Abstract
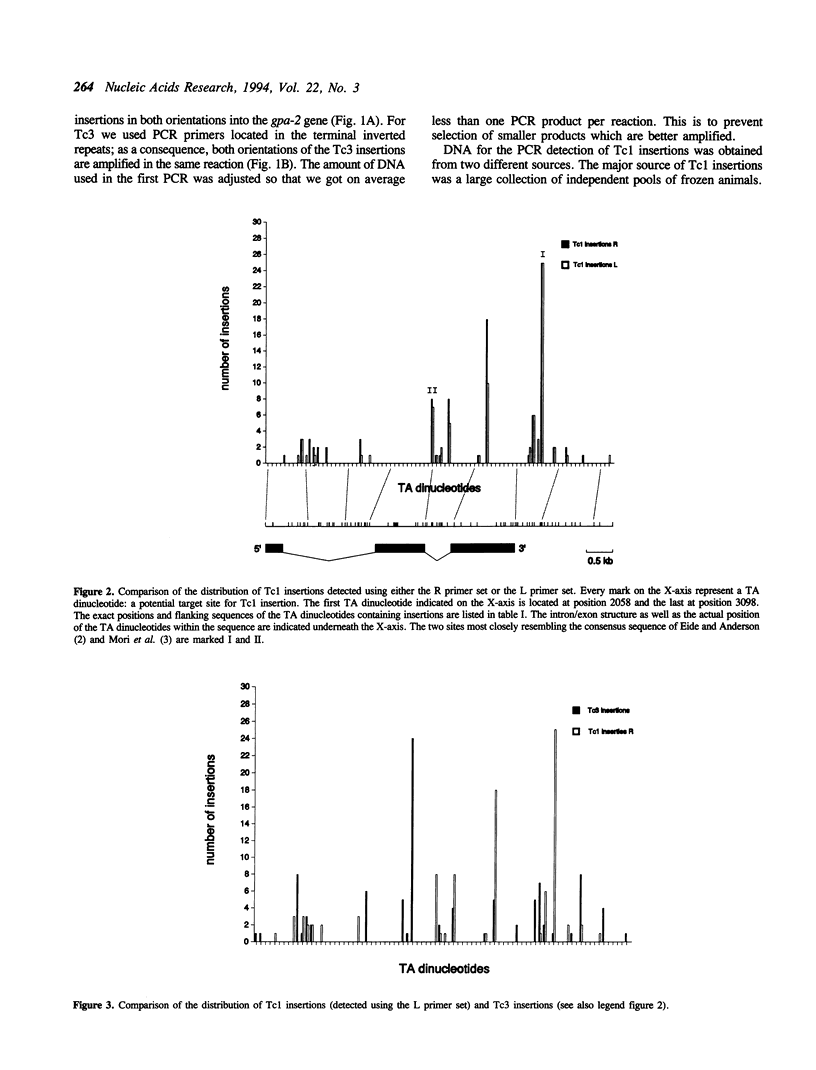
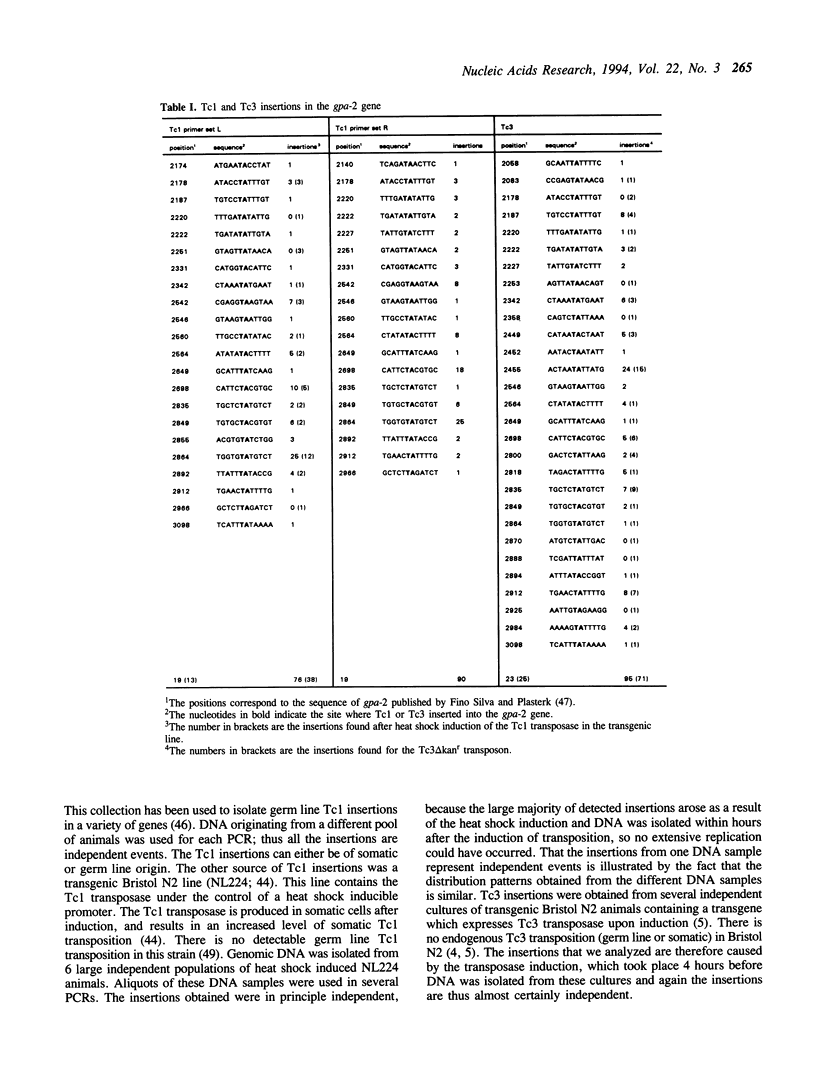
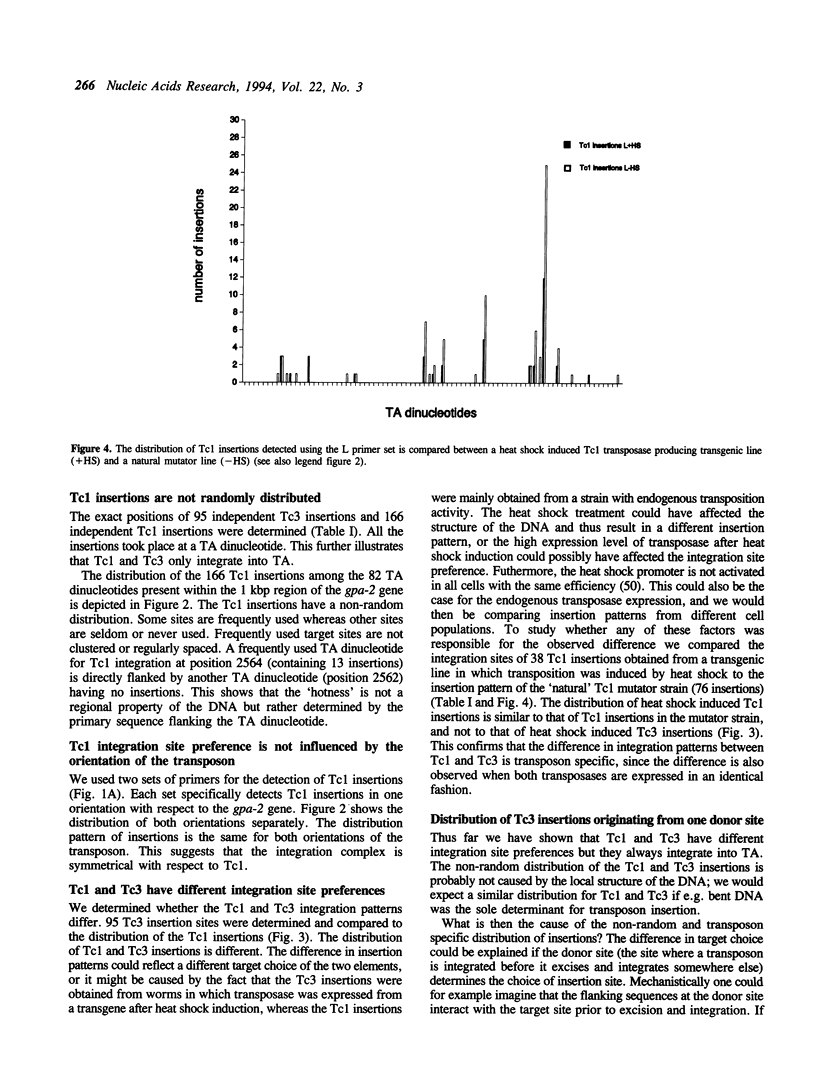
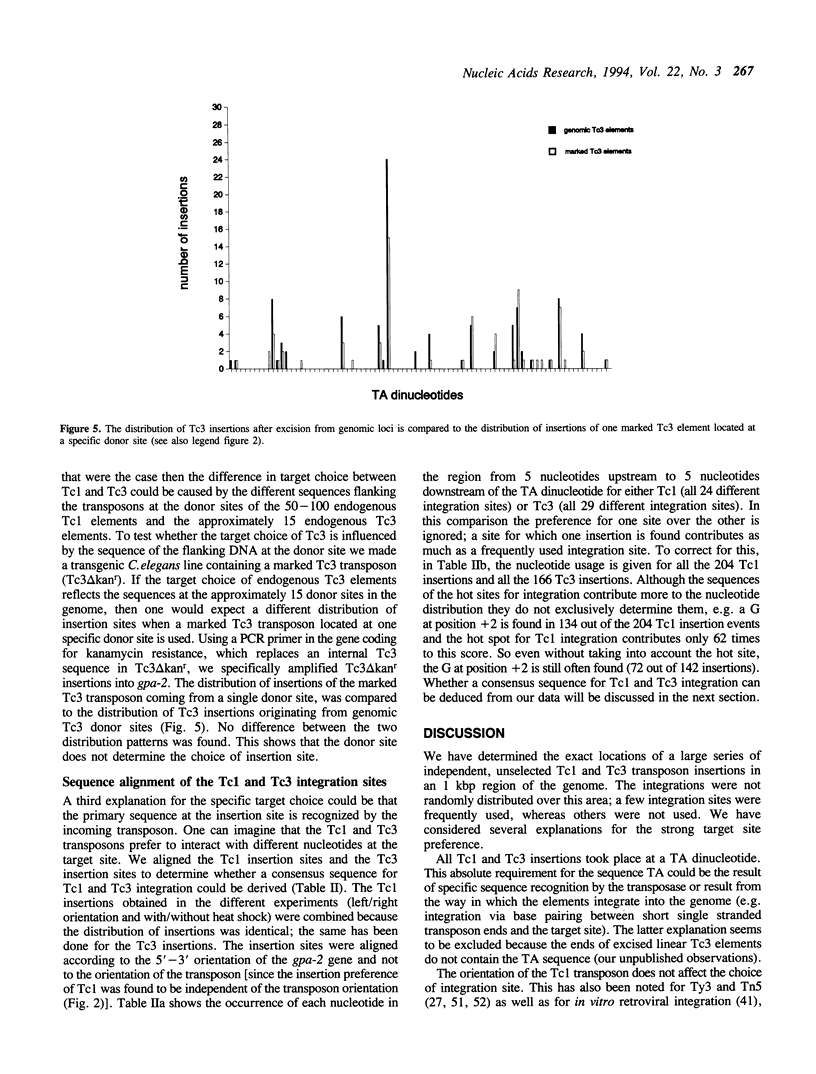
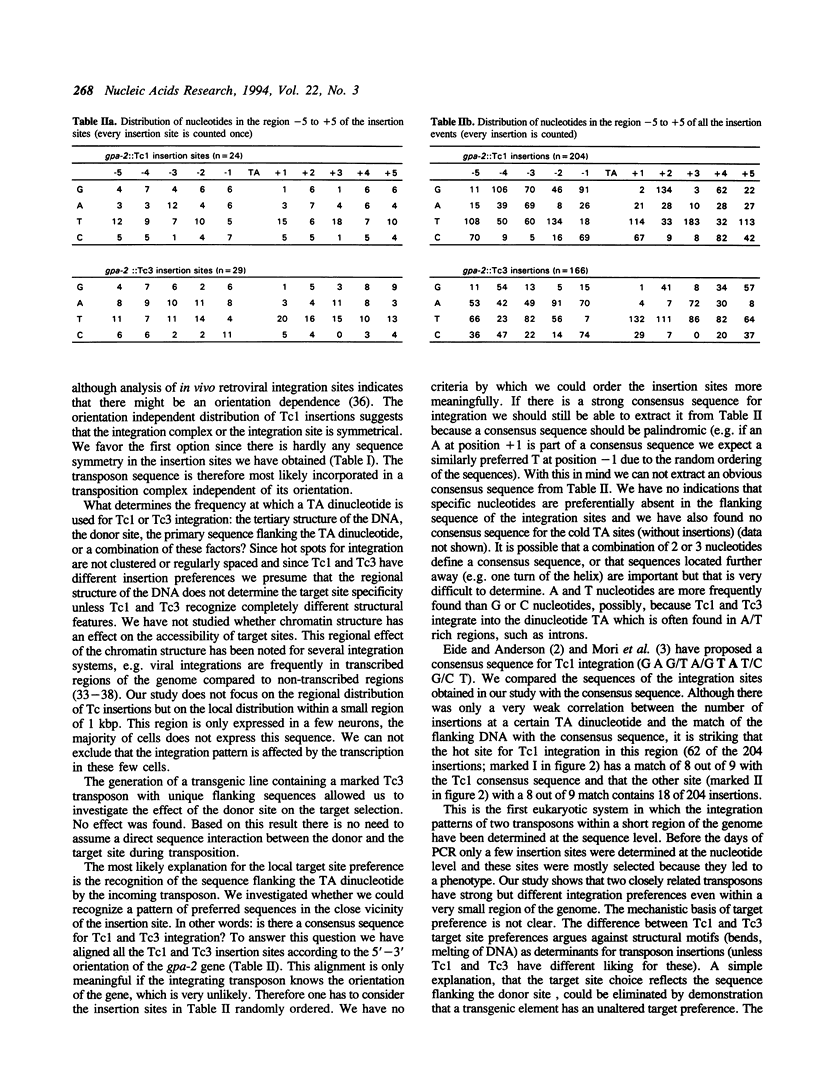
We have investigated the target choice of the related transposable elements Tc1 and Tc3 of the nematode C. elegans. The exact locations of 204 independent Tc1 insertions and 166 Tc3 insertions in an 1 kbp region of the genome were determined. There was no phenotypic selection for the insertions. All insertions were into the sequence TA. Both elements have a strong preference for certain positions in the 1 kbp region. Hot sites for integration are not clustered or regularly spaced. The orientation of the integrated transposon has no effect on the distribution pattern. We tested several explanations for the target site preference. If simple structural features of the DNA (e.g. bends) would mark hot sites, we would expect the patterns of the two related transposons Tc1 and Tc3 to be similar; however we found them to be completely different. Furthermore we found that the sequence at the donor site has no effect on the choice of the new insertion site, because the insertion pattern of a transposon that jumps from a transgenic donor site is identical to the insertion pattern of transposons jumping from endogenous genomic donor sites. The most likely explanation for the target choice is therefore that the primary sequence of the target site is recognized by the transposase. However, alignment of the Tc1 and Tc3 integration sites does not reveal a strong consensus sequence for either transposon.
