Abstract
Patients with protracted sepsis develop impaired immunity, which predisposes them to acquiring secondary infections. One of the most common and lethal secondary infections is Pseudomonas aeruginosa pneumonia. Immunoadjuvant therapy is a promising approach to reverse sepsis-induced immunosuppression and improve morbidity and mortality from secondary infections. Interleukin-7 is an immunoadjuvant that improves survival in clinically relevant animal models of polymicrobial peritonitis and in fungal sepsis. This study investigated the effect of recombinant human interleukin-7 (rhIL-7) on survival in a 2-hit model of sublethal cecal ligation and puncture followed by P. aeruginosa pneumonia. Potential immunologic mechanisms responsible for the rhIL-7 putative beneficial effect were also examined, focusing on IL-17, IL-22, IFN-γ, and TNF-α, cytokines that are critical in the control of sepsis and pulmonary Pseudomonas infections. Results showed that rhIL-7 was highly effective in preventing P. aeruginosa–induced death, i.e., 92% survival in rhIL-7–treated mice versus 56% survival in control mice. rhIL-7 increased absolute numbers of immune effector cells in lung and spleen and ameliorated the sepsis-induced loss of lung innate lymphoid cells (ILCs). rhIL-7 also significantly increased IL-17–, IFN-γ–, and TNF-α–producing lung ILCs and CD8 T cells as well as IFN-γ– and TNF-α–producing splenic T cell subsets and ILCs. Furthermore, rhIL-7 enhanced NF-κB and STAT3 signaling in lungs during sepsis and pneumonia. Given the high mortality associated with secondary P. aeruginosa pneumonia, the ability of rhIL-7 to improve immunity and increase survival in multiple animal models of sepsis, and the remarkable safety profile of rhIL-7, clinical trials with rhIL-7 should be considered.
Keywords: sepsis, immunosuppression, innate lymphoid cell, IL-17, IFN-γ, TNF-α
Introduction
Sepsis results in >250,000 deaths annually in the United States, and it still remains one of the leading causes of death in most intensive care units [1, 2]. After sepsis onset, both pro- and anti-inflammatory responses occur. However, if sepsis persists, massive apoptosis of immune cells occurs in both myeloid and lymphoid lineages, resulting in compromised host immunity. In addition, sepsis up-regulates expression of T cell inhibitory receptors and ligands and induces increases in T regulatory and myeloid-derived suppressor cell numbers. These pathologic processes result in immune effector cells that have greatly reduced production of key cytokines and a propensity to undergo apoptotic cell death [3, 4]. Consequently, the balance of immune status shifts to a predominant anti-inflammatory and immunosuppressive phase [3–8]. Despite advances in treatment algorithms and development of new antimicrobial agents, secondary nosocomial pneumonia remains a frequent cause of deaths in intensive care units, with mortality estimated to be between 33 and 50% [9]. One of the major causative bacterial pathogens is Pseudomonas aeruginosa, an opportunistic pathogen frequently occurring in patients with impaired immunity [9, 10].
Currently, there is a great deal of interest in the use of immunoadjuvant therapy to boost host immunity in patients with life-threatening infections occurring during the immunosuppressive phase of the disorder [3, 5]. IL-7 is a stromal cell–derived cytokine that stimulates proliferation of cells in the lymphoid lineage and has crucial roles for survival, development, and homeostasis of lymphocytes [11–13]. rhIL-7 is one of the most promising of these new immunoadjuvants; rhIL-7 has been effective in decreasing mortality in immunosuppressive animal models of sepsis and in patients with virulent viral infections [14, 15].
The purpose of this study was to determine whether rhIL-7 improves survival in a clinically relevant, 2-hit model of P. aeruginosa pneumonia. Potential beneficial immunologic mechanisms of rhIL-7 were also investigated. In particular, effects of rhIL-7 to increase the number of immune effector cells and to improve production of key cytokines that are essential in host defense against P. aeruginosa were examined. Specifically, effects of rhIL-7 on IL-17, a cytokine that enhances neutrophil recruitment to sites of infection; IL-22, a cytokine that has protective effects on nonhematopoietic cells, including lung epithelial cells; IFN-γ, a cytokine that stimulates antimicrobial properties of monocyte/macrophages; and TNF-α, a cytokine that has been shown to be beneficial in containing P. aeruginosa, were investigated.
MATERIALS AND METHODS
Mice
Eight- to 10-week-old, male, C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Procedures were approved by the Animal Studies Committee at Washington University School of Medicine.
Sepsis model with secondary Pseudomonas aeruginosa pneumonia: a 2-hit model of sepsis
The 2-hit sepsis model of polymicrobial sepsis from CLP, followed by P. aeruginosa pneumonia, was used. This 2-hit model produces an immunosuppressive state that reflects the impaired immunity occurring in patients with protracted sepsis [16, 17]. CLP was performed as the “first hit” to induce polymicrobial peritonitis. Intratracheal injection of P. aeruginosa was the “second hit.” For CLP, mice were anesthetized with isoflurane, and a midline incision was performed. The cecum was ligated at a position one-third from the distal end, was punctured once using a 27-gauge needle, and the abdomen was closed; 1 ml of 0.9% normal saline mixed with 0.05 mg/kg/body weight of buprenorphine was immediately administered postoperatively, and 1 mg imipenem with 1 ml saline was administered s.c. 4 h postoperatively. The same procedure was followed for sham surgery, except that the cecum was not ligated and not punctured in these mice.
P. aeruginosa (27853; ATCC, Manassas, VA, USA) was grown overnight at 37°C in tryptic soy broth. Cells were harvested by centrifugation, and the pellet was washed and resuspended in sterile saline to obtain an optical density of 0.5 A600. Colony counts of inoculum corresponded to a concentration of 1.8–5.3 × 108 CFU/ml. At d 3 after CLP, surviving mice were anesthetized with isoflurane. An outer sheath of a 24-gauge catheter was inserted orally into the trachea under direct vision. Using a pipette, 25 μl of 0.5-A600 P. aeruginosa suspension was injected through the sheath. Mice were held in the vertical position for 15–30 s. The timing to inject P. aeruginosa (d 3 after CLP) was determined so that pneumonia was induced at the most immunosuppressive point after CLP (Muenzer et al. [16] and Supplemental Fig. 1A). The inoculum dose of P. aeruginosa was also determined so that the mortality rate in the control group (mice with intratracheal P. aeruginosa infection after CLP and no rhIL-7 treatment) would be approximately 50%, and almost all sham-operated mice with P. aeruginosa infection would survive (Supplemental Fig. 1B). Imipenem (1 mg/mouse) diluted in 1 ml 0.9% saline was s.c. administered at 2 h after P. aeruginosa infection (Fig. 1A) to more accurately reflect the clinical situation in which patients with pneumonia receive antibiotic therapy. Imipenem treatment after P. aeruginosa infection prolonged the survival of CLP mice by 12–48 h (Supplemental Fig. 1C).
