary or reactivated infection (75, 134, 135). Intrathecal synthesis of VZV-specific antibody or the presence of anti-VZV immunoglobulin M (IgM) antibody in the CSF is also specific for encephalitis and may be positive when PCR studies are negative. With the availability of PCR, it is increasingly being recognized that both varicella meningitis and encephalitis can occur in the absence of a detectable rash: recent studies have reported that only about one-half of patients with acute CNS symptoms related to VZV had an associated typical rash (75, 97, 112, 134). In patients without a rash, VZV CSF PCR may play a pivotal role in making early diagnosis, which is necessary for successful treatment of CNS VZV.
(b) Zoster. In immunocompromised patients, and much more rarely in apparently immunocompetent individuals, meningoencephalitis can occur as a complication of zoster reactivation. Pathologically, a variety of discrete processes can be identified. A vasculopathy of large arteries (granulomatous arteritis) occurs in older patients (>60 years of age) following an outbreak of trigeminal zoster. Pathological studies have shown viral antigen and nucleic acid in the affected arteries. Encephalitis also results from a small-vessel vasculopathy that produces multiple infarcts in both cortical and subcortical gray and white matter combined with deep white matter ischemic or demyelinating lesions (8, 93, 95, 96, 129). Zoster infection in immunocompromised patients also may result in a necrotizing infection of ependymal cells resulting in ventriculitis and periventriculitis, usually presenting with hydrocephalus, altered mental status, and gait difficulty. CSF PCR is positive for VZV DNA in the majority of these cases, and intrathecal synthesis of VZV-specific antibody occurs. Other groups have documented lower sensitivity of CSF PCR in the setting of subacute to chronic CNS VZV infection (myelitis and encephalitis) and have stressed that intrathecal synthesis of VZV-specific antibody may be a more reliable diagnostic measure in such cases, particularly with intervals of weeks to months between zoster and onset of neurologic disease (94).
In a recent study of HIV-infected patients, a favorable influence of HAART on the prevalence of neurologic complications caused by VZV among AIDS patients was noted, with a large reduction in the prevalence of VZV-related disease noted since 1996, the point at which HAART became widely available. The sensitivity of PCR in detecting VZV DNA in CSF from these patients with known VZV neurologic disease was 80%, with a specificity of 98%, in the small number of patients that were available for evaluation (59). A larger multicenter retrospective study of the neurologic complications of VZV infection in 34 HIV-infected adults (including encephalitis, myelitis, radiculitis, and meningitis) reported CSF PCR positivity in 100% of cases, compared to culture positivity in only 33% (66).
(ii) Human herpes virus 6.
HHV-6 is a member of the herpesvirus family that, like CMV, is classified in the betaherpesvirus subfamily. Epidemiological studies suggest that infection occurs in early childhood (6 to 12 months), with two-thirds of children being seropositive by 1 year and up to 95% being seropositive by adulthood. Viral invasion of the CNS appears to be a common event during primary infection (38) and may account for the fact that one-third of infants with primary HHV-6 infection develop seizures. CSF PCR was positive for HHV-6 in 9 of 10 children with roseola in one study (132). A more recent study found that 10% of febrile infants under 3 months of age undergoing evaluation for sepsis had evidence of HHV-6 infection in plasma, CSF, or both, but HHV-6 DNA was found in infants with and without alternative explanations for their fevers (34).
HHV-6 has also been implicated as a cause of febrile convulsions. In one study, 80% of children with >3 febrile convulsions had HHV-6 DNA detectable in CSF, suggesting the possibility that HHV-6 can cause a syndrome of recurrent febrile convulsions (132). However, the incidence of primary HHV-6 infection is similar in patients with febrile seizures and age-matched controls (109), and HHV-6 DNA was not detectable in CSF from any of 23 prospectively analyzed febrile seizure patients in a separate study (228).
These results are consistent with results of PCR on brain tissue in which HHV-6 DNA is detectable in >75% of normal brains (42, 145). In a PCR-based study to investigate the role of herpesviruses in neurologic syndromes, HHV-6 was detected in 1% of adult immunocompetent patients presenting with a variety of neurologic symptoms and, in some of the cases, concurrent with diagnosis of other infections of the central nervous system (220). Quantification of HHV-6 viral DNA may be important in determining the relevance of HHV-6 DNA in clinical specimens. An HHV-6 RT-PCR that can distinguish between latent and replicating virus in PBMCs has also been developed, and its application for the analysis of CSF specimens may also be helpful with regard to this issue.
Severe encephalitis is a rare complication of HHV-6 infection in immunocompetent children and appears to result from direct viral invasion of the CNS rather than from an immune-mediated process (111, 259). HHV-6 also produces focal encephalitis in older children and adults. PCR testing for HHV-6 DNA of CSF from patients referred into Collaborative Antiviral Study Group trials as having suspected HSV encephalitis suggested that ∼7% of cases (9 of 138) might be due to HHV-6 (152). HHV-6 CSF PCR was also noted to be positive in 25% of children with influenza-associated encephalopathy, suggesting the possibility of reactivation in the presence of this disease (222).
Like CMV, HHV-6 becomes latent following primary infection and can reactivate during immunosuppression. Fatal HHV-6 encephalitis, presumably due to reactivation, has been reported in patients following bone marrow transplantation (22, 73). Several cases of fatal HHV-6 encephalitis have been reported in patients with AIDS (130, 131), and virus can be detected by PCR in the CSF of HIV-positive patients, although not always in association with clinical disease (27). Real-time quantitative PCR for HHV-6 has been developed and may be useful to monitor viral load in bone marrow transplantation patients treated with antiherpetic therapy (91).
HHV-6 has been associated with MS and demyelinating CNS disease The evidence for an association with MS is largely circumstantial, and the role of HHV-6, if any, in the etiology or pathogenesis of this disease remains unproven and controversial (234). Viral antigen and nucleic acid have been detected by some groups in oligodendrocytes in MS plaques. HHV-6 DNA has been detected by PCR in brain tissue from patients with MS (78%) but has also been detected at a similar frequency in brain tissue from non-MS patients (74%) (42). One study demonstrated no HHV-6 DNA in CSF from 32 MS patients and no difference in HHV-6 DNA positivity in PBMCs between MS patients and non-MS controls (227). Another group, using quantitative PCR, demonstrated an increased incidence of HHV-6 DNA positivity in PBMCs in MS patients compared to controls (53 versus 30%) but not differences in quantitative viral loads between groups or differences between MS patients during remission or relapse (7).
Other viruses. (i) Human immunodeficiency virus.
In addition to opportunistic CNS disease associated with EBV, JCV, and Toxoplasma in HIV-infected individuals (see individual sections above and below), CNS illness in AIDS patients may be caused by HIV disease itself, resulting in serious morbidity such as behavioral and motor disturbances, meningitis, or encephalitis. Analysis of CSF for the presence of the viral genome to allow early diagnosis of AIDS-related neurologic complications has been an impressive example of the clinical application of PCR (45-47, 49-51, 53-57). Although HIV type 1 RNA can be detected in CSF at any stage of HIV infection and irrespective of presence of neurologic symptoms, CSF viral load (as measured by quantitative CSF PCR) has been correlated with the likelihood of neurologic disease in several studies (48). The CSF (not plasma) HIV viral load has been correlated with the severity of cortical atrophy in children with symptomatic HIV disease (32), with the degree of neurologic dysfunction in adult patients with HIV-associated dementia (29, 77, 151, 184), and with the presence of HIV encephalitis (53). Quantitative PCR techniques have also been utilized to monitor responses to antiretroviral therapy, which is know to induce substantial decreases in CSF HIV RNA levels when effective (48, 99, 172).
(ii) Rabies virus.
Current serologic tests do not reliably diagnose rabies. One group has described a rapid PCR technique based on amplification of nucleic acid sequences to detect rabies-specific RNA in the saliva and CSF as early as day 2 after onset of symptoms (241). An optimized RT-PCR protocol for the detection of rabies virus genomic RNA performed on saliva combined with immunofluorescence assay of skin biopsy samples detected 9 of 9 patients with confirmed rabies by postmortem examination and 0 of 19 patients with suspected rabies who were later determined not to have rabies (61). Rapid RT-PCR with TaqMan genotype-specific probes can distinguish among the six established rabies virus and rabies virus-related genotypes (24).
(iii) Human T-cell lymphotrophic virus.
Human T-cell lymphotropic virus (HTLV) is the etiologic agent implicated in HTLV type 1 (HTLV-1)-associated myelopathy (HAM)/tropical spastic paraparesis (TSP). The combination of CSF PCR for proviral DNA and serologic analysis for intrathecal synthesis of HTLV-1-specific antibodies provides consistent criteria for the diagnosis of HAM/TSP. One study noted that 25 of 27 patients with disease consistent with HAM/TSP had detectable HTLV-1 proviral sequences in CSF, in contrast to only 3 of 27 control patients with other neurologic diseases (173). Amyotrophic lateral sclerosis-like manifestations of HAM/TSP have also been reported, and these patients were noted to have CSF proviral loads as high as those of patients with classical HAM/TSP (149). Another group noted that the prevalence of HAM/TSP rises exponentially with increasing HTLV proviral load in PBMCs, once the proviral load exceeds 1% of PBMCs (162).
Viral Entities for Which Molecular Diagnostic Methods Are Not Readily Available and/or Are of Unproven Sensitivity or Specificity
Arboviruses.
Although specific PCR techniques have been developed for many arboviral infections (138, 153), molecular techniques are not widely utilized for diagnosis of CNS disease in humans, due to the fact that sensitivity is often poor in the setting of clinical symptoms. Measurement of intrathecal synthesis of virus-specific IgM is the most widely accepted method of diagnosis in these cases (230). Specific PCR tests are available at various institutions and the Centers for Disease Control and Prevention on a research basis but are more reliably applied for surveillance of arboviral prevalence in mosquito pool populations (107, 108, 209, 249) (See additional discussion and references in “Multiplex and Consensus Primer PCRs” above).
(i) West Nile virus.
The development of molecular techniques for the diagnosis of West Nile virus in humans has been of particular interest and relevance since its emergence as an epidemic pathogen in the Western Hemisphere in 1999. Although traditional and quantitative (TaqMan) RT-PCRs of CSF and serum for WNV are available on a research basis at several institutions (30, 108, 140), PCR is not an adequately sensitive method in the setting of clinical disease to reliably rule out infection. Sensitivity is less than 60% in immunocompetent patients with CNS disease at the time of clinical presentation (57% of initial CSF specimens tested during the New York City epidemic of 1999) (167). This apparently low sensitivity may reflect timing of specimen collection, since peak viremia occurs 3 to 5 days prior to the onset of clinical symptoms and the early presence of IgM and IgG antibodies correlates with clearance of virus. Diagnosis has been based predominantly on serology, including the presence of IgM antibodies detected by capture enzyme-linked immunosorbent assay in CSF, a fourfold or greater increase in neutralizing antibody in acute- and convalescent-phase CSF specimens, or the presence of both IgM and IgG antibodies in serum. Identification of virus-specific antigen by immunocytochemistry or of WNV nucleic acid by PCR in brain tissue should also be considered diagnostic.
The sensitivity of WNV-PCR may be higher in immunocompromised hosts who do not mount a significant serologic response to infection and have persistent viremia, and PCR may be prove to be a more useful marker of infection in this population. PCR and nucleic acid test methods have been utilized for screening the blood supply and organs for transplantation (41, 113, 166). In these settings, PCR has higher sensitivity, since WNV-infected asymptomatic blood and organ donors are more likely to be viremic than symptomatic individuals. WNV RT-PCR was successfully used to identify the 1999 New York WNV epidemic strain as 99.9% genetically identical to an Israeli strain circulating in 1998 (92).
Measles virus.
