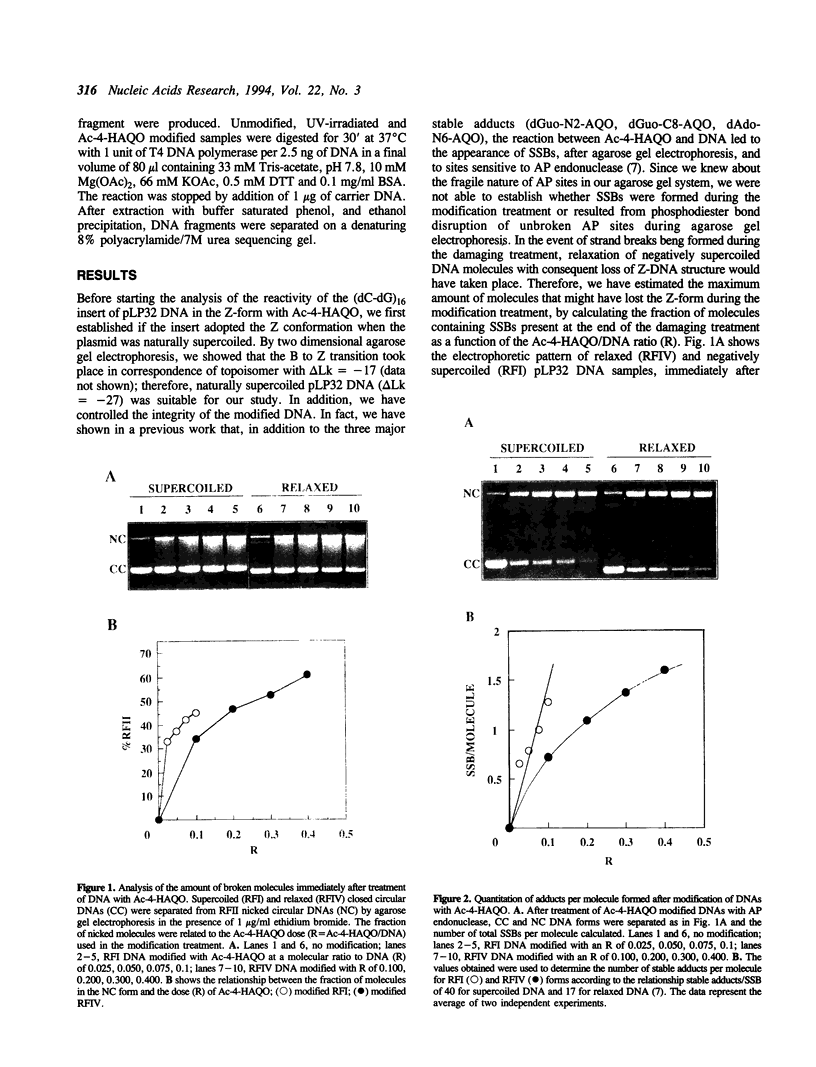
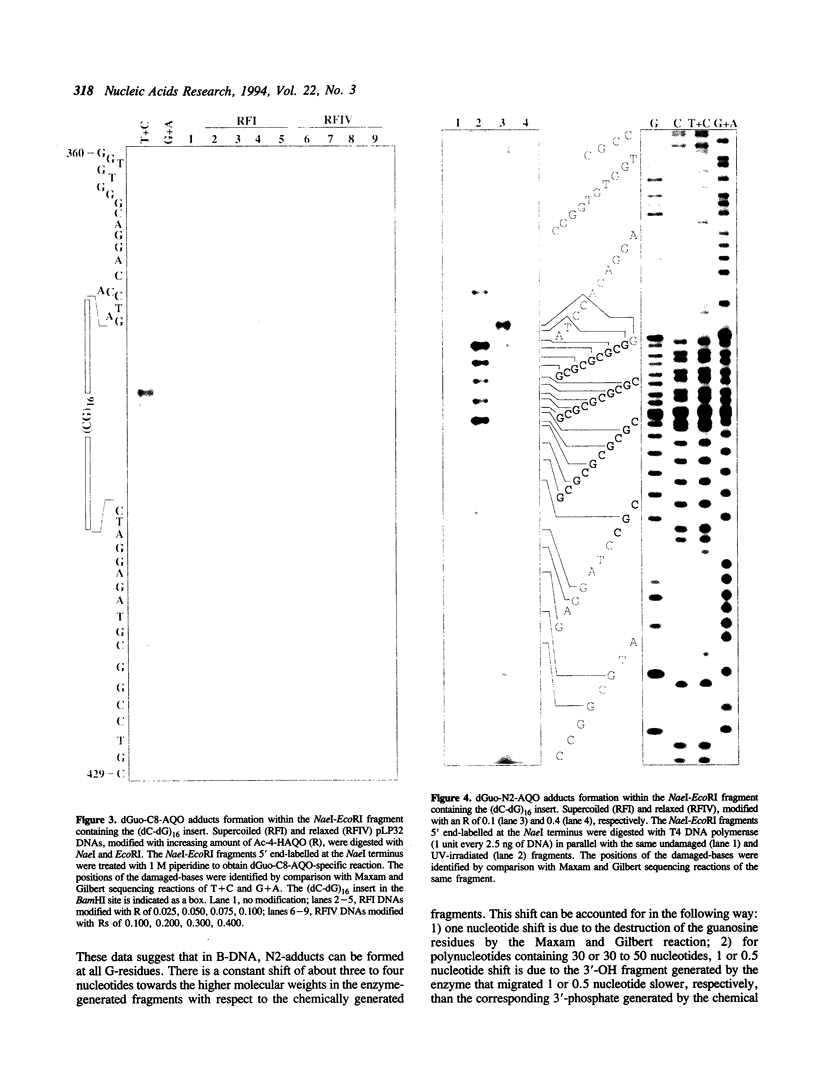
Abstract
DNA secondary and tertiary structures are known to affect the reaction between the double helix and several damaging agents. We have previously shown that the tertiary structure of DNA influences the reactivity of 4-acetoxyaminoquinoline 1-oxide (Ac-4-HAQO), the ultimate carcinogen of 4-nitroquinoline 1-oxide (4-NQO), being more reactive with naturally supercoiled DNA than with relaxed DNA. The relative proportion of the three main stable adducts and of an unstable adduct, that resulted in strand scission and/or AP sites, was also affected by the degree of supercoiling of plasmid DNA. In this study we examined the influence of Z-DNA structure on the reactivity of Ac-4-HAQO by mapping the distribution of the two main Ac-4-HAQO adducts, C8-guanine and N2-guanine, along a (dC-dG)16 sequence inserted at the BamHI site of pBR322 plasmid DNA. This insert adopted the left-handed Z and right-handed B structure depending on the superhelical density of the plasmid. Sites of C8-guanine adduct formation were determined by hot piperidine cleavage of Ac-4-HAQO modified DNA, while N2-guanine adducts were mapped by the arrest of the 3'-5' exonuclease activity of T4 DNA polymerase. The results showed that Ac-4-HAQO did not react with guanine residues when the (dC-dG)16 sequence was in Z conformation, while hyperreactivity at the B-Z junction was observed. These results indicate that Ac-4-HAQO can probe the polymorphism of DNA at the nucleotide level.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailleul B., Daubersies P., Galiègue-Zouitina S., Loucheux-Lefebvre M. H. Molecular basis of 4-nitroquinoline 1-oxide carcinogenesis. Jpn J Cancer Res. 1989 Aug;80(8):691–697. doi: 10.1111/j.1349-7006.1989.tb01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B., Galiègue-Zouitina S., Loucheux-Lefebvre M. H. Conformations of poly(dG-dC).poly(dG-dC) modified by the O-acetyl derivative of the carcinogen 4-hydroxyaminoquinoline 1-oxide. Nucleic Acids Res. 1984 Oct 25;12(20):7915–7927. doi: 10.1093/nar/12.20.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho J. A., Wells R. D. Left-handed Z-DNA and genetic recombination. Prog Nucleic Acid Res Mol Biol. 1989;37:107–126. doi: 10.1016/s0079-6603(08)60696-0. [DOI] [PubMed] [Google Scholar]