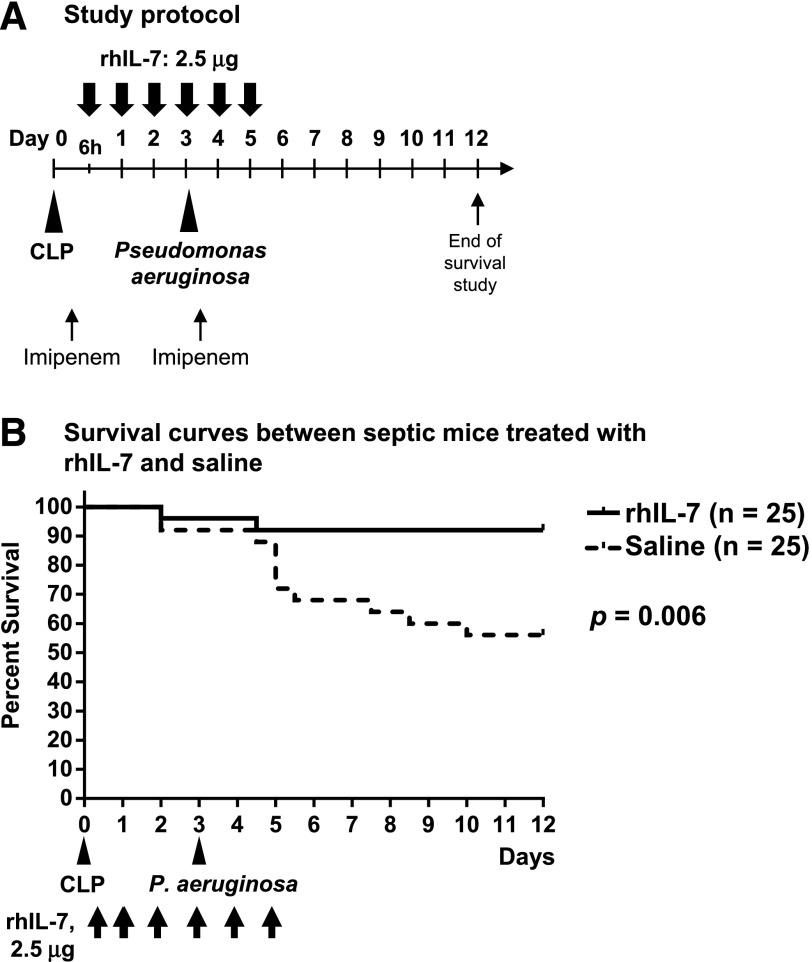
Figure 1. rhIL-7 treatment improves survival in an animal model of sepsis followed by P. aeruginosa pneumonia.
(A) Experimental design. Mice underwent CLP on d 0 by puncturing the cecum once using a 27-gauge needle. On d 3 after CLP, 25 μl of 0.5 A600 P. aeruginosa suspension (corresponding to 4.4–7.6 × 106 CFUs) was injected intratracheally. Mice received s.c. rhIL-7 injections on 6 d consecutively starting at 6 h after CLP; a dose of imipenem was administered both at 4 h after CLP and at 2 h after P. aeruginosa infection. (B) Survival curves of CLP mice treated with rhIL-7 (solid line) or saline (dotted line). Results of 3 independent experiments were combined. One mouse in the rhIL-7–treated group and 2 in the saline-treated (control) groups died before intratracheal P. aeruginosa injection. After the P. aeruginosa infection, 23 of 24 mice survived in rhIL-7–treated groups. However, 9 of 23 mice died in the control groups; of which, more than a half died within 48 h after P. aeruginosa injection.
Treatment with rhIL-7
rhIL-7 was provided by Revimmune (Bethesda, MD, USA) and was prepared as described previously [18]. Human IL-7 cross-reacts with mouse IL-7 receptors [19–21]; 2.5 μg of rhIL-7 in 100 μl of saline was administered s.c. on 6 d consecutively, beginning at 6 h after CLP, 3 d before intratracheal injection of P. aeruginosa (Fig. 1A). This experimental protocol was determined based on our previous studies and preliminary experiments [15, 18, 22]. Control groups of mice received 100 μl of 0.9% normal sterile saline s.c.. Survival of rhIL-7–treated and saline-treated CLP mice was monitored through d 12 after CLP. For in vitro studies, additional groups of sham mice received rhIL-7 or saline as controls. Note that all sham-operated and CLP mice had intratracheal injections of P. aeruginosa. One-half of the sham mice that had intratracheal injection of P. aeruginosa received rhIL-7, and one-half of the sham mice that had intratracheal injections of P. aeruginosa received saline diluent. All mice that underwent CLP had intratracheal injections of P. aeruginosa, with one-half receiving rhIL-7 and one-half receiving saline diluent.
Sampling procedures
To assess effects of sepsis and rhIL-7 treatment, mice were sacrificed on d 4 (at 16 h after intratracheal P. aeruginosa injection). Blood was drawn by cardiac puncture and was placed in sterile tubes with lithium heparin. The blood was centrifuged at 2000 g for 10 min, and the supernatants were frozen at −80°C for ELISA. BAL was performed with 3 ml sterile saline, as previously described [16]. The first 1 ml of BAL fluid was centrifuged at 460 g for 5 min, and the supernatant was stored at −80°C for ELISA. Cell pellets from 3 ml of BAL were used for staining to count neutrophils. Lungs and spleens were harvested for flow cytometric analyses or homogenized with a tissue disruptor in 1 ml sterile saline for ELISA. Perfusion of the lungs was not performed. The homogenates were centrifuged at 8160 g for 10 min at 4°C and the supernatants were stored at −80°C for ELISA. Harvested lungs were also immediately frozen in liquid nitrogen and stored at −80°C for immunoblot analysis.
Cell isolation
After lungs were harvested, the tissues were cut into small pieces (1∼2 mm) and were added to 5 ml of RPMI 1640. The tissues were digested with deoxyribonuclease I (Atlanta Biologicals, Flowery Branch, GA, USA) (5000 U/ml), hyaluronidase (Sigma-Aldrich, St. Louis. MO) (10,000 U/ml), and Liberase (Roche, Basel, Switzerland) (26 U/ml) at 37°C for 40 min with shaking. To stop the digestion, 500 μl of FBS was added for 1 min at room temperature. The lung tissues were then dissociated and filtered through a 70 μm cell strainer. Spleens were dissociated through 70-μm cell strainers in HBSS containing 2% FBS. After the suspensions of both lung cells and splenocytes were centrifuged, RBCs were lysed at room temperature for 2 min, and the cells were resuspended with IMDM containing 10% FBS.
Quantitation of cytokines by ELISA
Using stored plasma and supernatants of BAL fluid and lung and spleen homogenates, ELISA was performed to quantitate IL-17 and IL-22 by Mouse IL-17 DuoSet and Mouse IL-22 DuoSet (both from R&D Systems, Minneapolis, MN, USA). Similarly, IFN-γ and TNF-α were quantitated using IFN-γ Mouse Antibody Pair and TNF-α Mouse Antibody Pair (both from Life Technologies, Carlsbad, CA, USA). All procedures for ELISA were performed according to the manufacturers’ instructions. The lower limits of detection were as follows: 15.6 pg/ml (BAL fluid and plasma) and 7.8 pg/ml (lung and spleen homogenates) for IL-17; 31.2 pg/ml (BAL fluid and plasma) and 15.6 pg/ml (lung and spleen homogenates) for IL-22; and 15.6 pg/ml for IFN-γ; and 15.6 pg/ml for TNF-α.
Flow cytometry
For phenotypic and functional analysis of immune cells in lungs and spleens, the following Abs were used: fluorochrome-conjugated Abs to mouse CD45, CD3, TCR-γ/δ, CD8, NK1.1, CD19, CD90, NKp46, ST2, Ly6G, F4/80, CD11b, IL-17, and IFN-γ, as well as Zombie NIR dead/live stain, were purchased from BioLegend (San Diego, CA, USA). Fluorochrome-conjugated Abs to mouse IL-22 and TNF-α were from eBioscience (San Diego, CA, USA), and PE-CF594–conjugated anti-mouse CD4 was from BD Biosciences (San Jose, CA, USA). These Abs were used for phenotyping of T cells and ILCs. The combinations of fluorochrome-conjugated Abs in each T cell and ILC panel are shown in Supplemental Table 1. CD3 and CD19 were used as Lin markers [23, 24]. Lin−NK1.1+ cells were defined as NK cells (group 1 ILCs); Lin−NK1.1−CD90+ST2+ cells, as group ILC2s; and Lin−NK1.1−CD90+ST2–cells, as group ILC3s. Additional intranuclear staining for GATA3 and RORγt was performed for detection of ILC2s and ILC3s, respectively, in experiments in which cell proliferation was assessed. For samples that did not include ST2 staining, Lin−NK1.1−CD90+ cells were defined as Lin−CD90+ ILCs (which include both group 2 and 3 ILCs). Flow cytometric detection of proliferating cells was performed using fluorochrome-labeled anti–Ki-67 Abs (BioLegend). For this, cells were first stained for surface markers, followed by fixation and permeabilization using the Foxp3 buffer kit (eBioscience) for intranuclear staining of Ki-67 and transcription factors. To assess cell numbers, we used 123count eBeads (eBioscience) according to the manufacturer’s instructions. Samples were acquired on an LSR Fortessa (BD Biosciences) and analyzed with FlowJo (version 10.1; (Tree Star, Ashland, OR, USA). Flow cytometric analysis was also performed using a FACScan (BD Biosciences) to calculate cell numbers of infiltrating neutrophils (CD45+Ly6G+CD11b+) in the BAL fluid.