Full text
PDF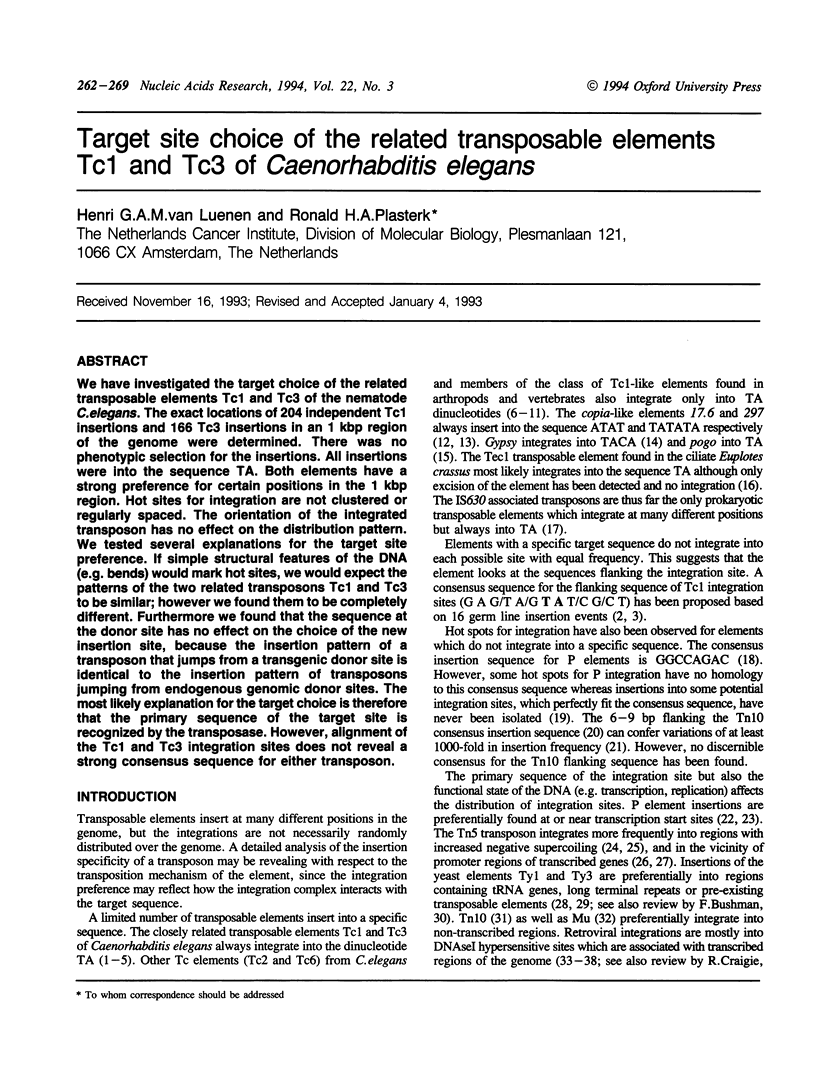


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender J., Kleckner N. Tn10 insertion specificity is strongly dependent upon sequences immediately adjacent to the target-site consensus sequence. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7996–8000. doi: 10.1073/pnas.89.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Schmandt M. A., Lowe J. B. Specificity of transposon Tn5 insertion. Genetics. 1983 Dec;105(4):813–828. doi: 10.1093/genetics/105.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezinsky L., Wang G. V., Humphreys T., Hunt J. The transposable element Uhu from Hawaiian Drosophila--member of the widely dispersed class of Tc1-like transposons. Nucleic Acids Res. 1990 Apr 25;18(8):2053–2059. doi: 10.1093/nar/18.8.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman F. Dodging the genes. Curr Biol. 1993 Aug 1;3(8):533–535. doi: 10.1016/0960-9822(93)90050-x. [DOI] [PubMed] [Google Scholar]
- Casadesus J., Roth J. R. Transcriptional occlusion of transposon targets. Mol Gen Genet. 1989 Apr;216(2-3):204–209. doi: 10.1007/BF00334357. [DOI] [PubMed] [Google Scholar]
- Collins J., Forbes E., Anderson P. The Tc3 family of transposable genetic elements in Caenorhabditis elegans. Genetics. 1989 Jan;121(1):47–55. doi: 10.1093/genetics/121.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R. Hotspots and warm spots: integration specificity of retroelements. Trends Genet. 1992 Jun;8(6):187–190. doi: 10.1016/0168-9525(92)90223-q. [DOI] [PubMed] [Google Scholar]
- Datta A. R., Rosner J. L. Reduced transposition in rho mutants of Escherichia coli K-12. J Bacteriol. 1987 Feb;169(2):888–890. doi: 10.1128/jb.169.2.888-890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D., Anderson P. Insertion and excision of Caenorhabditis elegans transposable element Tc1. Mol Cell Biol. 1988 Feb;8(2):737–746. doi: 10.1128/mcb.8.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L. High-frequency excision of transposable element Tc 1 in the nematode Caenorhabditis elegans is limited to somatic cells. Cell. 1984 Mar;36(3):599–605. doi: 10.1016/0092-8674(84)90339-8. [DOI] [PubMed] [Google Scholar]
- Fino Silva I., Plasterk R. H. Characterization of a G-protein alpha-subunit gene from the nematode Caenorhabditis elegans. J Mol Biol. 1990 Oct 20;215(4):483–487. doi: 10.1016/s0022-2836(05)80160-3. [DOI] [PubMed] [Google Scholar]
- Freund R., Meselson M. Long terminal repeat nucleotide sequence and specific insertion of the gypsy transposon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4462–4464. doi: 10.1073/pnas.81.14.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Kleckner N. A symmetrical six-base-pair target site sequence determines Tn10 insertion specificity. Cell. 1982 Jan;28(1):155–163. doi: 10.1016/0092-8674(82)90385-3. [DOI] [PubMed] [Google Scholar]
- Harris L. J., Baillie D. L., Rose A. M. Sequence identity between an inverted repeat family of transposable elements in Drosophila and Caenorhabditis. Nucleic Acids Res. 1988 Jul 11;16(13):5991–5998. doi: 10.1093/nar/16.13.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heierhorst J., Lederis K., Richter D. Presence of a member of the Tc1-like transposon family from nematodes and Drosophila within the vasotocin gene of a primitive vertebrate, the Pacific hagfish Eptatretus stouti. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6798–6802. doi: 10.1073/pnas.89.15.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Detection of Caenorhabditis transposon homologs in diverse organisms. New Biol. 1992 Apr;4(4):382–388. [PubMed] [Google Scholar]