Measles virus (MV) infection has been associated with acute encephalitis, encephalomyelitis, and subacute sclerosing panencephalitis (SSPE), a degenerative disease of the central nervous system that leads to death within a few years. RT-PCR has been utilized in the setting of both acute and subacute disease: in one study, in three of the four patients with central nervous system involvement, MV sequence was detected in CSF obtained within 1 day following the onset of the manifestations, suggesting transient and direct invasion of the CNS by MV at the initial stage of CNS involvement and that PCR can be used with sufficient sensitivity and specificity to detect the measles virus sequence in clinical samples (150). Quantitative real-time CSF RT-PCR has for measles been utilized to monitor viral load in SSPE patients over the course of the therapy with ribavarin and alpha interferon. Measles virus RNA decreases rapidly after treatment with alpha interferon, paralleling the decrease in the measles virus antibody titer in the cerebrospinal fluid and the improvement in the neurologic disability (105). RT-PCR has also been used in conjunction with serology and culture to confirm the etiology of an outbreak of acute encephalopathy without rash as measles in children in northern India (242). RT-PCR amplification of the 3′ terminus of the nucleocapsid gene, the region exhibiting the highest variability within the MV genome, from CSF samples has been utilized for retrospective molecular epidemiological studies to investigate strain variation in measles viruses circulating up to 20 years ago (136). MV CSF RT-PCR has also been used to identify the viral genome in a rare case of intractable frontal lobe epilepsy with mental deterioration (123).
Adenovirus.
Adenoviruses are common etiologic agents of upper respiratory infection and diarrhea but can occasionally produce CNS infections in immunocompetent and immunocompromised hosts (particularly adenovirus type 7). CNS cases are usually associated with pneumonia and epidemics of adenovirus in family outbreaks. Reye-like syndrome has also rarely been reported (120). CSF PCR is available for diagnosis, but standardization has not been achieved. CSF PCR for adenovirus was utilized in a prospective study of 27 adults with acute viral encephalitis (219).
BK virus.
Recently, PCR of CSF was utilized to investigate the possible etiologic role of BK and JC viruses in immunocompromised and immunocompetent children with suspected encephalitis and meningoencephalitis. JC virus DNA was not found in any of 266 CSF samples analyzed; however, BK virus was detected in 2 of 266 samples (1.6%) taken from older children age 10 to 16 years (20), suggesting the possibility of a pathogenic role in some cases of childhood encephalitis.
Use of PCR in the Diagnosis of Infections Which Can Mimic Viral CNS Disease
M. pneumoniae.
Mycoplasma pneumoniae commonly causes respiratory tract infections in humans, but it may also be associated with central nervous system manifestations in immunocompetent (particularly children) and immunocompromised patients, including aseptic meningitis as well as encephalitis and ADEM. Controversy exists as to whether encephalitis is more commonly the result of active infection of brain tissue or the result of secondary inflammatory or autoimmune-mediated mechanisms. PCR is available for detection of the Mycoplasma genome in CSF and has successfully been used to detect the Mycoplasma genome in CSF samples from patients with clinically and serologically confirmed Mycoplasma central nervous system infection (164). Although several groups have suggested that detection of the microbial genome in CSF supports the role of direct invasion of this organism into the central nervous system (23, 164, 210), others disagree (1). It is likely that both active infection and postinfectious forms occur, with the latter being more amenable to steroid therapy than the former, suggesting the possible therapeutic utility of antimicrobial agents.
B. burgdorferi (Lyme disease).
Borrelia burgdorferi infection is associated with subacute and late CNS and peripheral nervous system complications, including cranial neuropathies, aseptic meningitis, and encephalitis. Serologic detection of intrathecal Lyme-specific IgM is routinely used as the diagnostic method of choice, but IgM may not be present at the time of clinical symptoms. Recently, the yield of PCR testing of CSF and urine specimens from 30 patients with neuroborreliosis was analyzed and compared to they yield obtained by serologic methods (142, 143). The diagnostic sensitivities of PCR in CSF and urine samples were only 17 and 7%, respectively. Specific intrathecal antibody production was found in 90% of the patients, and 87% showed elevated B. burgdorferi antibodies in serum, suggesting that measurement of specific intrathecal antibody synthesis was superior to CSF PCR for diagnosis of neuroborreliosis. However, in patients with a short duration of disease (<14 days), CSF PCR may be a useful diagnostic supplement. PCR of skin biopsy specimens is currently the most sensitive and specific test for the diagnosis of patients with electron microscopy, being superior to culture and serologic testing. Detection of spirochetal DNA in clinical samples by PCR may have a role in diagnosis but has not yet been standardized for routine diagnostic use (33)
Toxoplasma.
Toxoplasma gondii is endemic worldwide, and infection causes significant morbidity and mortality in the developing fetus and as an opportunistic infection in immunocompromised individuals. Serologic detection methods are the standard for clinical diagnosis, but in the past decade, PCR has been applied for prenatal diagnosis of congenital toxoplasmosis and the detection of acute disease in the immunocompromised patient. Overall, PCR for Toxoplasma suffers from a lack of sensitivity, a lack of standardization, and variable performance depending on the laboratory in which it is performed. A wide variety of primers have been used in different assays, but few comparative tests have been performed (19).
Despite the variable performance described above, at least one group has demonstrated high sensitivity, specificity, and clinical utility of PCR in the diagnosis of Toxoplasma encephalitis (TE) in HIV-infected individuals. The diagnostic value of this PCR assay was assessed in human patients by analyzing CSF and serum samples from 12 AIDS patients with confirmed Toxoplasma encephalitis compared to controls, including HIV-infected patients with other opportunistic infections and no signs of cerebral toxoplasmosis and immunocompetent patients with neurocysticercosis. Eleven of the 12 patients with confirmed toxoplasmosis had positive PCR results in either blood or cerebrospinal fluid samples (6 of 9 blood samples and 8 of 12 cerebrospinal fluid samples). All samples from control patients were negative (116). An additional retrospective study using PCR to analyze CSF for the presence of T. gondii DNA in HIV-infected patients confirmed the high specificity of the test but showed lower sensitivity. CSF PCR testing was performed on HIV-infected patients with a clinical suspicion of TE, and the results were compared to those for control patients with other HIV-associated symptoms or for non-HIV-infected patients with neurologic disorders. PCR was positive in two of four of patients with a final diagnosis of TE and negative in all remaining patients. The two patients with positive PCR had a fulminant course and experienced treatment failure (117).
The switch from bradyzoites (latent infection) to tachyzoites (active infection) is the fundamental pathogenic event that leads to TE in patients with AIDS. Distinction between these stages is difficult, particularly when specific treatment has been started. One group has developed CSF PCR assays (nested PCR and a more sensitive RT-PCR) to evaluate CSF specimens from AIDS patients with TE before and after administration of antiparasitic therapy for target sequences expressed on bradyzoites (SAG4 and MAG1), tachyzoites (SAG1), or both stages (B1) of T. gondii. Specific primers for the genes SAG4, MAG1, and SAG1 may be useful in AIDS patients with relapse of TE, in whom the use of PCR targeting of the B1 gene may fail to detect DNA, especially when prophylaxis or treatment has been started (58, 62). These assays may aid in distinguishing asymptomatic latent infection from reactivated disease in these patients.
Mycobacterium tuberculosis meningitis.
Early diagnosis of Mycobacterium tuberculosis meningitis (TBM) by PCR and other molecular methods has been attempted with mixed success (101, 213, 230). A summary of six studies, each including more than 20 patients with suspected TBM, showed sensitivity ranging from 33 to 91% and specificity ranging from 88 to 100%. Commercially available PCRs such as the Amplicor MTB test (Roche Molecular) address false-positive results but require additional trials to document sensitivity and clinical usefulness (25). Under optimal conditions, these tests may have a high specificity (95 to 100%) but are limited by their sensitivity (50 to 75%). There have been several reports of higher sensitivity (75 to 94%) with IS6110 (insertion-like M. tuberculosis sequence element)-targeted PCR (40, 169). Rapid testing is promising but should be used with caution and performed with internal controls to monitor for potential amplification reaction inhibitors in specimens (18).
REFERENCES
- 1.Abele-Horn, M., U. Busch, H. Nitschko, E. Jacobs, R. Bax, F. Pfaff, B. Schaffer, and J. Heesemann. 1998. Molecular approaches to diagnosis of pulmonary diseases due to Mycoplasma pneumoniae. J. Clin. Microbiol. 36:548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle, S. W., and E. Puchhammer-Stockl. 2002. Diagnosis of herpesvirus infections of the central nervous system. J. Clin. Virol. 25(Suppl. 1):S79-S85. [DOI] [PubMed] [Google Scholar]
- 3.Abravaya, K., J. Huff, R. Marshall, B. Merchant, C. Mullen, G. Schneider, and J. Robinson. 2003. Molecular beacons as diagnostic tools: technology and applications. Clin. Chem. Lab. Med. 41:468-474. [DOI] [PubMed] [Google Scholar]
- 4.Abzug, M. J., G. Cloud, J. Bradley, P. J. Sanchez, J. Romero, D. Powell, M. Lepow, C. Mani, E. V. Capparelli, S. Blount, F. Lakeman, R. J. Whitley, and D. W. Kimberlin. 2003. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr. Infect. Dis.J. 22:335-341. [DOI] [PubMed] [Google Scholar]
- 5.Abzug, M. J., H. L. Keyserling, M. L. Lee, M. J. Levin, and H. A. Rotbart. 1995. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin. Infect. Dis. 20:1201-1206. [DOI] [PubMed] [Google Scholar]
- 6.Abzug, M. J., M. Loeffelholz, and H. A. Rotbart. 1995. Diagnosis of neonatal enterovirus infection by polymerase chain reaction. J. Pediatr. 126:447-450. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Lafuente, R., C. Martin-Estefania, V. de Las Heras, C. Castrillo, J. J. Picazo, E. Varela de Seijas, and R. A. Gonzalez. 2002. Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch. Neurol. 59:929-933. [DOI] [PubMed] [Google Scholar]
- 8.Amlie-Lefond, C., B. K. Kleinschmidt-DeMasters, R. Mahalingam, L. E. Davis, and D. H. Gilden. 1995. The vasculopathy of varicella-zoster virus encephalitis. Ann. Neurol. 37:784-790. [DOI] [PubMed] [Google Scholar]
- 9.Ando, Y., H. Kimura, H. Miwata, T. Kudo, M. Shibata, and T. Morishima. 1993. Quantitative analysis of herpes simplex virus DNA in cerebrospinal fluid of children with herpes simplex encephalitis. J. Med. Virol. 41:170-173. [DOI] [PubMed] [Google Scholar]
- 10.Antinori, A., A. Ammassari, A. De Luca, A. Cingolani, R. Murri, G. Scoppettuolo, M. Fortini, T. Tartaglione, L. M. Larocca, G. Zannoni, P. Cattani, R. Grillo, R. Roselli, M. Iacoangeli, M. Scerrati, and L. Ortona. 1997. Diagnosis of AIDS-related focal brain lesions: a decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology 48:687-694. [DOI] [PubMed] [Google Scholar]
- 11.Antinori, A., A. Cingolani, A. De Luca, G. Gaidano, A. Ammassari, L. M. Larocca, and L. Ortona. 1999. Epstein-Barr virus in monitoring the response to therapy of acquired immunodeficiency syndrome-related primary central nervous system lymphoma. Ann. Neurol. 45:259-261. [PubMed] [Google Scholar]
- 12.Antinori, A., L. M. Larocca, L. Fassone, P. Cattani, D. Capello, A. Cingolani, G. Saglio, G. Fadda, G. Gaidano, and L. Ortona. 1999. HHV-8/KSHV is not associated with AIDS-related primary central nervous system lymphoma. Brain Pathol. 9:199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arribas, J. R., D. B. Clifford, C. J. Fichtenbaum, D. L. Commins, W. G. Powderly, and G. A. Storch. 1995. Level of cytomegalovirus (CMV) DNA in cerebrospinal fluid of subjects with AIDS and CMV infection of the central nervous system. J. Infect. Dis. 172:527-531. [DOI] [PubMed] [Google Scholar]