Stimulation of immune cells for intracellular cytokine staining
Single-cell preparations (4 × 106/well for spleen; ≤4 × 106/well for lungs) from lungs and spleens were stimulated in culture in the presence of 50 ng/ml PMA, 1 µg/ml ionomycin (both from Sigma-Aldrich) and GolgiPlug (BD Biosciences) at 37°C for 4 h in 5% CO2. After the culture, cells were spun down and incubated in Fc receptor block (TruStain FcX; BioLegend). Cell surface markers were stained with Abs to surface markers as indicated in Supplemental Table 1. Cells were then fixed using BD Cytofix (BD Biosciences), permeabilized in 0.5% saponin buffer, and stained with the following 2 Ab cocktails: 1) IL-22-PE and IL-17-A647, and 2) IFN-γ-PE and TNF-α–APC (Supplemental Table 1).
Immunoblot analysis
Western blot analysis was performed to assess the activation status of NF-κB and STAT3 signaling in lung homogenates from mice after CLP and pneumonia induction. Abs for phospho-p65 and phospho-STAT3 were purchased from Cell Signaling Technology (Danvers, MA, USA). Procedures were performed according to the manufacturer’s instructions.
Statistical analysis
Survival times between rhIL-7–treated and saline-treated CLP mice were compared using the log-rank test and are presented as Kaplan-Meier curves. Data from the ELISA, FACS, and immunoblotting were analyzed with the Mann-Whitney U test to assess rhIL-7 treatment effect. The statistical software Prism (GraphPad Software, La Jolla, CA, USA) was used. A P value ≤ 0.05 was considered statistically significant.
RESULTS
rhIL-7 treatment improves survival in P. aeruginosa pneumonia
We used the well-developed animal model of sepsis induced by CLP to evaluate whether rhIL-7 treatment can prevent deaths from secondary P. aeruginosa pneumonia. rhIL-7 was administered to groups of mice at 6 h after CLP, followed by daily administration for 5 d consecutively. Pneumonia was elicited by intratracheal infection with P. aeruginosa on d 3 after CLP (Fig. 1A). In mice that underwent CLP followed by P. aeruginosa pneumonia, rhIL-7–treated mice had significantly improved survival compared with saline-treated control mice, i.e., 92% (23 of 25) vs. 56% (14 of 25) of mice surviving, respectively (Fig. 1B; P = 0.006). These results are the data from 3 separate survival studies, with a total of 25 mice cumulatively in each of the 2 groups.
rhIL-7 increases the number of lymphocytes in lung and spleen tissues during sepsis and pneumonia
Recent evidence suggests that neutrophils are early essential responders to P. aeruginosa infections [25]. A previous study from our group found that the rhIL-7 facilitated neutrophil recruitment to sites of infection [26]. To determine whether rhIL-7 increased neutrophil recruitment in the lung, we quantified absolute numbers of neutrophils within the BAL fluid at 16 h post P. aeruginosa infection. There were no significant differences in neutrophil numbers in BAL fluid between the different treatment groups (Supplement Fig. 2).
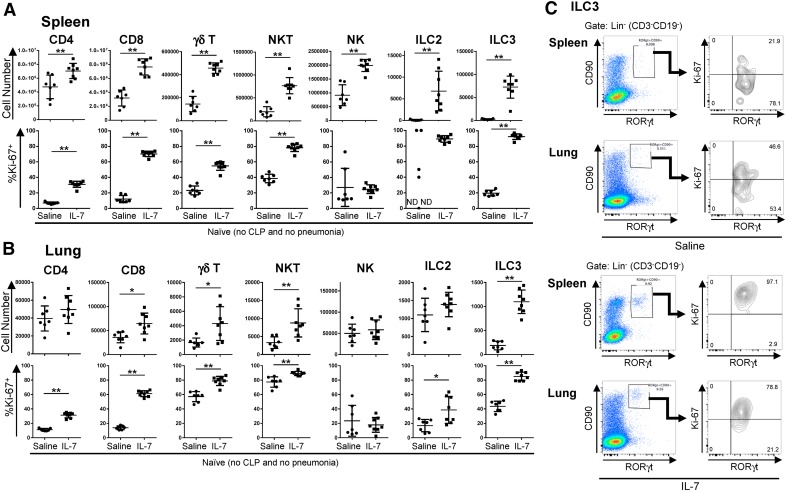
IL-7 has important roles in the development, proliferation, and maintenance of T cells and ILCs [11, 12]. IL-7 also increases antiapoptotic Bcl-2 family members, which decrease sepsis-induced apoptosis. Thus, we hypothesized that the improved survival of rhIL-7–treated mice in our 2-hit model may have been a result of expanded numbers or improved functionality of various lymphocyte subsets in rhIL-7–treated mice. Our previous studies showed that rhIL-7 treatment in septic mice increases lymphocyte numbers, in particular, CD4 and CD8 T cell numbers [18, 22], which could positively affect host immunity to P. aeruginosa infection. IL-7 is an important cytokine for ILC differentiation; however, the effect of rhIL-7 administration on ILC numbers or function is currently unknown. To assess the effects of rhIL-7 treatment on ILC and T cell numbers, we subjected naive mice to rhIL-7 treatment (4 doses on 4 d consecutively) in the absence of sepsis or infection and analyzed ILC and T cell numbers and the frequency of proliferating cells on d 5. We noted that, compared with saline-treated naive mice, rhIL-7–treated mice had significantly increased cell numbers in the spleen, not only of T cell subsets, such as CD4 and CD8 T cells, TCR-γδ T cells, and NKT cells, but also of the ILC populations examined: NK cells, ILC2s (Lin−CD90+ST2+GATA3+), and ILC3s (Lin−CD90+RORγt+) (Fig. 2A). The frequency of proliferating (Ki-67+) cells was significantly increased in all T cell subsets examined, as well as in ILC3s in the spleens in rhIL-7–treated mice. In the lungs, the effects of rhIL-7 treatment on lymphocyte numbers were more moderate, where, among T cells, only CD8+, TCR-γδ T cells, and NKT cells were significantly expanded in the lungs. However, we noted the presence of a distinct ILC3 population in the lungs of rhIL-7–treated mice, whereas control lungs were almost devoid of ILC3s (Fig. 2B and C). Similar to what we observed in the spleen, rhIL-7 treatment resulted in a significantly higher frequency of lung T cells that were actively proliferating. Among ILCs, we found higher frequencies of proliferating ILC2s and ILC3s in lung tissues, whereas NK cell numbers and proliferation were unchanged (Fig. 2B). Given that rhIL-7 treatment increased the numbers of both T cells and ILC subsets in naive mice, we hypothesized that rhIL-7 treatment could also increase the numbers of these cell types in sepsis and pneumonia, which might contribute to the survival benefit seen in our 2-hit model. To test this hypothesis, we subjected mice to CLP and pneumonia or to a sham procedure followed by pneumonia and assessed absolute numbers of T cell and ILC subsets in lungs and spleens of mice subjected to CLP and pneumonia (Fig. 3). When rhIL-7 was administered to sham (nonseptic) mice before P. aeruginosa infection, rhIL-7 significantly increased numbers of CD8 T cell, TCR-γδ T cells, and NKT cells in both lungs and spleens (Fig. 3A and B). Importantly, mice subjected to CLP before pneumonia also had significant increases in the numbers of CD8 T and NKT cells in both lungs and spleens, and TCR-γδ T cells in the spleen, when treated with rhIL-7, although the magnitude of the increase was not as high as in the sham-operated mice.