- Henikoff S., Plasterk R. H. Related transposons in C.elegans and D.melanogaster. Nucleic Acids Res. 1988 Jul 11;16(13):6234–6234. doi: 10.1093/nar/16.13.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenaga H., Saigo K. Insertion of a movable genetic element, 297, into the T-A-T-A box for the H3 histone gene in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4143–4147. doi: 10.1073/pnas.79.13.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Yuki S., Saigo K. Sequence-specific insertion of the Drosophila transposable genetic element 17.6. 1984 Jul 26-Aug 1Nature. 310(5975):332–333. doi: 10.1038/310332a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Syvanen M. DNA gyrase is a host factor required for transposition of Tn5. Cell. 1982 Aug;30(1):9–18. doi: 10.1016/0092-8674(82)90006-x. [DOI] [PubMed] [Google Scholar]
- Ji H., Moore D. P., Blomberg M. A., Braiterman L. T., Voytas D. F., Natsoulis G., Boeke J. D. Hotspots for unselected Ty1 transposition events on yeast chromosome III are near tRNA genes and LTR sequences. Cell. 1993 Jun 4;73(5):1007–1018. doi: 10.1016/0092-8674(93)90278-x. [DOI] [PubMed] [Google Scholar]
- Kelley M. R., Kidd S., Berg R. L., Young M. W. Restriction of P-element insertions at the Notch locus of Drosophila melanogaster. Mol Cell Biol. 1987 Apr;7(4):1545–1548. doi: 10.1128/mcb.7.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Patel M. D., Lobel L. I., Goff S. P., Nguyen-Huu M. C. Insertion mutagenesis of embryonal carcinoma cells by retroviruses. Science. 1985 May 3;228(4699):554–558. doi: 10.1126/science.3838595. [DOI] [PubMed] [Google Scholar]
- Kramer J. M., French R. P., Park E. C., Johnson J. J. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990 May;10(5):2081–2089. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon R. D., Waye J. S., Bautista D. S., Graham F. L. Nonrandom insertion of Tn5 into cloned human adenovirus DNA. Gene. 1985;40(1):31–38. doi: 10.1016/0378-1119(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991 Dec;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooslehner K., Karls U., Harbers K. Retroviral integration sites in transgenic Mov mice frequently map in the vicinity of transcribed DNA regions. J Virol. 1990 Jun;64(6):3056–3058. doi: 10.1128/jvi.64.6.3056-3058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Benian G. M., Moerman D. G., Waterston R. H. Transposable element Tc1 of Caenorhabditis elegans recognizes specific target sequences for integration. Proc Natl Acad Sci U S A. 1988 Feb;85(3):861–864. doi: 10.1073/pnas.85.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag D. K., DasGupta U., Adelt G., Berg D. E. IS50-mediated inverse transposition: specificity and precision. Gene. 1985;34(1):17–26. doi: 10.1016/0378-1119(85)90290-2. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Prasad S. S., Harris L. J., Baillie D. L., Rose A. M. Evolutionarily conserved regions in Caenorhabditis transposable elements deduced by sequence comparison. Genome. 1991 Feb;34(1):6–12. doi: 10.1139/g91-002. [DOI] [PubMed] [Google Scholar]