- 14.Aurelius, E. 1993. Herpes simplex encephalitis. Early diagnosis and immune activation in the acute stage and during long-term follow-up. Scand. J. Infect. Dis. Suppl. 89:3-62. [PubMed] [Google Scholar]
- 15.Aurelius, E., B. Johansson, B. Skoldenberg, and M. Forsgren. 1993. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J. Med. Virol. 39:179-186. [DOI] [PubMed] [Google Scholar]
- 16.Aurelius, E., B. Johansson, B. Skoldenberg, A. Staland, and M. Forsgren. 1991. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet 337:189-192. [DOI] [PubMed] [Google Scholar]
- 17.Bale, J. F., Jr. 1993. Viral encephalitis. Med. Clin. N. Am. 77:25-42. [DOI] [PubMed] [Google Scholar]
- 18.Baran, J., Jr., K. M. Riederer, and R. Khatib. 2000. Limits of detection of Mycobacterium tuberculosis in spiked cerebrospinal fluid using the polymerase chain reaction in tuberculous meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 19:47-50. [DOI] [PubMed] [Google Scholar]
- 19.Bastien, P. 2002. Molecular diagnosis of toxoplasmosis. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):S205-S215. [DOI] [PubMed] [Google Scholar]
- 20.Behzad-Behbahani, A., P. E. Klapper, P. J. Vallely, and G. M. Cleator. 2003. BK virus DNA in CSF of immunocompetent and immunocompromised patients. Arch. Dis. Child. 88:174-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bestetti, A., C. Pierotti, M. Terreni, A. Zappa, L. Vago, A. Lazzarin, and P. Cinque. 2001. Comparison of three nucleic acid amplification assays of cerebrospinal fluid for diagnosis of cytomegalovirus encephalitis. J. Clin. Microbiol. 39:1148-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bethge, W., R. Beck, G. Jahn, P. Mundinger, L. Kanz, and H. Einsele. 1999. Successful treatment of human herpesvirus-6 encephalitis after bone marrow transplantation. Bone Marrow Transplant. 24:1245-1248. [DOI] [PubMed] [Google Scholar]
- 23.Bitnun, A., E. L. Ford-Jones, M. Petric, D. MacGregor, H. Heurter, S. Nelson, G. Johnson, and S. Richardson. 2001. Acute childhood encephalitis and Mycoplasma pneumoniae. Clin. Infect. Dis. 32:1674-1684. [DOI] [PubMed] [Google Scholar]
- 24.Black, E. M., J. P. Lowings, J. Smith, P. R. Heaton, and L. M. McElhinney. 2002. A rapid RT-PCR method to differentiate six established genotypes of rabies and rabies-related viruses using TaqMan technology. J. Virol. Methods 105:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonington, A., J. I. Strang, P. E. Klapper, S. V. Hood, A. Parish, P. J. Swift, J. Damba, H. Stevens, L. Sawyer, G. Potgieter, A. Bailey, and E. G. Wilkins. 2000. TB PCR in the early diagnosis of tuberculous meningitis: evaluation of the Roche semi-automated COBAS Amplicor MTB test with reference to the manual Amplicor MTB PCR test. Tuber. Lung Dis. 80:191-196. [DOI] [PubMed] [Google Scholar]
- 26.Bossolasco, S., P. Cinque, M. Ponzoni, M. G. Vigano, A. Lazzarin, A. Linde, and K. I. Falk. 2002. Epstein-Barr virus DNA load in cerebrospinal fluid and plasma of patients with AIDS-related lymphoma. J. Neurovirol. 8:432-438. [DOI] [PubMed] [Google Scholar]
- 27.Bossolasco, S., R. Marenzi, H. Dahl, L. Vago, M. R. Terreni, F. Broccolo, A. Lazzarin, A. Linde, and P. Cinque. 1999. Human herpesvirus 6 in cerebrospinal fluid of patients infected with HIV: frequency and clinical significance. J. Neurol. Neurosurg. Psychiatry 67:789-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouquillon, C., A. Dewilde, L. Andreoletti, V. Lambert, V. Chieux, Y. Gerard, G. Lion, L. Bocket, and P. Wattre. 2000. Simultaneous detection of 6 human herpesviruses in cerebrospinal fluid and aqueous fluid by a single PCR using stair primers. J. Med. Virol. 62:349-353. [DOI] [PubMed] [Google Scholar]
- 29.Brew, B. J., L. Pemberton, P. Cunningham, and M. G. Law. 1997. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J. Infect. Dis. 175:963-966. [DOI] [PubMed] [Google Scholar]
- 30.Briese, T., W. G. Glass, and W. I. Lipkin. 2000. Detection of West Nile virus sequences in cerebrospinal fluid. Lancet 355:1614-1615. [DOI] [PubMed] [Google Scholar]
- 31.Broccolo, F., R. Iuliano, A. M. Careddu, R. Trovato, S. Lico, P. L. Blanc, F. Mazzotta, and L. Ceccherini-Nelli. 2000. Detection of lymphotropic herpesvirus DNA by polymerase chain reaction in cerebrospinal fluid of AIDS patients with neurological disease. Acta Virol. 44:137-143. [PubMed] [Google Scholar]
- 32.Brouwers, P., L. Civitello, C. DeCarli, P. Wolters, and S. Sei. 2000. Cerebrospinal fluid viral load is related to cortical atrophy and not to intracerebral calcifications in children with symptomatic HIV disease. J. Neurovirol. 6:390-397. [DOI] [PubMed] [Google Scholar]
- 33.Bunikis, J., and A. G. Barbour. 2002. Laboratory testing for suspected Lyme disease. Med. Clin. N. Am. 86:311-340. [DOI] [PubMed] [Google Scholar]
- 34.Byington, C. L., D. M. Zerr, E. W. Taggart, L. Nguy, D. R. Hillyard, K. C. Carroll, and L. Corey. 2002. Human herpesvirus 6 infection in febrile infants ninety days of age and younger. Pediatr. Infect. Dis. J. 21:996-999. [DOI] [PubMed] [Google Scholar]
- 35.Calvario, A., A. Bozzi, M. Scarasciulli, C. Ventola, R. Seccia, D. Stomati, and B. Brancasi. 2002. Herpes consensus PCR test: a useful diagnostic approach to the screening of viral diseases of the central nervous system. J. Clin. Virol. 25(Suppl. 1):S71-S78. [DOI] [PubMed] [Google Scholar]
- 36.Casas, I., F. Pozo, G. Trallero, J. M. Echevarria, and A. Tenorio. 1999. Viral diagnosis of neurological infection by RT multiplex PCR: a search for entero- and herpesviruses in a prospective study. J. Med. Virol. 57:145-151. [DOI] [PubMed] [Google Scholar]
- 37.Casas, I., A. Tenorio, J. M. Echevarria, P. E. Klapper, and G. M. Cleator. 1997. Detection of enteroviral RNA and specific DNA of herpesviruses by multiplex genome amplification. J. Virol. Methods 66:39-50. [DOI] [PubMed] [Google Scholar]
- 38.Caserta, M. T., C. B. Hall, K. Schnabel, K. McIntyre, C. Long, M. Costanzo, S. Dewhurst, R. Insel, and L. G. Epstein. 1994. Neuroinvasion and persistence of human herpesvirus 6 in children. J. Infect. Dis. 170:1586-1589. [DOI] [PubMed] [Google Scholar]
- 39.Cassinotti, P., H. Mietz, and G. Siegl. 1996. Suitability and clinical application of a multiplex nested PCR assay for the diagnosis of herpes simplex virus infections. J. Med. Virol. 50:75-81. [DOI] [PubMed] [Google Scholar]
- 40.Caws, M., S. M. Wilson, C. Clough, and F. Drobniewski. 2000. Role of IS6110-targeted PCR, culture, biochemical, clinical, and immunological criteria for diagnosis of tuberculous meningitis. J. Clin. Microbiol. 38:3150-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. 2003. Update: detection of West Nile virus in blood donations—US 2003. Morb. Mortal. Wkly. Rep. 52:916-919. [PubMed] [Google Scholar]
- 42.Challoner, P. B., K. T. Smith, J. D. Parker, D. L. MacLeod, S. N. Coulter, T. M. Rose, E. R. Schultz, J. L. Bennett, R. L. Garber, and M. Chang. 1995. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl. Acad. Sci. USA 92:7440-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu, K., D. W. Kang, J. J. Lee, and B. W. Yoon. 2002. Atypical brainstem encephalitis caused by herpes simplex virus 2. Arch. Neurol. 59:460-463. [DOI] [PubMed] [Google Scholar]
- 44.Cingolani, A., A. De Luca, L. M. Larocca, A. Ammassari, M. Scerrati, A. Antinori, and L. Ortona. 1998. Minimally invasive diagnosis of acquired immunodeficiency syndrome-related primary central nervous system lymphoma. J. Natl. Cancer Inst. 90:364-369. [DOI] [PubMed] [Google Scholar]
- 45.Cinque, P., F. Baldanti, L. Vago, M. R. Terreni, F. Lillo, M. Furione, A. Castagna, A. D. Monforte, A. Lazzarin, and A. Linde. 1995. Ganciclovir therapy for cytomegalovirus (CMV) infection of the central nervous system in AIDS patients: monitoring by CMV DNA detection in cerebrospinal fluid. J. Infect. Dis. 171:1603-1606. [DOI] [PubMed] [Google Scholar]
- 46.Cinque, P., A. Bestetti, P. Morelli, and S. Presi. 2000. Molecular analysis of cerebrospinal fluid: potential for the study of HIV-1 infection of the central nervous system. J. Neurovirol. 6(Suppl. 1):S95-S102. [PubMed] [Google Scholar]
- 47.Cinque, P., S. Bossolasco, A. Bestetti, S. Sala, C. Pierotti, and A. Lazzarin. 2002. Molecular studies of cerebrospinal fluid in human immunodeficiency virus type 1-associated opportunistic central nervous system diseases—an update. J. Neurovirol. 8(Suppl. 2):122-128. [DOI] [PubMed] [Google Scholar]
- 48.Cinque, P., S. Bossolasco, and A. Lundkvist. 2003. Molecular analysis of cerebrospinal fluid in viral diseases of the central nervous system. J. Clin. Virol. 26:1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cinque, P., S. Bossolasco, L. Vago, C. Fornara, S. Lipari, S. Racca, A. Lazzarin, and A. Linde. 1997. Varicella-zoster virus (VZV) DNA in cerebrospinal fluid of patients infected with human immunodeficiency virus: VZV disease of the central nervous system or subclinical reactivation of VZV infection? Clin. Infect. Dis. 25:634-639. [DOI] [PubMed] [Google Scholar]
- 50.Cinque, P., M. Brytting, L. Vago, A. Castagna, C. Parravicini, N. Zanchetta, M. A. d'Arminio, B. Wahren, A. Lazzarin, and A. Linde. 1993. Epstein-Barr virus DNA in cerebrospinal fluid from patients with AIDS-related primary lymphoma of the central nervous system. Lancet 342:398-401. [DOI] [PubMed] [Google Scholar]
- 51.Cinque, P., G. M. Cleator, T. Weber, P. Monteyne, C. Sindic, G. Gerna, A. M. van Loon, P. E. Klapper, et al. 1998. Diagnosis and clinical management of neurological disorders caused by cytomegalovirus in AIDS patients. J. Neurovirol. 4:120-132. [DOI] [PubMed] [Google Scholar]
- 52.Cinque, P., G. M. Cleator, T. Weber, P. Monteyne, C. J. Sindic, and A. M. van Loon. 1996. The role of laboratory investigation in the diagnosis and management of patients with suspected herpes simplex encephalitis: a consensus report. J. Neurol. Neurosurg. Psychiatry 61:339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cinque, P., B. Giudici, and S. Bossolasco. 1998. The application of the polymerase chain reaction of cerebrospinal fluid in the clinical management of AIDS-related CNS disorders. AIDS Patient Care STDS 12:287-294. [DOI] [PubMed] [Google Scholar]
- 54.Cinque, P., P. Scarpellini, L. Vago, A. Linde, and A. Lazzarin. 1997. Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. AIDS 11:1-17. [DOI] [PubMed] [Google Scholar]
- 55.Cinque, P., L. Vago, M. Brytting, A. Castagna, A. Accordini, V. A. Sundqvist, N. Zanchetta, A. D. Monforte, B. Wahren, and A. Lazzarin. 1992. Cytomegalovirus infection of the central nervous system in patients with AIDS: diagnosis by DNA amplification from cerebrospinal fluid. J. Infect. Dis. 166:1408-1411. [DOI] [PubMed] [Google Scholar]
- 56.Cinque, P., L. Vago, H. Dahl, M. Brytting, M. R. Terreni, C. Fornara, S. Racca, A. Castagna, A. D. Monforte, B. Wahren, A. Lazzarin, and A. Linde. 1996. Polymerase chain reaction on cerebrospinal fluid for diagnosis of virus-associated opportunistic diseases of the central nervous system in HIV-infected patients. AIDS 10:951-958. [DOI] [PubMed] [Google Scholar]
- 57.Cinque, P., L. Vago, M. R. Terreni, M. Brytting, R. Marenzi, A. Castagna, A. Lazzarin, and A. Linde. 1995. Diagnosis of cytomegalovirus infection of the nervous system in AIDS by polymerase chain reaction analysis of cerebrospinal fluid. Scand. J. Infect. Dis. Suppl. 99:92-94. [PubMed] [Google Scholar]
- 58.Contini, C., R. Cultrera, S. Seraceni, D. Segala, R. Romani, E. Fainardi, P. Cinque, A. Lazzarin, and S. Delia. 2002. The role of stage-specific oligonucleotide primers in providing effective laboratory support for the molecular diagnosis of reactivated Toxoplasma gondii encephalitis in patients with AIDS. J. Med. Microbiol. 51:879-890. [DOI] [PubMed] [Google Scholar]
- 59.Corral, I., C. Quereda, A. Antela, V. Pintado, J. L. Casado, P. Martin-Davila, E. Navas, and S. Moreno. 2003. Neurological complications of varicella-zoster virus in human immunodeficiency virus-infected patients: changes in prevalence and diagnostic utility of polymerase chain reaction in cerebrospinal fluid. J. Neurovirol. 9:129-135. [DOI] [PubMed] [Google Scholar]
- 60.Coyle, P. 2000. Postinfectious encephalomyelitis, p. 83-108. In K. P. Davis (ed.), Infectious diseases of the nervous system. Butterworth-Heinemann, Oxford, United Kingdom.