Figure 2. rhIL-7 induces proliferation of ILCs and other innate-like lymphocytes in spleen and lung.
Numbers of immune cells and frequencies of proliferating (Ki-67+) cells within each subset, in spleens (A) and lungs (B) were analyzed in naïve mice (no CLP or pneumonia) with either rhIL-7 or saline treatment for a consecutive 5 d. (C) Representative dot plots showing the gating strategies and the percentage of Ki-67+ cells in RORγt+ ILC3s in spleen and lung. Cells were gated on live CD45+ cells. For identification of T cells, cells were gated on CD3+ cells and on the corresponding additional receptors (CD4, CD8, TCR-γδ, or NK1.1). NK cells were identified as CD3−CD19−NK1.1+, ILC2s as CD3−CD19−CD90+ST2+GATA3+, and ILC3s as CD3−CD19−CD90+RORγt+. Results of 2 independent experiments were combined. Horizontal lines and error bars represent means ± sd. Asterisks indicate significant differences in values (*P < 0.05; **P < 0.01). ND (not detected) indicates that no ILC2s were detected in the spleens of 2 out of 7 saline-treated mice.
Figure 3. rhIL-7 increases the absolute number of lymphocytes in lung and spleen.
Numbers of immune cells within lungs (A) and spleens (B) were analyzed in mice after sham-surgery or CLP with either rhIL-7 or saline treatment at 16 h after P. aeruginosa infection. Cells were gated on live CD45+ cells. T cells and NK cells were gated as indicated in Fig. 2, with ILC2s as CD3−CD19−NK1.1−CD90+ST2+ and group ILC3s as CD3−CD19−NK1.1−CD90+ST2−. Results of 3 independent experiments were combined. Horizontal lines and error bars represent means ± sd. Asterisks indicate significant differences in values (*P < 0.05; **P < 0.01). (A) Lungs from CLP mice with P. aeruginosa pneumonia treated with saline tended to have decreased numbers of ILC2s and ILC3s compared with sham-operated mice with P. aeruginosa pneumonia treated with saline; however, rhIL-7 treatment significantly increased the number of ILC2s and ILC3s in CLP mice with P. aeruginosa pneumonia compared with the saline treatment. Pa, Pseudomonas aeruginosa infection.
When assessing numbers of NK cells, ILC2s (Lin−NK1.1−CD90+ST2+), and ILC3s (Lin−NK1.1−CD90+ST2−), we found similar effects of rhIL-7 in mediating increases in cell numbers in infected mice with sham surgery (Fig. 3A and B). CLP tended to induce the loss of lung NK cells, ILC2s and ILC3s (Fig. 3A). However, rhIL-7 treatment significantly increased the numbers of lung ILC2s and ILC3s and splenic ILC2s in CLP mice, compared with saline control (Fig. 3A and B). These results suggest that increased numbers of ILCs as well as those of T cells may be associated with the protective mechanisms of rhIL-7 treatment in this 2-hit model.
rhIL-7 does not enhance circulating and local level of cytokines during sepsis and pneumonia
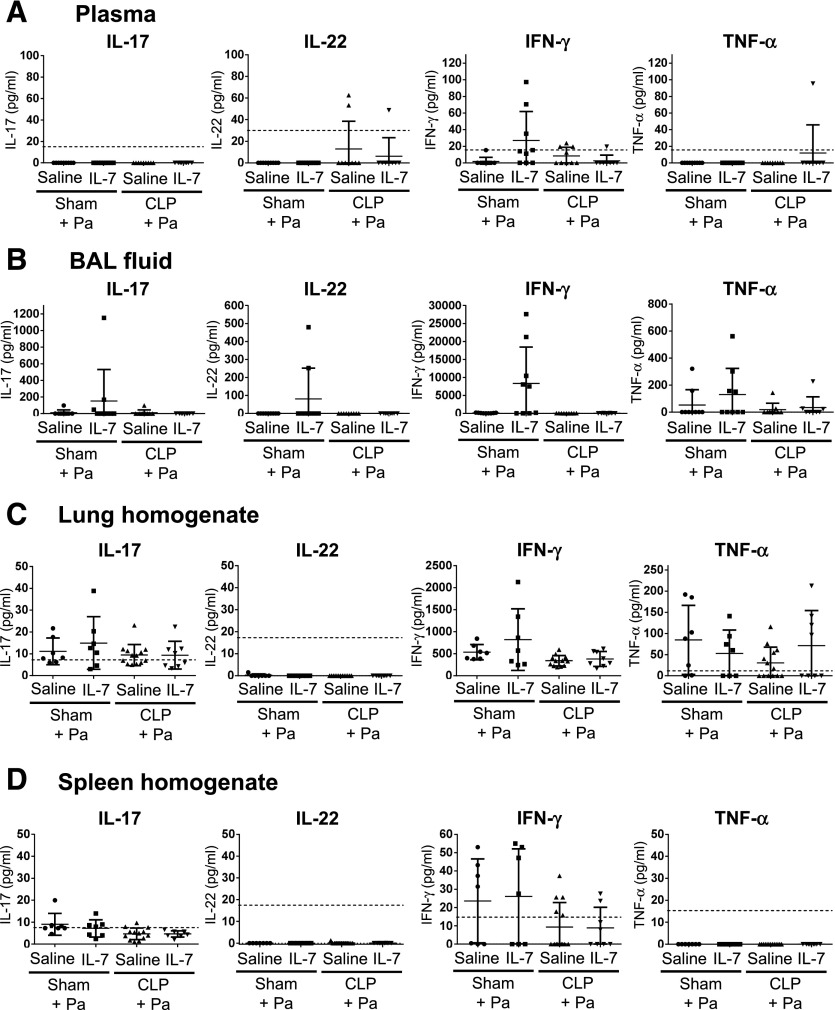
IL-17, IL-22, IFN-γ, and TNF-α are cytokines that have been reported to be beneficial for host defense during sepsis and during P. aeruginosa pneumonia [4, 10, 26–30]. We assessed levels of these cytokines in plasma, BAL fluid, and in lung and spleen homogenates of rhIL-7–treated mice at 16 h after pneumonia (Fig. 4). Although we found a larger proportion of plasma and BAL fluid samples with detectable IFN-γ levels in the sham-operated group with rhIL-7 treatment compared with the sham-operated mice treated with saline, these differences were not statistically significant (Fig. 4A and B). Similarly, there were no differences in IFN-γ levels in plasma, BAL fluid, and lung or spleen homogenates between CLP mice with or without rhIL-7 treatment (Fig. 4). Levels of IL-17 and TNF-α were similar between the individual treatment groups in the tested tissues; IL-22 levels were below the detection limit in most samples (Fig. 4).
Figure 4. rhIL-7 treatment before Pseudomonas pneumonia does not change levels of circulating or local cytokines.
All data were assessed by ELISA. Cytokine levels in plasma (A), BAL fluid (B), and lung (C) and spleen (D) homogenates were analyzed in mice after sham surgery or CLP with either rhIL-7 or saline treatments at 16 h after P. aeruginosa infection. Results of 3 independent experiments were combined. Horizontal lines and error bars represent means ± sd. Dotted lines indicate lower detection limits. Pa, Pseudomonas aeruginosa infection.
rhIL-7 increases IL-17–, IFN-γ–, and TNF-α–producing ILCs and CD8 T cells in lungs
Although circulating and local levels of cytokines were unchanged between CLP mice with and without rhIL-7, we reasoned that rhIL-7 treatment can alter local cytokine production by enhancing the immune function of a subset of lymphocytes, rather than all lymphocytes; an effect that may not readily be detected when testing whole lung homogenate or BAL fluid for cytokine levels.