- Pryciak P. M., Sil A., Varmus H. E. Retroviral integration into minichromosomes in vitro. EMBO J. 1992 Jan;11(1):291–303. doi: 10.1002/j.1460-2075.1992.tb05052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryciak P. M., Varmus H. E. Nucleosomes, DNA-binding proteins, and DNA sequence modulate retroviral integration target site selection. Cell. 1992 May 29;69(5):769–780. doi: 10.1016/0092-8674(92)90289-o. [DOI] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S. B., Bilanchone V. W., Clark D. J., Morcos P., Carle G. F., Brodeur G. M. Sigma elements are position-specific for many different yeast tRNA genes. Nucleic Acids Res. 1988 Feb 25;16(4):1499–1515. doi: 10.1093/nar/16.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S. B., Hansen L. J., Chalker D. L. Integration specificity of retrotransposons and retroviruses. Annu Rev Genet. 1990;24:491–518. doi: 10.1146/annurev.ge.24.120190.002423. [DOI] [PubMed] [Google Scholar]
- Scherdin U., Rhodes K., Breindl M. Transcriptionally active genome regions are preferred targets for retrovirus integration. J Virol. 1990 Feb;64(2):907–912. doi: 10.1128/jvi.64.2.907-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. C., Stoye J. P., Coffin J. M. Highly preferred targets for retrovirus integration. Cell. 1988 May 20;53(4):531–537. doi: 10.1016/0092-8674(88)90569-7. [DOI] [PubMed] [Google Scholar]
- Stringham E. G., Dixon D. K., Jones D., Candido E. P. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992 Feb;3(2):221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausta S. L., Turner L. R., Buckley L. K., Klobutcher L. A. High fidelity developmental excision of Tec1 transposons and internal eliminated sequences in Euplotes crassus. Nucleic Acids Res. 1991 Jun 25;19(12):3229–3236. doi: 10.1093/nar/19.12.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenzen T., Matsutani S., Ohtsubo E. Site-specific transposition of insertion sequence IS630. J Bacteriol. 1990 Jul;172(7):3830–3836. doi: 10.1128/jb.172.7.3830-3836.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota S., Ashburner M., Schedl P. P-element-induced control mutations at the r gene of Drosophila melanogaster. Mol Cell Biol. 1985 Oct;5(10):2567–2574. doi: 10.1128/mcb.5.10.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor M., Lobocka M., Goodell M., Pettitt J., O'Hare K. The pogo transposable element family of Drosophila melanogaster. Mol Gen Genet. 1992 Mar;232(1):126–134. doi: 10.1007/BF00299145. [DOI] [PubMed] [Google Scholar]
- Vijaya S., Steffen D. L., Robinson H. L. Acceptor sites for retroviral integrations map near DNase I-hypersensitive sites in chromatin. J Virol. 1986 Nov;60(2):683–692. doi: 10.1128/jvi.60.2.683-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. C., van Luenen H. G., Plasterk R. H. Characterization of the Caenorhabditis elegans Tc1 transposase in vivo and in vitro. Genes Dev. 1993 Jul;7(7A):1244–1253. doi: 10.1101/gad.7.7a.1244. [DOI] [PubMed] [Google Scholar]
- Zwaal R. R., Broeks A., van Meurs J., Groenen J. T., Plasterk R. H. Target-selected gene inactivation in Caenorhabditis elegans by using a frozen transposon insertion mutant bank. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7431–7435. doi: 10.1073/pnas.90.16.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luenen H. G., Colloms S. D., Plasterk R. H. Mobilization of quiet, endogenous Tc3 transposons of Caenorhabditis elegans by forced expression of Tc3 transposase. EMBO J. 1993 Jun;12(6):2513–2520. doi: 10.1002/j.1460-2075.1993.tb05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