- 61.Crepin, P., L. Audry, Y. Rotivel, A. Gacoin, C. Caroff, and H. Bourhy. 1998. Intravitam diagnosis of human rabies by PCR using saliva and cerebrospinal fluid. J. Clin. Microbiol. 36:1117-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cultrera, R., S. Seraceni, and C. Contini. 2002. Efficacy of a novel reverse transcriptase-polymerase chain reaction (RT-PCR) for detecting Toxoplasma gondii bradyzoite gene expression in human clinical specimens. Mol. Cell. Probes 16:31-39. [DOI] [PubMed] [Google Scholar]
- 63.d'Arminio, M. A., P. Cinque, L. Vago, A. Rocca, A. Castagna, C. Gervasoni, M. R. Terreni, R. Novati, A. Gori, A. Lazzarin, and M. Moroni. 1997. A comparison of brain biopsy and CSF-PCR in the diagnosis of CNS lesions in AIDS patients. J. Neurol. 244:35-39. [DOI] [PubMed] [Google Scholar]
- 64.Davis, L. E. 2000. Diagnosis and treatment of acute encephalitis. Neurologist 6:145-159. [Google Scholar]
- 65.DeBiasi, R. L., and K. L. Tyler. 1999. Polymerase chain reaction in the diagnosis and management of central nervous system infections. Arch. Neurol. 56:1215-1219. [DOI] [PubMed] [Google Scholar]
- 65a.DeBiasi, R. L., and K. L. Tyler. 2002. Viral meningitis and encephalitis. Continuum:Lifelong Learning in Neurology 8:59-88. [Google Scholar]
- 66.De La Blarchardiere, A., F. Rozenberg, E. Caumes, O. Picard, F. Lionnet, J. Livartowski, J. Coste, D. Sicard, P. Lebon, and D. Salmon-Ceron. 2000. Neurological complications of varicella-zoster virus infection in adults with human immunodeficiency virus infection. Scand. J. Infect. Dis. 32:263-269. [DOI] [PubMed] [Google Scholar]
- 67.De Luca, A., M. L. Giancola, A. Ammassari, S. Grisetti, M. G. Paglia, M. Gentile, A. Cingolani, R. Murri, G. Liuzzi, A. D. Monforte, and A. Antinori. 2000. The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS-associated progressive multifocal leukoencephalopathy. J. Infect. Dis. 182:1077-1083. [DOI] [PubMed] [Google Scholar]
- 68.Dennett, C., P. E. Klapper, G. M. Cleator, and A. G. Lewis. 1991. CSF pretreatment and the diagnosis of herpes encephalitis using the polymerase chain reaction. J. Virol. Methods 34:101-104. [DOI] [PubMed] [Google Scholar]
- 69.Domingues, R. B., F. D. Lakeman, M. S. Mayo, and R. J. Whitley. 1998. Application of competitive PCR to cerebrospinal fluid samples from patients with herpes simplex encephalitis. J. Clin. Microbiol. 36:2229-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Domingues, R. B., F. D. Lakeman, C. S. Pannuti, M. C. Fink, and A. M. Tsanaclis. 1997. Advantage of polymerase chain reaction in the diagnosis of herpes simplex encephalitis: presentation of 5 atypical cases. Scand. J. Infect. Dis. 29:229-231. [DOI] [PubMed] [Google Scholar]
- 71.Domingues, R. B., A. M. Tsanaclis, C. S. Pannuti, M. S. Mayo, and F. D. Lakeman. 1997. Evaluation of the range of clinical presentations of herpes simplex encephalitis by using polymerase chain reaction assay of cerebrospinal fluid samples. Clin. Infect. Dis. 25:86-91. [DOI] [PubMed] [Google Scholar]
- 72.Drews, K., T. Bashir, and K. Dorries. 2000. Quantification of human polyomavirus JC in brain tissue and cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy by competitive PCR. J. Virol. Methods 84:23-36. [DOI] [PubMed] [Google Scholar]
- 73.Drobyski, W. R., K. K. Knox, D. Majewski, and D. R. Carrigan. 1994. Fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N. Engl. J. Med. 330:1356-1360. [DOI] [PubMed] [Google Scholar]
- 74.Echevarria, J. M., I. Casas, and P. Martinez-Martin. 1997. Infections of the nervous system caused by varicella-zoster virus: a review. Intervirology 40:72-84. [DOI] [PubMed] [Google Scholar]
- 75.Echevarria, J. M., I. Casas, A. Tenorio, F. de Ory, and P. Martinez-Martin. 1994. Detection of varicella-zoster virus-specific DNA sequences in cerebrospinal fluid from patients with acute aseptic meningitis and no cutaneous lesions. J. Med. Virol. 43:331-335. [DOI] [PubMed] [Google Scholar]
- 76.Eggers, C., H. J. Stellbrink, T. Buhk, and K. Dorries. 1999. Quantification of JC virus DNA in the cerebrospinal fluid of patients with human immunodeficiency virus-associated progressive multifocal leukoencephalopathy—a longitudinal study. J. Infect. Dis. 180:1690-1694. [DOI] [PubMed] [Google Scholar]
- 77.Ellis, R. J., K. Hsia, S. A. Spector, J. A. Nelson, R. K. Heaton, M. R. Wallace, I. Abramson, J. H. Atkinson, I. Grant, J. A. McCutchan, et al. 1997. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. Ann. Neurol. 42:679-688. [DOI] [PubMed] [Google Scholar]
- 78.Espy, M. J., T. K. Ross, R. Teo, K. A. Svien, A. D. Wold, J. R. Uhl, and T. F. Smith. 2000. Evaluation of LightCycler PCR for implementation of laboratory diagnosis of herpes simplex virus infections. J. Clin. Microbiol. 38:3116-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Espy, M. J., J. R. Uhl, P. S. Mitchell, J. N. Thorvilson, K. A. Svien, A. D. Wold, and T. F. Smith. 2000. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J. Clin. Microbiol. 38:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang, X., Y. Mi, J. J. Li, T. Beck, S. Schuster, and W. Tan. 2002. Molecular beacons: fluorogenic probes for living cell study. Cell Biochem. Biophys. 37:71-81. [DOI] [PubMed] [Google Scholar]
- 81.Fedele, C. G., A. Avellon, M. Ciardi, S. Delia, and A. Tenorio. 2000. Quantitation of polyomavirus DNA by a competitive nested polymerase chain reaction. J. Virol. Methods 88:51-61. [DOI] [PubMed] [Google Scholar]
- 82.Fedele, C. G., M. Ciardi, S. Delia, J. M. Echevarria, and A. Tenorio. 1999. Multiplex polymerase chain reaction for the simultaneous detection and typing of polyomavirus JC, BK and SV40 DNA in clinical samples. J. Virol. Methods 82:137-144. [DOI] [PubMed] [Google Scholar]
- 83.Fodor, P. A., M. J. Levin, A. Weinberg, E. Sandberg, J. Sylman, and K. L. Tyler. 1998. Atypical herpes simplex virus encephalitis diagnosed by PCR amplification of viral DNA from CSF. Neurology 51:554-559. [DOI] [PubMed] [Google Scholar]
- 84.Fomsgaard, A., N. Kirkby, I. P. Jensen, and B. F. Vestergaard. 1998. Routine diagnosis of herpes simplex virus (HSV) encephalitis by an internal DNA controlled HSV PCR and an IgG-capture assay for intrathecal synthesis of HSV antibodies. Clin. Diagn. Virol. 9:45-56. [DOI] [PubMed] [Google Scholar]
- 85.Fox, J. D., S. Han, A. Samuelson, Y. Zhang, M. L. Neale, and D. Westmoreland. 2002. Development and evaluation of nucleic acid sequence based amplification (NASBA) for diagnosis of enterovirus infections using the NucliSens basic kit. J. Clin. Virol. 24:117-130. [DOI] [PubMed] [Google Scholar]
- 86.Frias, C., L. Matas, X. Ferre, M. Millan, S. Marti, A. Hernandez, and V. Ausina. 2001. Usefulness of adding multiplex nested-polymerase chain reaction assay of cerebrospinal fluid samples to routine diagnostic testing for herpesvirus encephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 20:670-672. [DOI] [PubMed] [Google Scholar]
- 87.Fujimoto, H., K. Asaoka, T. Imaizumi, M. Ayabe, H. Shoji, and M. Kaji. 2003. Epstein-Barr virus infections of the central nervous system. Intern. Med. 42:33-40. [DOI] [PubMed] [Google Scholar]
- 88.Garcia, D., V, R. Alonso, P. Miralles, J. Berenguer, M. Rodriguez-Creixems, and E. Bouza. 1999. Dual qualitative-quantitative nested PCR for detection of JC virus in cerebrospinal fluid: high potential for evaluation and monitoring of progressive multifocal leukoencephalopathy in AIDS patients receiving highly active antiretroviral therapy. J. Clin. Microbiol. 37:724-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garcia, D., V, I. M. Diaz, P. Miralles, J. Berenguer, M. Marin, L. Munoz, and E. Bouza. 2002. JC virus load in progressive multifocal leukoencephalopathy: analysis of the correlation between the viral burden in cerebrospinal fluid, patient survival, and the volume of neurological lesions. Clin. Infect. Dis. 34:1568-1575. [DOI] [PubMed] [Google Scholar]
- 90.Gateley, A., R. M. Gander, P. C. Johnson, S. Kit, H. Otsuka, and S. Kohl. 1990. Herpes simplex virus type 2 meningoencephalitis resistant to acyclovir in a patient with AIDS. J. Infect. Dis. 161:711-715. [DOI] [PubMed] [Google Scholar]
- 91.Gautheret-Dejean, A., C. Manichanh, F. Thien-Ah-Koon, A. M. Fillet, N. Mangeney, M. Vidaud, N. Dhedin, J. P. Vernant, and H. Agut. 2002. Development of a real-time polymerase chain reaction assay for the diagnosis of human herpesvirus-6 infection and application to bone marrow transplant patients. J. Virol. Methods 100:27-35. [DOI] [PubMed] [Google Scholar]
- 92.Giladi, M., E. Metzkor-Cotter, D. A. Martin, Y. Siegman-Igra, A. D. Korczyn, R. Rosso, S. A. Berger, G. L. Campbell, and R. S. Lanciotti. 2001. West Nile encephalitis in Israel, 1999: the New York connection. Emerg. Infect. Dis. 7:659-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilden, D. H. 2002. Varicella zoster virus vasculopathy and disseminated encephalomyelitis. J. Neurol. Sci. 195:99-101. [DOI] [PubMed] [Google Scholar]
- 94.Gilden, D. H., J. L. Bennett, B. K. Kleinschmidt-DeMasters, D. D. Song, A. S. Yee, and I. Steiner. 1998. The value of cerebrospinal fluid antiviral antibody in the diagnosis of neurologic disease produced by varicella zoster virus. J. Neurol. Sci. 159:140-144. [DOI] [PubMed] [Google Scholar]
- 95.Gilden, D. H., B. K. Kleinschmidt-DeMasters, J. J. LaGuardia, R. Mahalingam, and R. J. Cohrs. 2000. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 342:635-645. [DOI] [PubMed] [Google Scholar]
- 96.Gilden, D. H., R. Mahalingam, R. J. Cohrs, B. K. Kleinschmidt-DeMasters, and B. Forghani. 2002. The protean manifestations of varicella-zoster virus vasculopathy. J. Neurovirol. 8(Suppl. 2):75-79. [DOI] [PubMed] [Google Scholar]
- 97.Gilden, D. H., R. R. Wright, S. A. Schneck, J. M. Gwaltney, Jr., and R. Mahalingam. 1994. Zoster sine herpete, a clinical variant. Ann. Neurol. 35:530-533. [DOI] [PubMed] [Google Scholar]