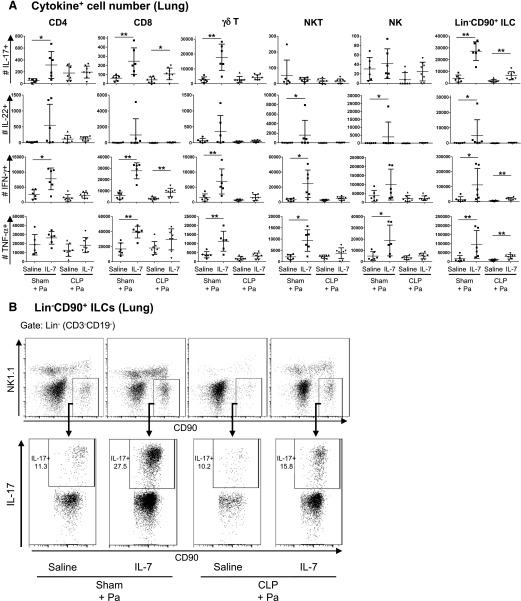
Subsets of T cells and ILCs are potent producers of IL-17, IL-22, IFN-γ, and TNF-α [11, 30–32]. Thus, we focused on the effect of rhIL-7 on the contribution of these cell types, i.e., CD4 and CD8 T cells, TCR-γδ T cells, NKT and NK cells, and ILCs on cytokine production during sepsis and pneumonia. When assessing cytokine production on a cellular level in the lungs of P. aeruginosa–infected mice, we noted that rhIL-7 treatment increased the frequencies and numbers of IL-17–, IL-22–, IFN-γ–, and TNF-α–producing T cells and ILCs in the lungs of sham-operated mice with pneumonia (Fig. 5A and B, and Supplemental Figs. 3A and 4). These cytokine-enhancing effects of rhIL-7 were attenuated by CLP. No significant IL-22–producing lymphocyte population was detected in the lungs of CLP mice. Importantly, lung Lin−CD90+ ILCs, which putatively represent a mixed ILC2 and ILC3 population that produced IL-17, IFN-γ, and TNF-α, were significantly increased with rhIL-7 treatment (Fig. 5A and B, and Supplemental Fig. 4A). In addition to increases in cytokine-producing Lin−CD90+ ILCs, rhIL-7–treated CLP mice had significantly increased IL-17– and IFN-γ–producing CD8 T cells compared with saline-treated CLP mice (Fig. 5A and Supplemental Fig. 4B).
Figure 5. rhIL-7 increases the number of cytokine-producing lymphocytes in lung.
The numbers of IL-17–, IL-22–, IFN-γ–, and TNF-α–producing cells within lungs were analyzed by flow cytometry in mice with sham surgery or CLP, treated with either rhIL-7- or saline, at 16 h after P. aeruginosa infection. (A) Graphs show the absolute numbers of IL-17–, IL-22–, IFN-γ–, and TNF-α–producing T cell and ILC subsets in lungs of infected mice. Cells were restimulated in vitro with PMA and ionomycin for 4 h before analysis. Cells were gated on live CD45+ cells. T cells and NK cells were gated as indicated in Fig. 2. CD3−CD19−NK1.1−CD90+ cells were defined as Lin−CD90+ ILCs. In CLP mice with P. aeruginosa pneumonia, rhIL-7 treatment increased the number of IL-17– and IFN-γ–producing CD8 T cells and that of IL-17–, IFN-γ–, and TNF-α–producing ILCs in lungs. Results of 3 independent experiments were combined. Horizontal lines and error bars represent means ± sd. Asterisks indicate significant differences in values (*P < 0.05; **P < 0.01). (B) Representative dot plots showing the gating strategies and the percentage of IL-17–producing cells in Lin−CD90+ ILCs in lungs. Pa, Pseudomonas aeruginosa infection.
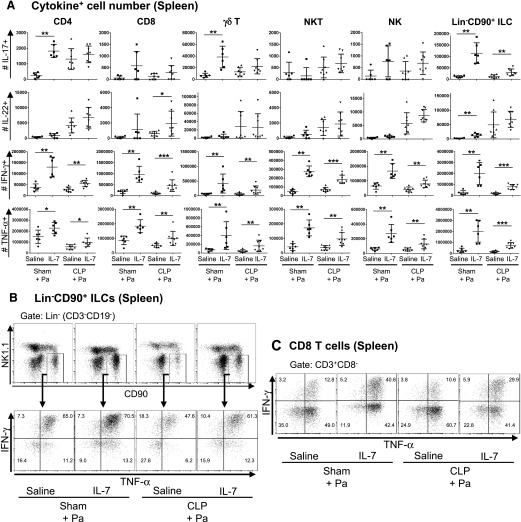
rhIL-7 increases the numbers of IFN-γ–, TNF-α–, IL-17–, and IL-22–producing lymphocytes in spleens
When examining cytokine-producing lymphocytes in the spleens of P. aeruginosa–infected mice, we observed that rhIL-7 treatment led to significant increases in the numbers of IFN-γ– and TNF-α–producing CD4 T, CD8 T, TCR-γδ T, and NKT and NK cells and Lin−CD90+ ILCs in mice after CLP and pneumonia induction (Fig. 6 and Supplemental Figs. 3B and 4). Furthermore, the number of IL-17–producing splenic Lin−CD90+ ILCs was increased significantly in the rhIL-7 treatment groups (Fig. 6A). These effects were even more pronounced in sham-operated mice. Intriguingly, although we detected a few IL-22–producing lymphocytes in the spleens of sham-operated mice, CLP mice had measurable numbers of splenic IL-22–producing CD4 T cells, NK cells, and Lin−CD90+ ILCs (Fig. 6A). There was a significant increase in the number of IL-22–producing splenic CD8 T cells. There was also a trend of more IL-22–producing NK cells with rhIL-7 treatment in CLP mice, although this was not statistically significant (P = 0.065).
Figure 6. rhIL-7 increases the number of cytokine-producing lymphocytes in spleen.
The numbers of IL-17–, IL-22–, IFN-γ–, and TNF-α–producing cells within spleens were analyzed by flow cytometry in mice with sham surgery or CLP, treated with either rhIL-7- or saline, at 16 h after P. aeruginosa infection. (A) Graphs show the absolute numbers of IL-17–, IL-22–, IFN-γ–, and TNF-α–producing T cell and ILC subsets in spleens of infected mice. Cells were restimulated and analyzed as in Fig. 5. In CLP mice with P. aeruginosa pneumonia, rhIL-7 treatment increased the numbers of IFN-γ–, TNF-α–, IL-17–, and IL-22–producing T cell subsets and ILCs in spleens. Results of 3 independent experiments were combined. Horizontal lines and error bars represent means ± sd. Asterisks indicate significant differences in values (*P < 0.05; **P < 0.01; ***P < 0.001). (B and C) Representative dot plots showing the gating strategies and the percentages of IFN-γ– and TNF-α–producing cells in Lin−CD90+ ILCs and CD8 T cells in spleens. Pa, Pseudomonas aeruginosa infection.
The increases in cytokine-producing T cell and ILC numbers were not due to a general expansion of these subsets during the rhIL-7 treatment; rather, it appears that their cytokine-producing functions were specifically enhanced by rhIL-7. For instance, although Lin−CD90+ ILC numbers were increased 2.1-fold in spleens of the rhIL-7–treated mice over those in saline-treated mice after CLP and pneumonia, the number of IFN-γ–producing Lin−CD90+ ILCs was increased 4.0-fold in these mice (Supplemental Fig. 4A). Similar effects were seen in CD8 T cells, which showed specific increases in IFN-γ responses (Supplemental Fig. 4B). Interestingly, although both the absolute number of splenic CD8 T cells and the number of TNF-α–producing CD8 T cells were increased to similar levels in rhIL-7–treated mice (Supplemental Fig. 4B), the number of TNF-α–producing splenic Lin−CD90+ ILCs was increased 4.5-fold, and this value was significantly higher than the 2.1-fold increase in absolute Lin−CD90+ ILC number (Supplemental Fig. 4A). Thus, rhIL-7 treatment not only expanded T cell and ILC numbers but also increased their functions.