- Boehm T., Mengle-Gaw L., Kees U. R., Spurr N., Lavenir I., Forster A., Rabbitts T. H. Alternating purine-pyrimidine tracts may promote chromosomal translocations seen in a variety of human lymphoid tumours. EMBO J. 1989 Sep;8(9):2621–2631. doi: 10.1002/j.1460-2075.1989.tb08402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., Costa de Oliveira R., Laval J. The Escherichia coli O6-methylguanine-DNA methyltransferase does not repair promutagenic O6-methylguanine residues when present in Z-DNA. J Biol Chem. 1985 Jul 25;260(15):8711–8715. [PubMed] [Google Scholar]
- Doetsch P. W., Chan G. L., Haseltine W. A. T4 DNA polymerase (3'-5') exonuclease, an enzyme for the detection and quantitation of stable DNA lesions: the ultraviolet light example. Nucleic Acids Res. 1985 May 10;13(9):3285–3304. doi: 10.1093/nar/13.9.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiègue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H. Adducts from in vivo action of the carcinogen 4-hydroxyaminoquinoline 1-oxide in rats and from in vitro reaction of 4-acetoxyaminoquinoline 1-oxide with DNA and polynucleotides. Cancer Res. 1985 Feb;45(2):520–525. [PubMed] [Google Scholar]
- Galiègue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H. In vitro DNA reaction with an ultimate carcinogen model of 4-nitroquinoline-1-oxide: the 4-acetoxyaminoquinoline-1-oxide. Enzymatic degradation of the modified DNA. Carcinogenesis. 1983;4(3):249–254. doi: 10.1093/carcin/4.3.249. [DOI] [PubMed] [Google Scholar]
- Galiègue-Zouitina S., Daubersies P., Loucheux-Lefebvre M. H., Bailleul B. Mutagenicity of N2 guanylarylation is SOS functions dependent and reminiscent of the high mutagenic property of 4NQO. Carcinogenesis. 1989 Oct;10(10):1961–1966. doi: 10.1093/carcin/10.10.1961. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. H., Shaper N. L., Grossman L. Human placental apurinic/apyrimidinic endonuclease. Mechanism of action. J Biol Chem. 1982 Nov 25;257(22):13459–13464. [PubMed] [Google Scholar]
- Hingerty B., Broyde S. AAF linked to the guanine amino group: a B-Z junction. Nucleic Acids Res. 1983 May 25;11(10):3241–3254. doi: 10.1093/nar/11.10.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa A., Kuboya T., Aoyama T., Sugiura Y. Activation of DNA cleavage by dynemicin A in a B-Z conformational junction. Biochemistry. 1992 Jul 28;31(29):6784–6787. doi: 10.1021/bi00144a019. [DOI] [PubMed] [Google Scholar]