- 98.Ginocchio, C. C. 2000. Laboratory diagnosis of human cytomegalovirus (HCMV) central nervous system disease in AIDS patients. Int. J. Antimicrob. Agents 16:447-453. [DOI] [PubMed] [Google Scholar]
- 99.Gisolf, E. H., R. H. Enting, S. Jurriaans, F. de Wolf, M. E. van der Ende, R. M. Hoetelmans, P. Portegies, and S. A. Danner. 2000. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS 14:1583-1589. [DOI] [PubMed] [Google Scholar]
- 100.Glaser, C. A., S. Gilliam, D. Schnurr, B. Forghani, S. Honarmand, N. Khetsuriani, M. Fischer, C. K. Cossen, and L. J. Anderson. 2003. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998-2000. Clin. Infect. Dis. 36:731-742. [DOI] [PubMed] [Google Scholar]
- 101.Glennon, M., and M. Cormican. 2001. Detection and diagnosis of mycobacterial pathogens using PCR. Expert Rev. Mol. Diagn. 1:163-174. [DOI] [PubMed] [Google Scholar]
- 102.Guffond, T., A. Dewilde, P. E. Lobert, D. Caparros-Lefebvre, D. Hober, and P. Wattre. 1994. Significance and clinical relevance of the detection of herpes simplex virus DNA by the polymerase chain reaction in cerebrospinal fluid from patients with presumed encephalitis. Clin. Infect. Dis. 18:744-749. [DOI] [PubMed] [Google Scholar]
- 103.Halwachs-Baumann, G., B. Genser, S. Pailer, H. Engele, H. Rosegger, A. Schalk, H. H. Kessler, and M. Truschnig-Wilders. 2002. Human cytomegalovirus load in various body fluids of congenitally infected newborns. J. Clin. Virol. 25(Suppl. 3):S81-S87. [DOI] [PubMed] [Google Scholar]
- 104.Hamilton, R. L., C. Achim, M. R. Grafe, J. C. Fremont, D. Miners, and C. A. Wiley. 1995. Herpes simplex virus brainstem encephalitis in an AIDS patient. Clin. Neuropathol. 14:45-50. [PubMed] [Google Scholar]
- 105.Hara, S., H. Kimura, Y. Hoshino, N. Hayashi, T. Negoro, A. Okumura, Y. Kajita, T. Sakuma, T. Nakayama, M. Hosoya, A. Tomoda, and T. Morisima. 2003. Combination therapy with intraventricular interferon-alpha and ribavirin for subacute sclerosing panencephalitis and monitoring measles virus RNA by quantitative PCR assay. Brain Dev. 25:367-369. [DOI] [PubMed] [Google Scholar]
- 106.Hirsch, H. H., P. R. Meylan, A. Iten, M. Battegay, and P. Erb. 1998. HIV-1-infected patients with focal neurologic signs: diagnostic role of PCR for Toxoplasma gondii, Epstein-Barr virus, and JC virus. Clin. Microbiol. Infect. 4:577-584. [DOI] [PubMed] [Google Scholar]
- 107.Huang, C., B. Slater, W. Campbell, J. Howard, and D. White. 2001. Detection of arboviral RNA directly from mosquito homogenates by reverse-transcription-polymerase chain reaction. J. Virol. Methods 94:121-128. [DOI] [PubMed] [Google Scholar]
- 108.Huang, C., B. Slater, R. Rudd, N. Parchuri, R. Hull, M. Dupuis, and A. Hindenburg. 2002. First isolation of West Nile virus from a patient with encephalitis in the United States. Emerg. Infect. Dis. 8:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hukin, J., K. Farrell, L. M. MacWilliam, M. Colbourne, E. Waida, R. Tan, L. Mroz, and E. Thomas. 1998. Case-control study of primary human herpesvirus 6 infection in children with febrile seizures. Pediatrics 101:E3. [DOI] [PubMed] [Google Scholar]
- 110.Imai, S., N. Usui, M. Sugiura, T. Osato, T. Sato, H. Tsutsumi, N. Tachi, S. Nakata, T. Yamanaka, S. Chiba, and. 1993. Epstein-Barr virus genomic sequences and specific antibodies in cerebrospinal fluid in children with neurologic complications of acute and reactivated EBV infections. J. Med. Virol. 40:278-284. [DOI] [PubMed] [Google Scholar]
- 111.Ishiguro, N., S. Yamada, T. Takahashi, Y. Takahashi, T. Togashi, T. Okuno, and K. Yamanishi. 1990. Meningo-encephalitis associated with HHV-6 related exanthem subitum. Acta Paediatr. Scand. 79:987-989. [DOI] [PubMed] [Google Scholar]
- 112.Iten, A., P. Chatelard, P. Vuadens, J. Miklossy, R. Meuli, R. Sahli, and P. R. Meylan. 1999. Impact of cerebrospinal fluid PCR on the management of HIV-infected patients with varicella-zoster virus infection of the central nervous system. J. Neurovirol. 5:172-180. [DOI] [PubMed] [Google Scholar]
- 113.Iwamoto, M., D. B. Jernigan, A. Guasch, M. J. Trepka, C. G. Blackmore, W. C. Hellinger, S. M. Pham, S. Zaki, R. S. Lanciotti, S. E. Lance-Parker, C. A. DiazGranados, A. G. Winquist, C. A. Perlino, S. Wiersma, K. L. Hillyer, J. L. Goodman, A. A. Marfin, M. E. Chamberland, and L. R. Petersen. 2003. Transmission of West Nile virus from an organ donor to four transplant recipients. N. Engl. J. Med. 348:2196-2203. [DOI] [PubMed] [Google Scholar]
- 114.Johnson, R. T. 1996. Acute encephalitis. Clin. Infect. Dis. 23:219-224. [DOI] [PubMed] [Google Scholar]
- 115.Johnson, R. T. 1998. Viral infections of the nervous system, 2nd ed. Lippincott-Raven Press, Philadelphia, Pa.
- 116.Joseph, P., M. M. Calderon, R. H. Gilman, M. L. Quispe, J. Cok, E. Ticona, V. Chavez, J. A. Jimenez, M. C. Chang, M. J. Lopez, and C. A. Evans. 2002. Optimization and evaluation of a PCR assay for detecting toxoplasmic encephalitis in patients with AIDS. J. Clin. Microbiol. 40:4499-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Julander, I., C. Martin, M. Lappalainen, E. Guy, B. Isberg, and B. Evengard. 2001. Polymerase chain reaction for diagnosis of cerebral toxoplasmosis in cerebrospinal fluid in HIV-positive patients. Scand. J. Infect. Dis. 33:538-541. [DOI] [PubMed] [Google Scholar]
- 118.Kahlon, J., S. Chatterjee, F. D. Lakeman, F. Lee, A. J. Nahmias, and R. J. Whitley. 1987. Detection of antibodies to herpes simplex virus in the cerebrospinal fluid of patients with herpes simplex encephalitis. J. Infect. Dis. 155:38-44. [DOI] [PubMed] [Google Scholar]
- 119.Kaji, M., and H. Shoji. 1995. Detection of Epstein-Barr virus genome in peripheral leucocytes and CSF by the polymerase chain reaction in two patients with Epstein-Barr virus related to aseptic meningitis. J. Neurol. Neurosurg. Psychiatry 59:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaji, M., and H. Shoji. 1997. Adenovirus CNS infections. Nippon Rinsho 55:876-879. [PubMed] [Google Scholar]
- 121.Kapusta, M., D. Dzierzanowska, D. Dunin-Wasowicz, B. Milewska-Bobula, A. Dobrzanska, U. Wojda, E. Swiatkowska, and G. Blaszczyk. 2001. Detection of cytomegalovirus in infant cerebrospinal fluid by conventional PCR, nested PCR and PCR-Digene. Acta Microbiol. Pol. 50:263-274. [PubMed] [Google Scholar]
- 122.Karlsen, F., H. B. Steen, and J. M. Nesland. 1995. SYBR green I DNA staining increases the detection sensitivity of viruses by polymerase chain reaction. J. Virol. Methods 55:153-156. [DOI] [PubMed] [Google Scholar]
- 123.Kawashima, H., T. Miyajima, T. Mori, L. Yuan, M. Ogihara, K. Kinoue, K. Takekuma, and A. Hoshika. 1996. A case of intractable epilepsy positive for the detection of measles virus genome in the cerebrospinal fluid and peripheral mononuclear cells using reverse transcriptase-polymerase chain reaction. Brain Dev. 18:220-223. [DOI] [PubMed] [Google Scholar]
- 124.Kimberlin, D., D. Powell, W. Gruber, P. Diaz, A. Arvin, M. Kumar, R. Jacobs, R. Van Dyke, S. Burchett, S. J. Soong, A. Lakeman, and R. Whitley. 1996. Administration of oral acyclovir suppressive therapy after neonatal herpes simplex virus disease limited to the skin, eyes and mouth: results of a phase I/II trial. Pediatr. Infect. Dis. J. 15:247-254. [DOI] [PubMed] [Google Scholar]
- 125.Kimberlin, D. W., F. D. Lakeman, A. M. Arvin, C. G. Prober, L. Corey, D. A. Powell, S. K. Burchett, R. F. Jacobs, S. E. Starr, R. J. Whitley, et al. 1996. Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. J. Infect. Dis. 174:1162-1167. [DOI] [PubMed] [Google Scholar]
- 126.Kimura, H., K. Aso, K. Kuzushima, N. Hanada, M. Shibata, and T. Morishima. 1992. Relapse of herpes simplex encephalitis in children. Pediatrics 89:891-894. [PubMed] [Google Scholar]
- 127.Kimura, H., Y. Ito, M. Futamura, Y. Ando, Y. Yabuta, Y. Hoshino, Y. Nishiyama, and T. Morishima. 2002. Quantitation of viral load in neonatal herpes simplex virus infection and comparison between type 1 and type 2. J. Med. Virol. 67:349-353. [DOI] [PubMed] [Google Scholar]
- 128.Kleinschmidt-DeMasters, B. K., R. L. DeBiasi, and K. L. Tyler. 2001. Polymerase chain reaction as a diagnostic adjunct in herpesvirus infections of the nervous system. Brain Pathol. 11:452-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kleinschmidt-DeMasters, B. K., and D. H. Gilden. 2001. Varicella-zoster virus infections of the nervous system: clinical and pathologic correlates. Arch. Pathol. Lab Med. 125:770-780. [DOI] [PubMed] [Google Scholar]
- 130.Knox, K. K., and D. R. Carrigan. 1995. Active human herpesvirus (HHV-6) infection of the central nervous system in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9:69-73. [PubMed] [Google Scholar]
- 131.Knox, K. K., D. P. Harrington, and D. R. Carrigan. 1995. Fulminant human herpesvirus six encephalitis in a human immunodeficiency virus-infected infant. J. Med. Virol. 45:288-292. [DOI] [PubMed] [Google Scholar]
- 132.Kondo, K., H. Nagafuji, A. Hata, C. Tomomori, and K. Yamanishi. 1993. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J. Infect. Dis. 167:1197-1200. [DOI] [PubMed] [Google Scholar]
- 133.Koralnik, I. J., D. Boden, V. X. Mai, C. I. Lord, and N. L. Letvin. 1999. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology 52:253-260. [DOI] [PubMed] [Google Scholar]
- 134.Koskiniemi, M., H. Piiparinen, T. Rantalaiho, P. Eranko, M. Farkkila, K. Raiha, E. M. Salonen, P. Ukkonen, and A. Vaheri. 2002. Acute central nervous system complications in varicella zoster virus infections. J. Clin. Virol. 25:293-301. [DOI] [PubMed] [Google Scholar]
- 135.Koskiniemi, M., T. Rantalaiho, H. Piiparinen, C. H. von Bonsdorff, M. Farkkila, A. Jarvinen, E. Kinnunen, S. Koskiniemi, L. Mannonen, M. Muttilainen, K. Linnavuori, J. Porras, M. Puolakkainen, K. Raiha, E. M. Salonen, P. Ukkonen, A. Vaheri, and V. Valtonen. 2001. Infections of the central nervous system of suspected viral origin: a collaborative study from Finland. J. Neurovirol. 7:400-408. [DOI] [PubMed] [Google Scholar]