Taken together, rhIL-7 treatment during sepsis and pneumonia led to an increase in the numbers of IL-17–, IFN-γ–, and TNF-α–producing lung ILCs and CD8 T cells; and IFN-γ–, TNF-α–, IL-17–, and IL-22–producing splenic T cells and ILCs, consistent with enhanced lymphocyte functions as a possible mechanism to explain the protective effect of rhIL-7 treatment in sepsis and P. aeruginosa pneumonia.
rhIL-7 treatment increases NF-κB and STAT3 signaling in lungs
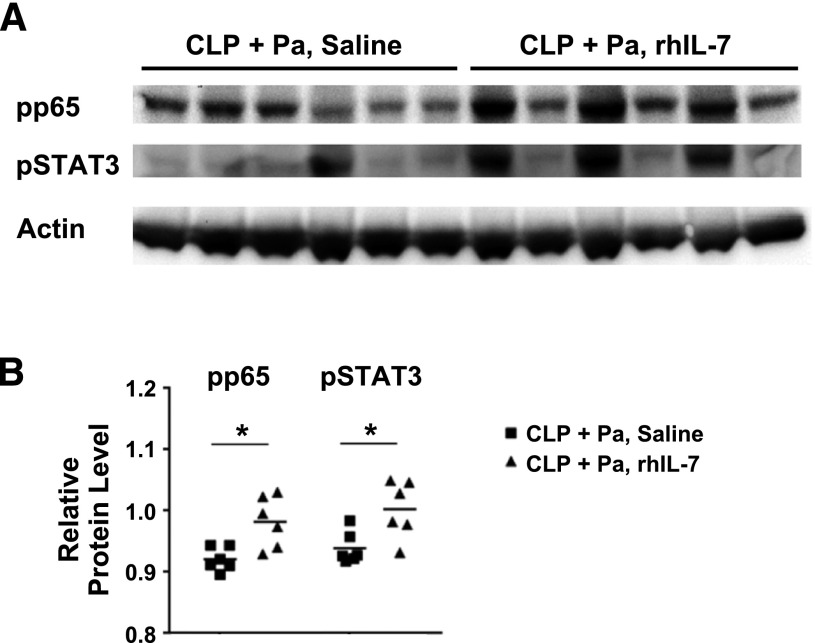
Given the effect of rhIL-7 to increase several key cytokines, we examined whether cells from lung homogenates of CLP mice treated with rhIL-7 had increased NF-κB activation, a transcription factor that is essential for cytokine production. Additionally, we investigated whether lung homogenates from rhIL-7–treated, septic mice had increased phosphorylated STAT3 expression; STAT3 has been reported to be an important mediator of repair following lung injury [33]. Compared with saline-treated mice that had CLP and pneumonia, rhIL-7-treated mice that had CLP and pneumonia had a significant increase in the level of phosphorylated p65, the active form of the NF-κB, in lung homogenates (Fig. 7A and B). Similarly, rhIL-7 also increased the levels of phosphorylated STAT3 within lungs of CLP mice with pneumonia compared to saline-treated controls (Fig. 7A and B).
Figure 7. NF-κB and STAT3 signaling in lungs.
(A) Using lungs that were harvested on d 4 after CLP (at 16 h after intratracheal P. aeruginosa injection), Western blotting was performed to detect activation of the transcriptional factors NF-κB and STAT3. Phosphorylated p65 (pp65) is one of the active forms of NF-κB. β-actin was used as a total protein loading control. Lanes represent individual animals. (B) Quantification of pp65 and phosphorylated STAT3 (pSTAT3) expression was performed with Image J. Densitometric analysis of pp65 and pSTAT3 bands was performed relative to the actin band. In CLP mice, both pp65 and pSTAT3 protein levels were significantly increased in rhIL-7–treated mice over those in saline-treated mice. An asterisk indicates a significant difference in values (*P < 0.05). Pa, Pseudomonas aeruginosa infection.
DISCUSSION
The present results demonstrating that rhIL-7 improves survival in secondary P. aeruginosa pneumonia are an important addition to the growing evidence that IL-7 immunoadjuvant therapy represents an effective new approach in sepsis. Our group has previously shown that rhIL-7 improves immunity and survival in a primary peritonitis model of sepsis and in secondary infections from fungal pathogens [15, 18, 22, 26]. Other investigators [34, 35] have shown that rhIL-7 improves host immunity in viral infections. Multiple clinical case reports document that rhIL-7 decreased viral load to undetectable levels and resulted in survival of patients with JC virus–induced, progressive, multifocal leukoencephalopathy [14, 36]. Thus, rhIL-7 is an effective immunoadjuvant against a number of diverse bacterial, fungal, and viral pathogens. The present results are particularly significant because P. aeruginosa is a leading cause of hospital-acquired pneumonia and carries an extremely high mortality rate. Importantly, many patients succumb to P. aeruginosa despite therapy with antimicrobial drugs to which the organism is susceptible, thereby suggesting that impaired host immunity may be one factor in the poor outcome [37]. In that regard, P. aeruginosa particularly targets patients with known immunosuppression [38]. Therefore, therapy with agents that boost host immunity may offer a significant additive benefit to conventional antimicrobial therapy.
IL-7 has been termed the “maestro” of the immune system because of its multitude of effects to enhance host immunity [39]. One of the major pathophysiologic events in sepsis is depletion of key immune effector cells, including various subsets of T cells [4]. In this study, we demonstrated that administration of rhIL-7 induced the proliferation of ILCs and innate-like T cells, in addition to CD4 and CD8 T cells, in naive mice. Thus, we speculated that increased proliferation of these cells also occurs during the 2-hit model of sepsis and P. aeruginosa pneumonia, which could contribute to protection from lethal pneumonia. In addition, IL-7 also acted to ameliorate depletion of immune cells by blocking sepsis-induced apoptosis [40]. The antiapoptotic effects of IL-7 are due to its action to increase anti-apoptotic Bcl-2 family members and decrease proapoptotic Bcl-2 family members [12]. In the present study, rhIL-7–treated mice that had CLP followed by P. aeruginosa pneumonia had increased absolute numbers of lung and spleen immune effector cells, i.e., CD8, TCR-γδ T cells, NKT cells, and ILCs, compared with control mice that received saline diluent (Fig. 3). This lymphocyte sparring effect of rhIL-7 was most likely due to both its ability to decrease sepsis-induced apoptosis and its effect on inducing lymphocyte proliferation, as shown in the present study, as well as in 2 of our previous sepsis studies [18, 22].
A major pathophysiologic effect of sepsis is impaired T cell and monocyte/macrophage production of cytokines, which are essential for containing and killing pathogens [4]. In the present study, we investigated IFN-γ, IL-17, and TNF-α because of their reported beneficial roles for improving host defenses in sepsis and P. aeruginosa infection [4, 10, 32]. The present results show that, in the 2-hit CLP and pneumonia model, rhIL-7 caused an increase in IFN-γ–producing lung Lin−CD90+ ILCs and CD8 T cells and splenic T cell subsets and ILCs (Figs. 5 and 6). These results showing rhIL-7’s effects of increasing IFN-γ are particularly important, given recent work by Pastille et al. [41] who demonstrated the importance of IFN-γ in the host defense against P. aeruginosa pneumonia after CLP.
The effect of rhIL-7 of increasing cytokines was not restricted to IFN-γ, i.e., rhIL-7 also increased the absolute number of spleen and lung cells producing IL-17 and TNF-α. Interestingly, the cytokine-promoting effect of rhIL-7 was more pronounced in the sham-operated mice with pneumonia compared with mice that had undergone CLP and then developed pneumonia (Figs. 5 and 6). The presence of preceding polymicrobial peritonitis blunted, but did not abolish, the ability of rhIL-7 to increase cytokine-producing cells in lung and spleen. The ability of rhIL-7 to increase IL-17 may also contribute to rhIL-7’s salutary effect of improving survival in P. aeruginosa pneumonia. In this regard, independent groups [28, 42] have reported that IL-17 and its receptor pathway are pivotal in facilitating pulmonary clearance of gram-negative bacteria and thereby improving survival. TNF-α also has an important role in mouse models of P. aeruginosa pneumonia [10]. Particularly relevant to the present study were findings from Song et al. [43], who also conducted experiments in a 2-hit model of CLP followed by P. aeruginosa pneumonia. These investigators showed that neutralization of TNF-α reversed the protective effect of IL-4 knockout in this 2-hit pneumonia model, consistent with a protective role for TNF-α. In short, rhIL-7 increased production of 3 unique cytokines that have been reported to have essential roles in resolving P. aeruginosa pneumonia, thereby underscoring the pleiotropic effects of rhIL-7 during infection. This ability of rhIL-7 to increase multiple cytokines is likely partly due to its effect of activating CD4 T helper cells that are master regulators of host immunity and induce crosstalk between the innate and adaptive immune systems.
