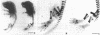Abstract
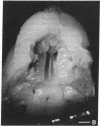
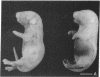
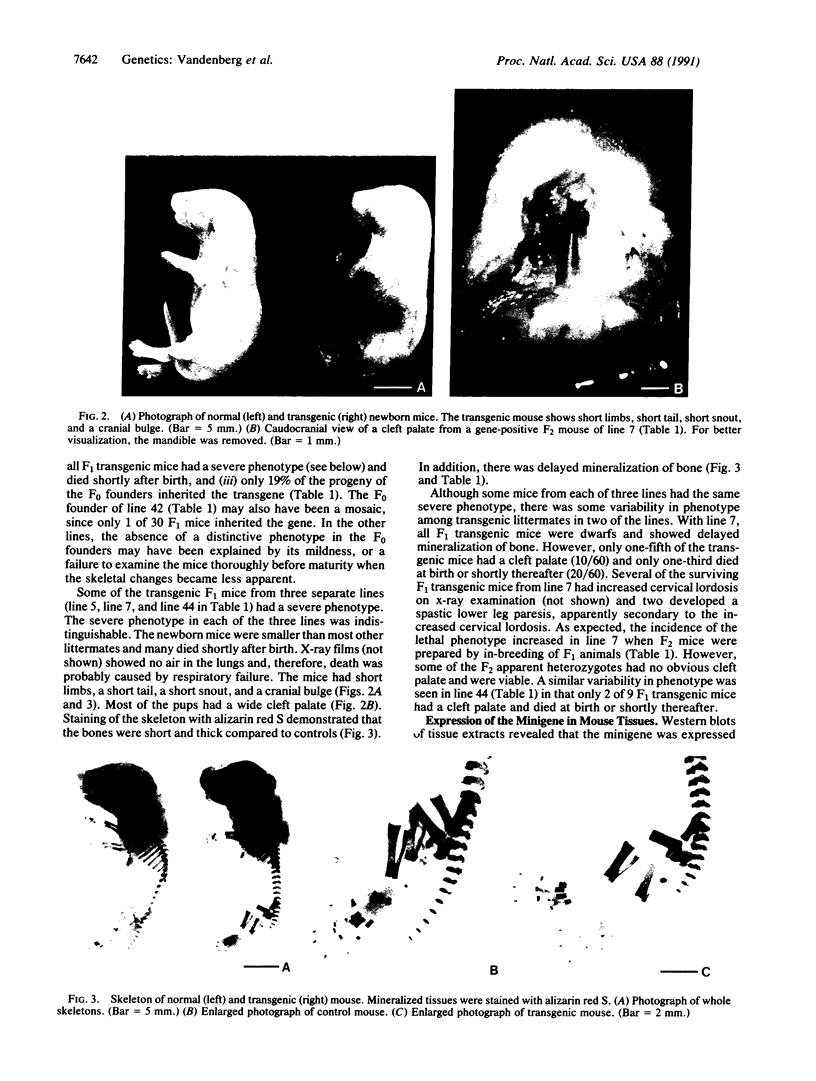
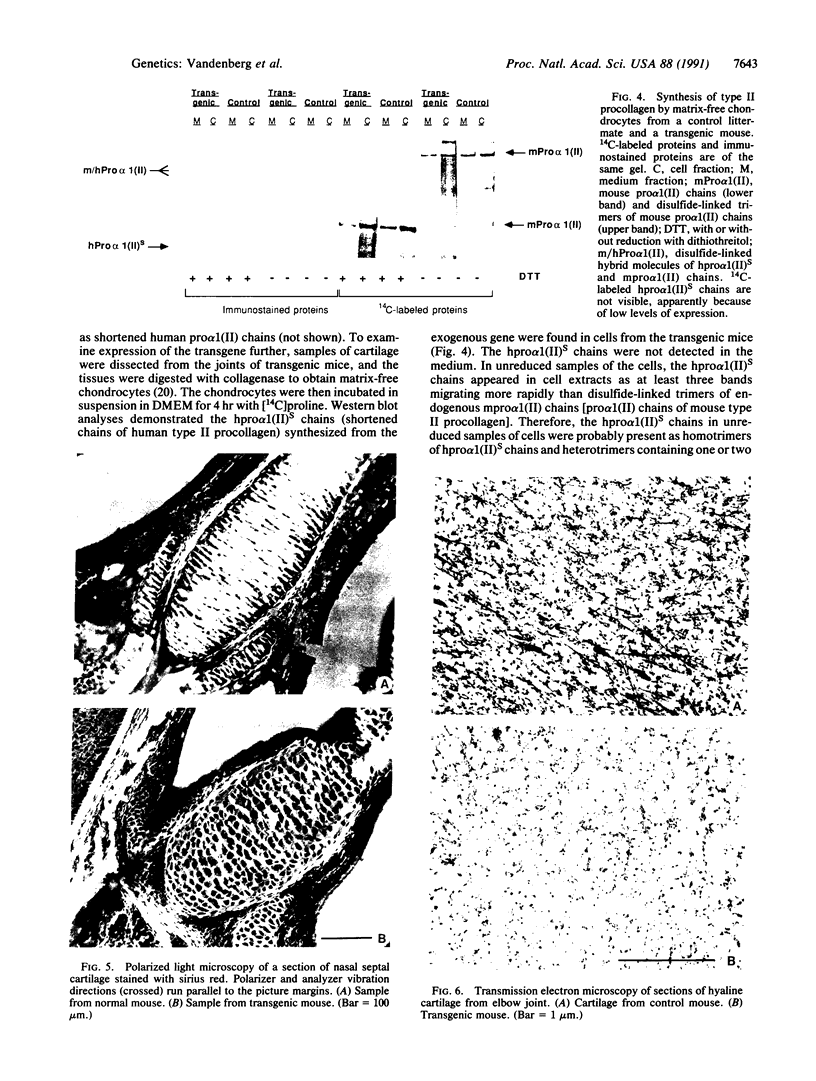
A minigene version of the human gene for type II procollagen (COL2A1) was prepared that lacked a large central region containing 12 of the 52 exons and therefore 291 of the 1523 codons of the gene. The construct was modeled after sporadic in-frame deletions of collagen genes that cause synthesis of shortened pro alpha chains that associate with normal pro alpha chains and thereby cause degradation of the shortened and normal pro alpha chains through a process called procollagen suicide. The gene construct was used to prepare five lines of transgenic mice expressing the minigene. A large proportion of the mice expressing the minigene developed a phenotype of a chondrodysplasia with dwarfism, short and thick limbs, a short snout, a cranial bulge, a cleft palate, and delayed mineralization of bone. A number of mice died shortly after birth. Microscopic examination of cartilage revealed decreased density and organization of collagen fibrils. In cultured chondrocytes from the transgenic mice, the minigene was expressed as shortened pro alpha 1(II) chains that were disulfide-linked to normal mouse pro alpha 1(II) chains. Therefore, the phenotype is probably explained by depletion of the endogenous mouse type II procollagen through the phenomenon of procollagen suicide.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N. N., Ala-Kokko L., Knowlton R. G., Jimenez S. A., Weaver E. J., Maguire J. I., Tasman W., Prockop D. J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Prockop D. J. Completion of the intron-exon structure of the gene for human type II procollagen (COL2A1): variations in the nucleotide sequences of the alleles from three chromosomes. Genomics. 1990 Nov;8(3):454–460. doi: 10.1016/0888-7543(90)90031-o. [DOI] [PubMed] [Google Scholar]
- Ala-Kokko L., Prockop D. J. Efficient procedure for preparing cosmid libraries from microgram quantities of genomic DNA fragments size fractionated by gel electrophoresis. Matrix. 1990 Oct;10(5):279–284. doi: 10.1016/s0934-8832(11)80182-4. [DOI] [PubMed] [Google Scholar]
- Aulthouse A. L., Beck M., Griffey E., Sanford J., Arden K., Machado M. A., Horton W. A. Expression of the human chondrocyte phenotype in vitro. In Vitro Cell Dev Biol. 1989 Jul;25(7):659–668. doi: 10.1007/BF02623638. [DOI] [PubMed] [Google Scholar]