- 136.Kreis, S., and B. D. Schoub. 1998. Partial amplification of the measles virus nucleocapsid gene from stored sera and cerebrospinal fluids for molecular epidemiological studies. J. Med. Virol. 56:174-177. [PubMed] [Google Scholar]
- 137.Kricka, L. J. 2002. Stains, labels and detection strategies for nucleic acids assays. Ann. Clin. Biochem. 39:114-129. [DOI] [PubMed] [Google Scholar]
- 138.Kuno, G. 1998. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 72:27-41. [DOI] [PubMed] [Google Scholar]
- 139.Lakeman, F. D., R. J. Whitley, et al. 1995. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J. Infect. Dis. 171:857-863. [DOI] [PubMed] [Google Scholar]
- 140.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Landgren, M., M. Kyllerman, T. Bergstrom, L. Dotevall, L. Ljungstrom, and A. Ricksten. 1994. Diagnosis of Epstein-Barr virus-induced central nervous system infections by DNA amplification from cerebrospinal fluid. Ann. Neurol. 35:631-635. [DOI] [PubMed] [Google Scholar]
- 142.Lebech, A. M. 2002. Polymerase chain reaction in diagnosis of Borrelia burgdorferi infections and studies on taxonomic classification. APMIS Suppl. 105:1-40. [PubMed]
- 143.Lebech, A. M., K. Hansen, F. Brandrup, O. Clemmensen, and L. Halkier-Sorensen. 2000. Diagnostic value of PCR for detection of Borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and Lyme neuroborreliosis. Mol. Diagn. 5:139-150. [DOI] [PubMed] [Google Scholar]
- 144.Lekanne Deprez, R. H., A. C. Fijnvandraat, J. M. Ruijter, and A. F. Moorman. 2002. Sensitivity and accuracy of quantitative real-time polymerase chain reaction using SYBR green I depends on cDNA synthesis conditions. Anal. Biochem. 307:63-69. [DOI] [PubMed] [Google Scholar]
- 145.Luppi, M., P. Barozzi, A. Maiorana, R. Marasca, R. Trovato, R. Fano, L. Ceccherini-Nelli, and G. Torelli. 1995. Human herpesvirus-6: a survey of presence and distribution of genomic sequences in normal brain and neuroglial tumors. J. Med. Virol. 47:105-111. [DOI] [PubMed] [Google Scholar]
- 146.Madhoun, Z. T., D. B. DuBois, J. Rosenthal, J. C. Findlay, and D. C. Aron. 1991. Central diabetes insipidus: a complication of herpes simplex type 2 encephalitis in a patient with AIDS. Am. J. Med. 90:658-659. [PubMed] [Google Scholar]
- 147.Markoulatos, P., A. Georgopoulou, N. Siafakas, E. Plakokefalos, G. Tzanakaki, and J. Kourea-Kremastinou. 2001. Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay. J. Clin. Microbiol. 39:4426-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marshall, G. S., M. A. Hauck, G. Buck, and G. P. Rabalais. 1997. Potential cost savings through rapid diagnosis of enteroviral meningitis. Pediatr. Infect. Dis. J. 16:1086-1087. [DOI] [PubMed] [Google Scholar]
- 149.Matsuzaki, T., M. Nakagawa, M. Nagai, Y. Nobuhara, K. Usuku, I. Higuchi, K. Takahashi, T. Moritoyo, K. Arimura, S. Izumo, S. Akiba, and M. Osame. 2000. HTLV-I-associated myelopathy (HAM)/tropical spastic paraparesis (TSP) with amyotrophic lateral sclerosis-like manifestations. J. Neurovirol. 6:544-548. [DOI] [PubMed] [Google Scholar]
- 150.Matsuzono, Y., M. Narita, N. Ishiguro, and T. Togashi. 1994. Detection of measles virus from clinical samples using the polymerase chain reaction. Arch. Pediatr. Adolesc. Med. 148:289-293. [DOI] [PubMed] [Google Scholar]
- 151.McArthur, J. C., D. R. McClernon, M. F. Cronin, T. E. Nance-Sproson, A. J. Saah, M. St Clair, and E. R. Lanier. 1997. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann. Neurol. 42:689-698. [DOI] [PubMed] [Google Scholar]
- 152.McCullers, J. A., F. D. Lakeman, and R. J. Whitley. 1995. Human herpesvirus 6 is associated with focal encephalitis. Clin. Infect. Dis. 21:571-576. [DOI] [PubMed] [Google Scholar]
- 153.McMinn, P. C., P. G. Carman, and D. W. Smith. 2000. Early diagnosis of Murray Valley encephalitis by reverse transcriptase-polymerase chain reaction. Pathology 32:49-51. [DOI] [PubMed] [Google Scholar]
- 154.Medhurst, A. D., D. C. Harrison, S. J. Read, C. A. Campbell, M. J. Robbins, and M. N. Pangalos. 2000. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J. Neurosci. Methods 98:9-20. [DOI] [PubMed] [Google Scholar]
- 155.Meerbach, A., B. Gruhn, R. Egerer, U. Reischl, F. Zintl, and P. Wutzler. 2001. Semiquantitative PCR analysis of Epstein-Barr virus DNA in clinical samples of patients with EBV-associated diseases. J. Med. Virol. 65:348-357. [DOI] [PubMed] [Google Scholar]
- 156.Mertens, G., M. Ieven, D. Ursi, S. R. Pattyn, J. J. Martin, and P. M. Parizel. 1993. Detection of herpes simplex virus in the cerebrospinal fluid of patients with encephalitis using the polymerase chain reaction. J. Neurol. Sci. 118:213-216. [DOI] [PubMed] [Google Scholar]
- 157.Meylan, P. R., P. Vuadens, P. Maeder, R. Sahli, and M. C. Tagan. 1999. Monitoring the response of AIDS-related progressive multifocal leukoencephalopathy to HAART and cidofovir by PCR for JC virus DNA in the CSF. Eur. Neurol. 41:172-174. [DOI] [PubMed] [Google Scholar]
- 158.Minjolle, S., C. Michelet, I. Jusselin, M. Joannes, F. Cartier, and R. Colimon. 1999. Amplification of the six major human herpesviruses from cerebrospinal fluid by a single PCR. J. Clin. Microbiol. 37:950-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mitchell, P. S., M. J. Espy, T. F. Smith, D. R. Toal, P. N. Rys, E. F. Berbari, D. R. Osmon, and D. H. Persing. 1997. Laboratory diagnosis of central nervous system infections with herpes simplex virus by PCR performed with cerebrospinal fluid specimens. J. Clin. Microbiol. 35:2873-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Monpoeho, S., M. Coste-Burel, M. Costa-Mattioli, B. Besse, J. J. Chomel, S. Billaudel, and V. Ferre. 2002. Application of a real-time polymerase chain reaction with internal positive control for detection and quantification of enterovirus in cerebrospinal fluid. Eur.J. Clin. Microbiol. Infect. Dis. 21:532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moulignier, A., M. Baudrimont, M. L. Martin-Negrier, J. Mikol, C. Lapresle, and B. Dupont. 1996. Fatal brain stem encephalitis due to herpes simplex virus type 1 in AIDS. J. Neurol. 243:491-493. [DOI] [PubMed] [Google Scholar]
- 162.Nagai, M., K. Usuku, W. Matsumoto, D. Kodama, N. Takenouchi, T. Moritoyo, S. Hashiguchi, M. Ichinose, C. R. Bangham, S. Izumo, and M. Osame. 1998. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 4:586-593. [DOI] [PubMed] [Google Scholar]
- 163.Nakajima, H., D. Furutama, F. Kimura, K. Shinoda, N. Ohsawa, T. Nakagawa, A. Shimizu, and H. Shoji. 1998. Herpes simplex virus myelitis: clinical manifestations and diagnosis by the polymerase chain reaction method. Eur. Neurol. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 164.Narita, M., Y. Matsuzono, T. Togashi, and N. Kajii. 1992. DNA diagnosis of central nervous system infection by Mycoplasma pneumoniae. Pediatrics 90:250-253. [PubMed] [Google Scholar]
- 165.Parasuraman, T. V., K. Frenia, and J. Romero. 2001. Enteroviral meningitis. Cost of illness and considerations for the economic evaluation of potential therapies. Pharmacoeconomics 19:3-12. [DOI] [PubMed] [Google Scholar]
- 166.Pealer, L. N., A. A. Marfin, L. R. Petersen, R. S. Lanciotti, P. L. Page, S. L. Stramer, M. G. Stobierski, K. Signs, B. Newman, H. Kapoor, J. L. Goodman, and M. E. Chamberland. 2003. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 349:1236-1245. [DOI] [PubMed] [Google Scholar]
- 167.Petersen, L. R., and A. A. Marfin. 2002. West Nile virus: a primer for the clinician. Ann. Intern. Med. 137:173-179. [DOI] [PubMed] [Google Scholar]
- 168.Pichichero, M. E., S. McLinn, H. A. Rotbart, M. A. Menegus, M. Cascino, and B. E. Reidenberg. 1998. Clinical and economic impact of enterovirus illness in private pediatric practice. Pediatrics 102:1126-1134. [DOI] [PubMed] [Google Scholar]
- 169.Portillo-Gomez, L., S. L. Morris, and A. Panduro. 2000. Rapid and efficient detection of extra-pulmonary Mycobacterium tuberculosis by PCR analysis. Int. J. Tuberc. Lung Dis. 4:361-370. [PubMed] [Google Scholar]
- 170.Portolani, M., C. Cermelli, M. Meacci, P. Pietrosemoli, A. M. Sabbatini, M. C. Cerri, G. Guaraldi, and B. De Rienzo. 1999. Epstein-Barr virus DNA in the cerebrospinal fluid of patients with human immunodeficiency virus infection and central nervous system disorders. New Microbiol. 22:369-374. [PubMed] [Google Scholar]
- 171.Portolani, M., P. Pietrosemoli, M. Meacci, A. M. Sabbatini, M. Pecorari, G. Mantovani, and C. Cermelli. 1998. Detection of Epstein-Barr virus DNA in cerebrospinal fluid from immunocompetent individuals with brain disorders. New Microbiol. 21:77-79. [PubMed] [Google Scholar]
- 172.Price, R. W., E. E. Paxinos, R. M. Grant, B. Drews, A. Nilsson, R. Hoh, N. S. Hellmann, C. J. Petropoulos, and S. G. Deeks. 2001. Cerebrospinal fluid response to structured treatment interruption after virological failure. AIDS 15:1251-1259. [DOI] [PubMed] [Google Scholar]
- 173.Puccioni-Sohler, M., M. Rios, S. M. Carvalho, R. R. Goncalves, C. Oliveira, R. B. Correa, S. Novis, M. S. de Oliveira, and C. Bianco. 2001. Diagnosis of HAM/TSP based on CSF proviral HTLV-I DNA and HTLV-I antibody index. Neurology 57:725-727. [DOI] [PubMed] [Google Scholar]
- 174.Puchhammer-Stockl, E., F. X. Heinz, M. Kundi, T. Popow-Kraupp, G. Grimm, M. M. Millner, and C. Kunz. 1993. Evaluation of the polymerase chain reaction for diagnosis of herpes simplex virus encephalitis. J. Clin. Microbiol. 31:146-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Puchhammer-Stockl, E., T. Popow-Kraupp, F. X. Heinz, C. W. Mandl, and C. Kunz. 1990. Establishment of PCR for the early diagnosis of herpes simplex encephalitis. J. Med. Virol. 32:77-82. [DOI] [PubMed] [Google Scholar]