A novel finding in the present study was the effect of rhIL-7 on ILCs. IL-7 is known to be important for ILC development and maintenance [11]. The current investigation was, to our knowledge, the first work to document the capability of rhIL-7 treatment to affect the number and function of ILCs. Administration of rhIL-7 to naïve mice (nonsepsis and nonpneumonia) induced ILC proliferation (Fig. 2). rhIL-7 ameliorated sepsis-induced cell loss of ILC2s and ILC3s in lungs during sepsis and P. aeruginosa pneumonia. rhIL-7 significantly increased the number of IL-17–, IFN-γ–, and TNF-α–producing ILCs in lungs and spleens of sham-operated and CLP mice with P. aeruginosa pneumonia (Figs. 5 and 6 and Supplemental Fig. 4). This ability of rhIL-7 to activate ILCs may be vital in enabling the host to rapidly mobilize immune defenses and contain the invading pathogens before the time when CD4 and CD8 T cells are able to respond.
NF-κB and STAT3 are essential transcriptional factors involved in pathogen elimination and recovery from lung injury in pneumonia [33]. In this study, we revealed that rhIL-7 treatment increased NF-κB and STAT3 signaling in lungs during sepsis and P. aeruginosa pneumonia (Fig. 7). The activation of NF-κB may be associated with increased numbers of activated lymphocytes that produce cytokines, including IL-17, IFN-γ, and TNF-α (Fig. 5). STAT3 is a transcription factor downstream of cytokine receptor signaling. Recent work [33] demonstrated that STAT3 is activated in epithelial cells after IL-22 exposure and orchestrates epithelial repair mechanisms during pneumonia. Although there were no differences in IL-22 production from lung immune cells between CLP mice with and without rhIL-7 treatment, activation status of STAT3 was significantly increased in rhIL-7–treated CLP mice (Fig. 7). Thus, activated NF-κB and STAT3 signaling in lungs is consistent with the protective effect of rhIL-7 during sepsis and P. aeruginosa pneumonia.
rhIL-7 treatment has been well tolerated in >300 patients with a variety of infectious and oncologic diseases [12]. Unlike IL-2, a closely-related cytokine, rhIL-7 treatment has an excellent safety profile and rarely induces fever, capillary leak syndrome, or other clinical findings associated with excessive proinflammatory cytokines [12]. Given its multiple beneficial effects on host immunity; its reported efficacy in bacterial, fungal, and animal sepsis models; and its excellent clinical track record [12, 14, 18, 22, 26, 36], we believe that rhIL-7 represents an excellent candidate for clinical testing in patients with sepsis. Importantly, Venet et al. [44] recently demonstrated the ability of rhIL-7 to reverse sepsis-induced T cell alterations in patients with septic shock. Their studies showed that ex vivo treatment of patients’ cells with rhIL-7 corrected multiple sepsis-induced defects, including CD4 and CD8 T cell proliferation and IFN-γ production. Thus, functional restoration by rhIL-7 indicates that the IL-7 pathway remains fully operative during sepsis and provides further support for clinical trials with this cytokine, as advocated by the authors of that study [44].
In conclusion, rhIL-7 provided potent protection in a 2-hit model of CLP followed by P. aeruginosa pneumonia. rhIL-7 increased the number of immune effector cells in lungs and spleens. Moreover, rhIL-7 increased the number of lymphocytes that produce IFN-γ, IL-17, and TNF-α, cytokines that are important in the host defense against sepsis and P. aeruginosa pneumonia. The effects of rhIL-7 were observed not only on CD4 and CD8 T cells but also on TCR-γδ T cells, NK cells, and ILCs. Furthermore, rhIL-7 activated NF-κB and STAT3 in lungs during sepsis and P. aeruginosa pneumonia. Thus, rhIL-7 acts broadly on multiple components of host immunity to enhance the ability to fight pathogens. Given the excellent safety record of rhIL-7 in clinical trials to date and the high mortality associated with sepsis, immunoadjuvant therapeutic trials with rhIL-7 should be considered strongly.
AUTHORSHIP
Y.S. and R.S.H. designed the survival study. C.G.D. assisted the survival study. Y.S., A.G.F., and R.S.H. designed the experiments. Y.S. and A.G.F. performed the experiments and analyzed the data. T.E., J.U., and C.A.B. assisted the experiments. Y.S., A.G.F., C.G.D., T.E., J.U., C.A.B., J.M.G., G.V.B., and R.S.H. discussed the results and contributed to data interpretation. Y.S. and A.G.F. wrote the first draft of the manuscript. C.G.D. and T.E. assisted with manuscript preparation. M.M. contributed to experimental design and data interpretation and provided recombinant human interleukin-7. C.A.B., J.M.G., G.V.B., and R.S.H. contributed to the critical revision of the manuscript. All authors approved the final draft.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health Grants GM44118 and GM098391. We thank Nemani Rateri for assistance with animal surgery; Jacquelyn McDonough for advising about the survival study protocol; Dale Osborne, Sarbani Ghosh, Shin-Wen Hughes, and Meghan Wallace for assistance with cell preparation and bacterial preparation; Andrew Walton for assistance with manuscript preparation; and Lucas Sjeklocha for performing ELISA of IL-10 and IL-12.
Glossary
- BAL
bronchoalveolar lavage
- CLP
cecal ligation and puncture
- ILC
innate lymphoid cell
- ILC2
group 2 innate lymphoid cell
- ILC3
group 3 innate lymphoid cell
- Lin
lineage
- rhIL-7
recombinant human interleukin-7
- ROR
retinoic acid–related orphan receptor
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
T.E. is a full-time employee of GlaxoSmithKline. M.M. is a full-time employee of Revimmune, Inc. R.S.H. received research funding from Bristol-Myers Squibb and from MedImmune, and he is also an advisor to GlaxoSmithKline plc. and MSD (Merck & Co., Inc.). Other authors declare no conflicts of interest.