- Byers P. H. Brittle bones--fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990 Sep;6(9):293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Biosynthesis of cartilage procollagen. Eur J Biochem. 1973 May;35(1):159–166. doi: 10.1111/j.1432-1033.1973.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Francomano C. A., Liberfarb R. M., Hirose T., Maumenee I. H., Streeten E. A., Meyers D. A., Pyeritz R. E. The Stickler syndrome: evidence for close linkage to the structural gene for type II collagen. Genomics. 1987 Dec;1(4):293–296. doi: 10.1016/0888-7543(87)90027-9. [DOI] [PubMed] [Google Scholar]
- Fryer A. E., Upadhyaya M., Littler M., Bacon P., Watkins D., Tsipouras P., Harper P. S. Exclusion of COL2A1 as a candidate gene in a family with Wagner-Stickler syndrome. J Med Genet. 1990 Feb;27(2):91–93. doi: 10.1136/jmg.27.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira L. C., Bignolas G., Brentani R. R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979 Jul;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kimmel C. A., Trammell C. A rapid procedure for routine double staining of cartilage and bone in fetal and adult animals. Stain Technol. 1981 Sep;56(5):271–273. doi: 10.3109/10520298109067325. [DOI] [PubMed] [Google Scholar]
- Knowlton R. G., Weaver E. J., Struyk A. F., Knobloch W. H., King R. A., Norris K., Shamban A., Uitto J., Jimenez S. A., Prockop D. J. Genetic linkage analysis of hereditary arthro-ophthalmopathy (Stickler syndrome) and the type II procollagen gene. Am J Hum Genet. 1989 Nov;45(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990 Sep 15;265(26):15349–15352. [PubMed] [Google Scholar]
- Sangiorgi F. O., Benson-Chanda V., de Wet W. J., Sobel M. E., Tsipouras P., Ramirez F. Isolation and partial characterization of the entire human pro alpha 1(II) collagen gene. Nucleic Acids Res. 1985 Apr 11;13(7):2207–2225. doi: 10.1093/nar/13.7.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Tiller G. E., Rimoin D. L., Murray L. W., Cohn D. H. Tandem duplication within a type II collagen gene (COL2A1) exon in an individual with spondyloepiphyseal dysplasia. Proc Natl Acad Sci U S A. 1990 May;87(10):3889–3893. doi: 10.1073/pnas.87.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]
- Williams C. J., Prockop D. J. Synthesis and processing of a type I procollagen containing shortened pro-alpha 1(I) chains by fibroblasts from a patient with osteogenesis imperfecta. J Biol Chem. 1983 May 10;258(9):5915–5921. [PubMed] [Google Scholar]