- Ishii K., Hasegawa T., Fujisawa K., Andoh T. Rapid purification and characterization of DNA topoisomerase I from cultured mouse mammary carcinoma FM3A cells. J Biol Chem. 1983 Oct 25;258(20):12728–12732. [PubMed] [Google Scholar]
- Johnston B. H., Rich A. Chemical probes of DNA conformation: detection of Z-DNA at nucleotide resolution. Cell. 1985 Oct;42(3):713–724. doi: 10.1016/0092-8674(85)90268-5. [DOI] [PubMed] [Google Scholar]
- Kochel T. J., Sinden R. R. Hyperreactivity of B-Z junctions to 4,5',8-trimethylpsoralen photobinding assayed by an exonuclease III/photoreversal mapping procedure. J Mol Biol. 1989 Jan 5;205(1):91–102. doi: 10.1016/0022-2836(89)90367-7. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Manes T., Kohwi Y. Unusual conformational effect exerted by Z-DNA upon its neighboring sequences. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2223–2227. doi: 10.1073/pnas.84.8.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunala S., Brash D. E. Excision repair at individual bases of the Escherichia coli lacI gene: relation to mutation hot spots and transcription coupling activity. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11031–11035. doi: 10.1073/pnas.89.22.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagravère C., Malfoy B., Leng M., Laval J. Ring-opened alkylated guanine is not repaired in Z-DNA. 1984 Aug 30-Sep 5Nature. 310(5980):798–800. doi: 10.1038/310798a0. [DOI] [PubMed] [Google Scholar]
- Menichini P., Fronza G., Tornaletti S., Galiègue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H., Abbondandolo A., Pedrini A. M. In vitro DNA modification by the ultimate carcinogen of 4-nitroquinoline-1-oxide: influence of superhelicity. Carcinogenesis. 1989 Sep;10(9):1589–1593. doi: 10.1093/carcin/10.9.1589. [DOI] [PubMed] [Google Scholar]
- Milligan J. R., Skotnicki S. M., Archer M. C. The reaction of N-methyl-N-nitrosourea with DNA in B and non-B conformations. Carcinogenesis. 1990 Jun;11(6):947–951. doi: 10.1093/carcin/11.6.947. [DOI] [PubMed] [Google Scholar]