REFERENCES
- 1.Martin G. S., Mannino D. M., Moss M. (2006) The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 34, 15–21. [DOI] [PubMed] [Google Scholar]
- 2.Kung H. C., Hoyert D. L., Xu J., Murphy S. L. (2008) Deaths: final data for 2005. Natl. Vital Stat. Rep. 56, 1–120. [PubMed] [Google Scholar]
- 3.Hotchkiss R. S., Monneret G., Payen D. (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 13, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss R. S., Monneret G., Payen D. (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus D. C., van der Poll T. (2013) Severe sepsis and septic shock. N. Engl. J. Med. 369, 840–851. [DOI] [PubMed] [Google Scholar]
- 6.Kasten K. R., Tschöp J., Adediran S. G., Hildeman D. A., Caldwell C. C. (2010) T cells are potent early mediators of the host response to sepsis. Shock 34, 327–336. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood E. R., Hotchkiss R. S. (2013) BTLA as a biomarker and mediator of sepsis-induced immunosuppression. Crit. Care 17, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boomer J. S., To K., Chang K. C., Takasu O., Osborne D. F., Walton A. H., Bricker T. L., Jarman S. D. II, Kreisel D., Krupnick A. S., Srivastava A., Swanson P. E., Green J. M., Hotchkiss R. S. (2011) Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Thoracic Society; Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171, 388–416. [DOI] [PubMed] [Google Scholar]
- 10.Williams B. J., Dehnbostel J., Blackwell T. S. (2010) Pseudomonas aeruginosa: host defence in lung diseases. Respirology 15, 1037–1056. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg G. F., Artis D. (2015) Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 21, 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackall C. L., Fry T. J., Gress R. E. (2011) Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 11, 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corfe S. A., Paige C. J. (2012) The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin. Immunol. 24, 198–208. [DOI] [PubMed] [Google Scholar]
- 14.Patel A., Patel J., Ikwuagwu J. (2010) A case of progressive multifocal leukoencephalopathy and idiopathic CD4+ lymphocytopenia. J. Antimicrob. Chemother. 65, 2697–2698. [DOI] [PubMed] [Google Scholar]
- 15.Unsinger J., Burnham C. A., McDonough J., Morre M., Prakash P. S., Caldwell C. C., Dunne W. M. Jr., Hotchkiss R. S. (2012) Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 206, 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muenzer J. T., Davis C. G., Chang K., Schmidt R. E., Dunne W. M., Coopersmith C. M., Hotchkiss R. S. (2010) Characterization and modulation of the immunosuppressive phase of sepsis. Infect. Immun. 78, 1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muenzer J. T., Davis C. G., Dunne B. S., Unsinger J., Dunne W. M., Hotchkiss R. S. (2006) Pneumonia after cecal ligation and puncture: a clinically relevant “two-hit” model of sepsis. Shock 26, 565–570. [DOI] [PubMed] [Google Scholar]
- 18.Unsinger J., McGlynn M., Kasten K. R., Hoekzema A. S., Watanabe E., Muenzer J. T., McDonough J. S., Tschoep J., Ferguson T. A., McDunn J. E., Morre M., Hildeman D. A., Caldwell C. C., Hotchkiss R. S. (2010) IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 184, 3768–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capitini C. M., Chisti A. A., Mackall C. L. (2009) Modulating T-cell homeostasis with IL-7: preclinical and clinical studies. J. Intern. Med. 266, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin R. G., Friend D., Ziegler S. F., Jerzy R., Falk B. A., Gimpel S., Cosman D., Dower S. K., March C. J., Namen A. E., Park L. S. (1990) Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell 60, 941–951. [DOI] [PubMed] [Google Scholar]
- 21.Johnson S. E., Shah N., Panoskaltsis-Mortari A., LeBien T. W. (2005) Murine and human IL-7 activate STAT5 and induce proliferation of normal human pro-B cells. J. Immunol. 175, 7325–7331. [DOI] [PubMed] [Google Scholar]
- 22.Shindo Y., Unsinger J., Burnham C. A., Green J. M., Hotchkiss R. S. (2015) Interleukin-7 and anti-programmed cell death 1 antibody have differing effects to reverse sepsis-induced immunosuppression. Shock 43, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs A., Vermi W., Lee J. S., Lonardi S., Gilfillan S., Newberry R. D., Cella M., Colonna M. (2013) Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12– and IL-15–responsive IFN-γ–producing cells. Immunity 38, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinette M. L., Fuchs A., Cortez V. S., Lee J. S., Wang Y., Durum S. K., Gilfillan S., Colonna M., Immunological Genome C.; Immunological Genome Consortium (2015) Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patankar Y. R., Mabaera R., Berwin B. (2015) Differential ASC requirements reveal a key role for neutrophils and a noncanonical IL-1β response to Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L902–L913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasten K. R., Prakash P. S., Unsinger J., Goetzman H. S., England L. G., Cave C. M., Seitz A. P., Mazuski C. N., Zhou T. T., Morre M., Hotchkiss R. S., Hildeman D. A., Caldwell C. C. (2010) Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through γδ T-cell IL-17 production in a murine model of sepsis. Infect. Immun. 78, 4714–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudva A., Scheller E. V., Robinson K. M., Crowe C. R., Choi S. M., Slight S. R., Khader S. A., Dubin P. J., Enelow R. I., Kolls J. K., Alcorn J. F. (2011) Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J. Immunol. 186, 1666–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye P., Rodriguez F. H., Kanaly S., Stocking K. L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., Shellito J. E., Bagby G. J., Nelson S., Charrier K., Peschon J. J., Kolls J. K. (2001) Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aujla S. J., Chan Y. R., Zheng M., Fei M., Askew D. J., Pociask D. A., Reinhart T. A., McAllister F., Edeal J., Gaus K., Husain S., Kreindler J. L., Dubin P. J., Pilewski J. M., Myerburg M. M., Mason C. A., Iwakura Y., Kolls J. K. (2008) IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolk K., Witte E., Witte K., Warszawska K., Sabat R. (2010) Biology of interleukin-22. Semin. Immunopathol. 32, 17–31. [DOI] [PubMed] [Google Scholar]
- 31.Onishi R. M., Gaffen S. L. (2010) Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAleer J. P., Kolls J. K. (2014) Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol. Rev. 260, 129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinton L. J., Mizgerd J. P. (2015) Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu. Rev. Physiol. 77, 407–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanjappa S. G., Kim E. H., Suresh M. (2011) Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood 117, 5123–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellegrini M., Calzascia T., Toe J. G., Preston S. P., Lin A. E., Elford A. R., Shahinian A., Lang P. A., Lang K. S., Morre M., Assouline B., Lahl K., Sparwasser T., Tedder T. F., Paik J. H., DePinho R. A., Basta S., Ohashi P. S., Mak T. W. (2011) IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144, 601–613. [DOI] [PubMed] [Google Scholar]
- 36.Alstadhaug K. B., Croughs T., Henriksen S., Leboeuf C., Sereti I., Hirsch H. H., Rinaldo C. H. (2014) Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol. 71, 1030–1035. [DOI] [PubMed] [Google Scholar]
- 37.Shindo Y., Ito R., Kobayashi D., Ando M., Ichikawa M., Goto Y., Fukui Y., Iwaki M., Okumura J., Yamaguchi I., Yagi T., Tanikawa Y., Sugino Y., Shindoh J., Ogasawara T., Nomura F., Saka H., Yamamoto M., Taniguchi H., Suzuki R., Saito H., Kawamura T., Hasegawa Y.; Central Japan Lung Study Group (2015) Risk factors for 30-day mortality in patients with pneumonia who receive appropriate initial antibiotics: an observational cohort study. Lancet Infect. Dis. 15, 1055–1065. [DOI] [PubMed] [Google Scholar]
- 38.Shindo Y., Ito R., Kobayashi D., Ando M., Ichikawa M., Shiraki A., Goto Y., Fukui Y., Iwaki M., Okumura J., Yamaguchi I., Yagi T., Tanikawa Y., Sugino Y., Shindoh J., Ogasawara T., Nomura F., Saka H., Yamamoto M., Taniguchi H., Suzuki R., Saito H., Kawamura T., Hasegawa Y.; Central Japan Lung Study (2013) Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 188, 985–995. [DOI] [PubMed] [Google Scholar]
- 39.Sprent J., Surh C. D. (2012) Interleukin 7, maestro of the immune system. Semin. Immunol. 24, 149–150. [DOI] [PubMed] [Google Scholar]
- 40.Hutchins N. A., Unsinger J., Hotchkiss R. S., Ayala A. (2014) The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol. Med. 20, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pastille E., Pohlmann S., Wirsdörfer F., Reib A., Flohé S. B. (2015) A disturbed interaction with accessory cells upon opportunistic infection with Pseudomonas aeruginosa contributes to an impaired IFN-γ production of NK cells in the lung during sepsis-induced immunosuppression. Innate Immun. 21, 115–126. [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Feng Y., Yang K., Li Q., Ye L., Han L., Wan H. (2011) Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol. Med. Microbiol. 61, 179–188. [DOI] [PubMed] [Google Scholar]
- 43.Song Z., Zhang J., Zhang X., Li D., Wang H., Xu X., Xu W., Yin Y., Cao J. (2015) Interleukin 4 deficiency reverses development of secondary Pseudomonas aeruginosa pneumonia during sepsis-associated immunosuppression. J. Infect. Dis. 211, 1616–1627. [DOI] [PubMed] [Google Scholar]
- 44.Venet F., Foray A. P., Villars-Méchin A., Malcus C., Poitevin-Later F., Lepape A., Monneret G. (2012) IL-7 restores lymphocyte functions in septic patients. J. Immunol. 189, 5073–5081. [DOI] [PubMed] [Google Scholar]