- 176.Puchhammer-Stockl, E., E. Presterl, C. Croy, S. Aberle, T. Popow-Kraupp, M. Kundi, H. Hofmann, U. Wenninger, and I. Godl. 2001. Screening for possible failure of herpes simplex virus PCR in cerebrospinal fluid for the diagnosis of herpes simplex encephalitis. J. Med. Virol. 64:531-536. [DOI] [PubMed] [Google Scholar]
- 177.Quereda, C., I. Corral, F. Laguna, M. E. Valencia, A. Tenorio, J. E. Echeverria, E. Navas, P. Martin-Davila, A. Moreno, V. Moreno, J. M. Gonzalez-Lahoz, J. R. Arribas, and A. Guerrero. 2000. Diagnostic utility of a multiplex herpesvirus PCR assay performed with cerebrospinal fluid from human immunodeficiency virus-infected patients with neurological disorders. J. Clin. Microbiol. 38:3061-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rabenau, H. F., A. M. Clarici, G. Muhlbauer, A. Berger, A. Vince, S. Muller, E. Daghofer, B. I. Santner, E. Marth, and H. H. Kessler. 2002. Rapid detection of enterovirus infection by automated RNA extraction and real-time fluorescence PCR. J. Clin. Virol. 25:155-164. [DOI] [PubMed] [Google Scholar]
- 179.Ramers, C., G. Billman, M. Hartin, S. Ho, and M. H. Sawyer. 2000. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. JAMA 283:2680-2685. [DOI] [PubMed] [Google Scholar]
- 180.Read, S. J., and J. B. Kurtz. 1999. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J. Clin. Microbiol. 37:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180a.Read, S. J., K. J. Jeffery, and C. R. Bangham. 1997. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J. Clin. Microbiol. 35:691-696. (Erratum, 35:1649.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Read, S. J., J. L. Mitchell, and C. G. Fink. 2001. LightCycler multiplex PCR for the laboratory diagnosis of common viral infections of the central nervous system. J. Clin. Microbiol. 39:3056-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Revello, M. G., F. Baldanti, A. Sarasini, D. Zella, M. Zavattoni, and G. Gerna. 1997. Quantitation of herpes simplex virus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis by the polymerase chain reaction. Clin. Diagn. Virol. 7:183-191. [DOI] [PubMed] [Google Scholar]
- 183.Roberts, T. C., and G. A. Storch. 1997. Multiplex PCR for diagnosis of AIDS-related central nervous system lymphoma and toxoplasmosis. J. Clin. Microbiol. 35:268-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Robertson, K., S. Fiscus, C. Kapoor, W. Robertson, G. Schneider, R. Shepard, L. Howe, S. Silva, and C. Hall. 1998. CSF, plasma viral load and HIV associated dementia. J. Neurovirol. 4:90-94. [DOI] [PubMed] [Google Scholar]
- 185.Robinson, C. C., M. Willis, A. Meagher, K. E. Gieseker, H. Rotbart, and M. P. Glode. 2002. Impact of rapid polymerase chain reaction results on management of pediatric patients with enteroviral meningitis. Pediatr. Infect. Dis. J. 21:283-286. [DOI] [PubMed] [Google Scholar]
- 186.Roman-Campos, G., C. A. Phillips, and C. M. Poser. 1980. Herpes simplex encephalitis. Lancet i:489. [DOI] [PubMed] [Google Scholar]
- 187.Romero, J. R. 1999. Reverse-transcription polymerase chain reaction detection of the enteroviruses. Arch. Pathol. Lab. Med. 123:1161-1169. [DOI] [PubMed] [Google Scholar]
- 188.Romero, J. R. 2001. Pleconaril: a novel antipicornaviral drug. Expert. Opin. Investig. Drugs 10:369-379. [DOI] [PubMed] [Google Scholar]
- 189.Romero, J. R. 2002. Diagnosis and management of enteroviral infections of the central nervous system. Curr. Infect. Dis. Rep. 4:309-316. [DOI] [PubMed] [Google Scholar]
- 190.Roos, K. L. 1999. Encephalitis. Neurol. Clin. 17:813-833. [DOI] [PubMed] [Google Scholar]
- 191.Rose, J. W., W. G. Stroop, F. Matsuo, and J. Henkel. 1992. Atypical herpes simplex encephalitis: clinical, virologic, and neuropathologic evaluation. Neurology 42:1809-1812. [DOI] [PubMed] [Google Scholar]
- 192.Rotbart, H. A. 1990. Diagnosis of enteroviral meningitis with the polymerase chain reaction. J. Pediatr. 117:85-89. [DOI] [PubMed] [Google Scholar]
- 193.Rotbart, H. A. 1991. Nucleic acid detection systems for enteroviruses. Clin. Microbiol. Rev. 4:156-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rotbart, H. A. 1995. Enteroviral infections of the central nervous system. Clin. Infect. Dis. 20:971-981. [DOI] [PubMed] [Google Scholar]
- 195.Rotbart, H. A. 1997. Reproducibility of AMPLICOR enterovirus PCR test results. J. Clin. Microbiol. 35:3301-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rotbart, H. A. 1999. Antiviral therapy for enteroviral infections. Pediatr. Infect. Dis.J. 18:632-633. [DOI] [PubMed] [Google Scholar]
- 197.Rotbart, H. A. 2002. Treatment of picornavirus infections. Antiviral Res. 53:83-98. [DOI] [PubMed] [Google Scholar]
- 198.Rotbart, H. A., G. H. McCracken, Jr., R. J. Whitley, J. F. Modlin, M. Cascino, S. Shah, and D. Blum. 1999. Clinical significance of enteroviruses in serious summer febrile illnesses of children. Pediatr. Infect. Dis. J. 18:869-874. [DOI] [PubMed] [Google Scholar]
- 199.Rotbart, H. A., J. F. O'Connell, and M. A. McKinlay. 1998. Treatment of human enterovirus infections. Antiviral Res. 38:1-14. [DOI] [PubMed] [Google Scholar]
- 200.Rotbart, H. A., M. H. Sawyer, S. Fast, C. Lewinski, N. Murphy, E. F. Keyser, J. Spadoro, S. Y. Kao, and M. Loeffelholz. 1994. Diagnosis of enteroviral meningitis by using PCR with a colorimetric microwell detection assay. J. Clin. Microbiol. 32:2590-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rotbart, H. A., and A. D. Webster. 2001. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin. Infect. Dis. 32:228-235. [DOI] [PubMed] [Google Scholar]
- 202.Sadler, M., R. Al Shahi, P. Weeks, M. R. Nelson, and B. G. Gazzard. 1998. Clinical utility of PCR on cerebrospinal fluid for the diagnosis and management of HIV neurological complications. AIDS 12:2352-2353. [PubMed] [Google Scholar]
- 203.Sawyer, M. H. 1999. Enterovirus infections: diagnosis and treatment. Pediatr. Infect. Dis. J. 18:1033-1039. [DOI] [PubMed] [Google Scholar]
- 204.Sawyer, M. H. 2001. Enterovirus infections: diagnosis and treatment. Curr. Opin. Pediatr. 13:65-69. [DOI] [PubMed] [Google Scholar]
- 205.Sawyer, M. H. 2002. Enterovirus infections: diagnosis and treatment. Semin. Pediatr. Infect. Dis. 13:40-47. [DOI] [PubMed] [Google Scholar]
- 206.Sawyer, M. H., D. Holland, N. Aintablian, J. D. Connor, E. F. Keyser, and N. J. Waecker, Jr. 1994. Diagnosis of enteroviral central nervous system infection by polymerase chain reaction during a large community outbreak. Pediatr. Infect. Dis. J. 13:177-182. [DOI] [PubMed] [Google Scholar]
- 207.Scaramozzino, N., J. M. Crance, A. Jouan, D. A. DeBriel, F. Stoll, and D. Garin. 2001. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J. Clin. Microbiol. 39:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schmidbauer, M., H. Budka, and P. Ambros. 1989. Herpes simplex virus (HSV) DNA in microglial nodular brainstem encephalitis. J. Neuropathol. Exp. Neurol. 48:645-652. [DOI] [PubMed] [Google Scholar]
- 209.Schwaiger, M., and P. Cassinotti. 2003. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J. Clin. Virol. 27:136-145. [DOI] [PubMed] [Google Scholar]
- 210.Sgarabotto, D., S. Buoro, C. Mengoli, F. Scano, P. Cadrobbi, D. Di Luca, and G. Palu. 2000. PCR in meningoencephalitis diagnosis. Scand. J. Infect. Dis. 32:689-692. [DOI] [PubMed] [Google Scholar]
- 211.Shinkai, M., and S. A. Spector. 1995. Quantitation of human cytomegalovirus (HCMV) DNA in cerebrospinal fluid by competitive PCR in AIDS patients with different HCMV central nervous system diseases. Scand. J. Infect. Dis. 27:559-561. [DOI] [PubMed] [Google Scholar]
- 212.Skoldenberg, B. 1996. Herpes simplex encephalitis. Scand. J. Infect. Dis. Suppl. 100:8-13. [PubMed] [Google Scholar]
- 213.Soini, H., and J. M. Musser. 2001. Molecular diagnosis of mycobacteria. Clin. Chem. 47:809-814. [PubMed] [Google Scholar]
- 214.Soong, S. J., N. E. Watson, G. R. Caddell, C. A. Alford, Jr., R. J. Whitley, et al. 1991. Use of brain biopsy for diagnostic evaluation of patients with suspected herpes simplex encephalitis: a statistical model and its clinical implications. J. Infect. Dis. 163:17-22. [DOI] [PubMed] [Google Scholar]
- 215.Spector, S. A., K. Hsia, D. Wolf, M. Shinkai, and I. Smith. 1995. Molecular detection of human cytomegalovirus and determination of genotypic ganciclovir resistance in clinical specimens. Clin. Infect. Dis. 21(Suppl. 2):S170-S173. [DOI] [PubMed] [Google Scholar]
- 216.Starlin, R., N. Reed, B. Leeman, J. Black, E. Trulock, and L. M. Mundy. 2001. Acute flaccid paralysis syndrome associated with echovirus 19, managed with pleconaril and intravenous immunoglobulin. Clin. Infect. Dis. 33:730-732. [DOI] [PubMed] [Google Scholar]
- 217.Stellrecht, K. A., I. Harding, A. M. Woron, M. L. Lepow, and R. A. Venezia. 2002. The impact of an enteroviral RT-PCR assay on the diagnosis of aseptic meningitis and patient management. J. Clin. Virol. 25(Suppl. 1):S19-S26. [DOI] [PubMed] [Google Scholar]
- 218.Stocher, M., V. Leb, M. Bozic, H. H. Kessler, G. Halwachs-Baumann, O. Landt, H. Stekel, and J. Berg. 2003. Parallel detection of five human herpes virus DNAs by a set of real-time polymerase chain reactions in a single run. J. Clin. Virol. 26:85-93. [DOI] [PubMed] [Google Scholar]
- 219.Studahl, M., T. Bergstrom, and L. Hagberg. 1998. Acute viral encephalitis in adults—a prospective study. Scand. J. Infect. Dis. 30:215-220. [DOI] [PubMed] [Google Scholar]
- 220.Studahl, M., L. Hagberg, E. Rekabdar, and T. Bergstrom. 2000. Herpesvirus DNA detection in cerebral spinal fluid: differences in clinical presentation between alpha-, beta-, and gamma-herpesviruses. Scand. J. Infect. Dis. 32:237-248. [DOI] [PubMed] [Google Scholar]
- 221.Studahl, M., A. Ricksten, T. Sandberg, S. Elowson, S. Herner, C. Sall, and T. Bergstrom. 1994. Cytomegalovirus infection of the CNS in non-compromised patients. Acta Neurol. Scand. 89:451-457. [DOI] [PubMed] [Google Scholar]