- Palecek E. Local supercoil-stabilized DNA structures. Crit Rev Biochem Mol Biol. 1991;26(2):151–226. doi: 10.3109/10409239109081126. [DOI] [PubMed] [Google Scholar]
- Panigrahi G. B., Walker I. G. The N2-guanine adduct but not the C8-guanine or N6-adenine adducts formed by 4-nitroquinoline 1-oxide blocks the 3'-5' exonuclease action of T4 DNA polymerase. Biochemistry. 1990 Feb 27;29(8):2122–2126. doi: 10.1021/bi00460a023. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989 Oct 20;246(4928):358–363. doi: 10.1126/science.2678475. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Rio P., Leng M. N-hydroxyaminofluorene: a chemical probe for DNA conformation. J Mol Biol. 1986 Oct 5;191(3):569–572. doi: 10.1016/0022-2836(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Broyde S., Hingerty B. E. Z-DNA conformation of N-2-acetylaminofluorene modified poly(dG-dC).poly(dG-dC) determined by reactivity with anti cytidine antibodies and minimized potential energy calculations. Nucleic Acids Res. 1981 Oct 24;9(20):5459–5467. doi: 10.1093/nar/9.20.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth G. P., Chou P. J., Ho P. S. Mapping Z-DNA in the human genome. Computer-aided mapping reveals a nonrandom distribution of potential Z-DNA-forming sequences in human genes. J Biol Chem. 1992 Jun 15;267(17):11846–11855. [PubMed] [Google Scholar]
- Suh D., Sheardy R. D., Chaires J. B. Unusual binding of ethidium to a deoxyoligonucleotide containing a B-Z junction. Biochemistry. 1991 Sep 10;30(36):8722–8726. doi: 10.1021/bi00100a002. [DOI] [PubMed] [Google Scholar]
- Tornaletti S., Pedrini A. M. Studies on the interaction of 4-quinolones with DNA by DNA unwinding experiments. Biochim Biophys Acta. 1988 Mar 31;949(3):279–287. doi: 10.1016/0167-4781(88)90153-4. [DOI] [PubMed] [Google Scholar]
- Walker I. G. Alkaline sucrose sedimentation analysis as an indicator of repair capability of xeroderma pigmentosum fibroblasts for 4-nitroquinoline-1-oxide damage. Carcinogenesis. 1981;2(8):691–695. doi: 10.1093/carcin/2.8.691. [DOI] [PubMed] [Google Scholar]
- Wittig B., Dorbic T., Rich A. Transcription is associated with Z-DNA formation in metabolically active permeabilized mammalian cell nuclei. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2259–2263. doi: 10.1073/pnas.88.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]