- 222.Sugaya, N., T. Yoshikawa, M. Miura, T. Ishizuka, C. Kawakami, and Y. Asano. 2002. Influenza encephalopathy associated with infection with human herpesvirus 6 and/or human herpesvirus 7. Clin. Infect. Dis. 34:461-466. [DOI] [PubMed] [Google Scholar]
- 223.Tachikawa, N., M. Goto, Y. Hoshino, H. Gatanaga, A. Yasuoka, T. Wakabayashi, H. Katano, S. Kimura, S. Oka, and A. Iwamoto. 1999. Detection of Toxoplasma gondii, Epstein-Barr virus, and JC virus DNAs in the cerebrospinal fluid in acquired immunodeficiency syndrome patients with focal central nervous system complications. Intern. Med. 38:556-562. [DOI] [PubMed] [Google Scholar]
- 224.Tang, Y. W., P. S. Mitchell, M. J. Espy, T. F. Smith, and D. H. Persing. 1999. Molecular diagnosis of herpes simplex virus infections in the central nervous system. J. Clin. Microbiol. 37:2127-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Taoufik, Y., J. F. Delfraissy, and J. Gasnault. 2000. Highly active antiretroviral therapy does not improve survival of patients with high JC virus load in the cerebrospinal fluid at progressive multifocal leukoencephalopathy diagnosis. AIDS 14:758-759. [DOI] [PubMed] [Google Scholar]
- 226.Taoufik, Y., J. Gasnault, A. Karaterki, F. M. Pierre, E. Marchadier, C. Goujard, A. Lannuzel, J. F. Delfraissy, and E. Dussaix. 1998. Prognostic value of JC virus load in cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. J. Infect. Dis. 178:1816-1820. [DOI] [PubMed] [Google Scholar]
- 227.Taus, C., E. Pucci, E. Cartechini, A. Fie, G. Giuliani, M. Clementi, and S. Menzo. 2000. Absence of HHV-6 and HHV-7 in cerebrospinal fluid in relapsing-remitting multiple sclerosis. Acta Neurol. Scand. 101:224-228. [DOI] [PubMed] [Google Scholar]
- 228.Teach, S. J., H. L. Wallace, M. J. Evans, P. K. Duffner, J. Hay, and H. S. Faden. 1999. Human herpesviruses types 6 and 7 and febrile seizures. Pediatr. Neurol. 21:699-703. [DOI] [PubMed] [Google Scholar]
- 229.Tebas, P., R. F. Nease, and G. A. Storch. 1998. Use of the polymerase chain reaction in the diagnosis of herpes simplex encephalitis: a decision analysis model. Am. J. Med. 105:287-295. [DOI] [PubMed] [Google Scholar]
- 230.Thomson, R. B., Jr., and H. Bertram. 2001. Laboratory diagnosis of central nervous system infections. Infect. Dis. Clin. N. Am. 15:1047-1071. [DOI] [PubMed] [Google Scholar]
- 231.Troendle, A. J., G. J. Demmler, W. D. Williamson, J. M. McDonald, A. S. Istas, and G. J. Buffone. 1994. Polymerase chain reaction to detect cytomegalovirus DNA in the cerebrospinal fluid of neonates with congenital infection. J. Infect. Dis. 169:1334-1337. [DOI] [PubMed] [Google Scholar]
- 232.Troendle-Atkins, J., G. J. Demmler, and G. J. Buffone. 1993. Rapid diagnosis of herpes simplex virus encephalitis by using the polymerase chain reaction. J. Pediatr. 123:376-380. [DOI] [PubMed] [Google Scholar]
- 233.Tselis, A., R. Duman, G. A. Storch, and R. P. Lisak. 1997. Epstein-Barr virus encephalomyelitis diagnosed by polymerase chain reaction: detection of the genome in the CSF. Neurology 48:1351-1355. [DOI] [PubMed] [Google Scholar]
- 234.Tyler, K. L. 2003. Human herpesvirus 6 and multiple sclerosis: the continuing conundrum. J. Infect. Dis. 187:1360-1364. [DOI] [PubMed] [Google Scholar]
- 235.Tyler, K. L., D. G. Tedder, L. J. Yamamoto, J. A. Klapper, R. Ashley, K. A. Lichtenstein, and M. J. Levin. 1995. Recurrent brainstem encephalitis associated with herpes simplex virus type 1 DNA in cerebrospinal fluid. Neurology 45:2246-2250. [DOI] [PubMed] [Google Scholar]
- 236.Uren, E. C., P. D. Johnson, J. Montanaro, and G. L. Gilbert. 1993. Herpes simplex virus encephalitis in pediatrics: diagnosis by detection of antibodies and DNA in cerebrospinal fluid. Pediatr. Infect. Dis. J. 12:1001-1006. [DOI] [PubMed] [Google Scholar]
- 237.van der Meer, J. T., W. L. Drew, R. A. Bowden, G. J. Galasso, P. D. Griffiths, D. A. Jabs, C. Katlama, S. A. Spector, and R. J. Whitley. 1996. Summary of the International Consensus Symposium on Advances in the Diagnosis, Treatment and Prophylaxis and Cytomegalovirus Infection. Antiviral Res. 32:119-140. [DOI] [PubMed] [Google Scholar]
- 238.VanLandingham, K. E., H. B. Marsteller, G. W. Ross, and F. G. Hayden. 1988. Relapse of herpes simplex encephalitis after conventional acyclovir therapy. JAMA 259:1051-1053. [PubMed] [Google Scholar]
- 239.Verstrepen, W. A., P. Bruynseels, and A. H. Mertens. 2002. Evaluation of a rapid real-time RT-PCR assay for detection of enterovirus RNA in cerebrospinal fluid specimens. J. Clin. Virol. 25(Suppl. 1):S39-S43. [DOI] [PubMed] [Google Scholar]
- 240.Verstrepen, W. A., S. Kuhn, M. M. Kockx, M. E. Van De Vyvere, and A. H. Mertens. 2001. Rapid detection of enterovirus RNA in cerebrospinal fluid specimens with a novel single-tube real-time reverse transcription-PCR assay. J. Clin. Microbiol. 39:4093-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wacharapluesadee, S., and T. Hemachudha. 2001. Nucleic-acid sequence based amplification in the rapid diagnosis of rabies. Lancet 358:892-893. [DOI] [PubMed] [Google Scholar]
- 242.Wairagkar, N. S., N. J. Shaikh, R. K. Ratho, D. Ghosh, R. C. Mahajan, S. Singhi, and D. A. Gadkari. 2001. Isolation of measles virus from cerebrospinal fluid of children with acute encephalopathy without rash. Indian Pediatr. 38:589-595. [PubMed] [Google Scholar]
- 243.Wang, L., A. K. Gaigalas, J. Blasic, M. J. Holden, D. T. Gallagher, and R. Pires. 2003. Fluorescence resonance energy transfer between donor-acceptor pair on two oligonucleotides hybridized adjacently to DNA template. Biopolymers 72:401-412. [DOI] [PubMed] [Google Scholar]
- 244.Weber, T., S. Frye, M. Bodemer, M. Otto, and W. Luke. 1996. Clinical implications of nucleic acid amplification methods for the diagnosis of viral infections of the nervous system. J. Neurovirol. 2:175-190. [DOI] [PubMed] [Google Scholar]
- 245.Weber, T., P. E. Klapper, G. M. Cleator, M. Bodemer, W. Luke, W. Knowles, P. Cinque, A. M. van Loon, M. Grandien, A. L. Hammarin, M. Ciardi, G. Bogdanovic, et al. 1997. Polymerase chain reaction for detection of JC virus DNA in cerebrospinal fluid: a quality control study. J. Virol. Methods 69:231-237. [DOI] [PubMed] [Google Scholar]
- 246.Webster, A. D., H. A. Rotbart, T. Warner, P. Rudge, and N. Hyman. 1993. Diagnosis of enterovirus brain disease in hypogammaglobulinemic patients by polymerase chain reaction. Clin. Infect. Dis. 17:657-661. [DOI] [PubMed] [Google Scholar]
- 247.Weil, A. A., C. A. Glaser, Z. Amad, and B. Forghani. 2002. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin. Infect. Dis. 34:1154-1157. [DOI] [PubMed] [Google Scholar]
- 248.Weinberg, A., D. Spiers, G. Y. Cai, C. M. Long, R. Sun, and V. Tevere. 1998. Evaluation of a commercial PCR kit for diagnosis of cytomegalovirus infection of the central nervous system. J. Clin. Microbiol. 36:3382-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.White, D. J., L. D. Kramer, P. B. Backenson, G. Lukacik, G. Johnson, J. A. Oliver, J. J. Howard, R. G. Means, M. Eidson, I. Gotham, V. Kulasekera, and S. Campbell. 2001. Mosquito surveillance and polymerase chain reaction detection of West Nile virus, New York State. Emerg. Infect. Dis. 7:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Whitley, R. J. 1990. Viral encephalitis. N. Engl. J. Med. 323:242-250. [DOI] [PubMed] [Google Scholar]
- 251.Whitley, R. J., and J. W. Gnann. 2002. Viral encephalitis: familiar infections and emerging pathogens. Lancet 359:507-513. [DOI] [PubMed] [Google Scholar]
- 252.Whitley, R. J., and D. W. Kimberlin. 1999. Viral encephalitis. Pediatr. Rev. 20:192-198. [DOI] [PubMed] [Google Scholar]
- 253.Wildemann, B., K. Ehrhart, B. Storch-Hagenlocher, U. Meyding-Lamade, S. Steinvorth, W. Hacke, and J. Haas. 1997. Quantitation of herpes simplex virus type 1 DNA in cells of cerebrospinal fluid of patients with herpes simplex virus encephalitis. Neurology 48:1341-1346. [DOI] [PubMed] [Google Scholar]
- 254.Wildemann, B., J. Haas, N. Lynen, K. Stingele, and B. Storch-Hagenlocher. 1998. Diagnosis of cytomegalovirus encephalitis in patients with AIDS by quantitation of cytomegalovirus genomes in cells of cerebrospinal fluid. Neurology 50:693-697. [DOI] [PubMed] [Google Scholar]
- 255.Wittwer, C. T., M. G. Herrmann, A. A. Moss, and R. P. Rasmussen. 1997. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques 22:130-138. [DOI] [PubMed] [Google Scholar]
- 256.Wolf, D. G., D. J. Lee, and S. A. Spector. 1995. Detection of human cytomegalovirus mutations associated with ganciclovir resistance in cerebrospinal fluid of AIDS patients with central nervous system disease. Antimicrob. Agents Chemother. 39:2552-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wolf, D. G., and S. A. Spector. 1992. Diagnosis of human cytomegalovirus central nervous system disease in AIDS patients by DNA amplification from cerebrospinal fluid. J. Infect. Dis. 166:1412-1415. [DOI] [PubMed] [Google Scholar]
- 258.Yiannoutsos, C. T., E. O. Major, B. Curfman, P. N. Jensen, M. Gravell, J. Hou, D. B. Clifford, and C. D. Hall. 1999. Relation of JC virus DNA in the cerebrospinal fluid to survival in acquired immunodeficiency syndrome patients with biopsy-proven progressive multifocal leukoencephalopathy. Ann. Neurol. 45:816-821. [DOI] [PubMed] [Google Scholar]
- 259.Yoshikawa, T., and Y. Asano. 2000. Central nervous system complications in human herpesvirus-6 infection. Brain Dev. 22:307-314. [DOI] [PubMed] [Google Scholar]
- 260.Zhang, F., S. Tetali, X. P. Wang, M. H. Kaplan, F. V. Cromme, and C. C. Ginocchio. 2000. Detection of human cytomegalovirus pp67 late gene transcripts in cerebrospinal fluid of human immunodeficiency virus type 1-infected patients by nucleic acid sequence-based amplification. J. Clin. Microbiol. 38:1920-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zunt, J. R., and C. M. Marra. 1999. Cerebrospinal fluid testing for the diagnosis of central nervous system infection. Neurol. Clin. 17:675-689. [DOI] [PubMed] [Google Scholar]