Abstract
Immunosuppressive, naturally occurring CD4+CD25+forkhead box p3+ (Foxp3+) regulatory T cells (nTregs) offer potential for the treatment of immune-mediated inflammatory disorders. However, potential instability of ex vivo-expanded nTregs following their adoptive transfer may be a significant limitation. LPS-stimulated hepatic stellate cells (HSCs) induce expansion and enhance the suppressive function and stability of allogeneic nTregs. We aimed to delineate mechanisms underlying HSC-induced expansion and increased potency of nTregs. HSCs and nTregs were isolated from mouse livers and spleens, respectively. Following coculture with LPS-pretreated allogeneic HSCs (LPS/HSCs), proliferation of nTregs was measured by CFSE dilution, and Foxp3 expression and acetylation were determined by immunoprecipitation (IP) and Western blotting analysis. Expression of various genes associated with immunologic tolerance was determined by quantitative RT-PCR (qRT-PCR). LPS stimulation increased the expression and activity of the immunoregulatory enzyme IDO1 in HSCs, and LPS/HSCs stimulated aryl hydrocarbon receptor (AhR) signaling in cocultured nTregs. Reciprocally, Tregs increased IDO1 expression in HSCs. IDO1−/− LPS/HSCs were inferior to WT LPS/HSCs in stimulating nTreg expansion. Pharmacologic inhibition of IDO1 in HSCs by 1-methyltryptophan (1MT) inhibited LPS/HSC-induced AhR signaling in nTregs, which was responsible for their expansion, Foxp3 expression, and stabilization of Foxp3 by increasing acetylation of lysine residues. Finally, HSCs cryopreserved, following 2–3 passages, were as potent as primary-cultured HSCs in expanding nTregs. In conclusion, LPS/HSCs expand allogeneic nTregs through an IDO-dependent, AhR-mediated mechanism and increase their stability through lysine-acetylation of Foxp3. nTregs expanded by cryopreserved HSCs may have potential for clinical use.
Keywords: endotoxin, tolerance, acetylation, l-kynurenine, cytokines
Introduction
CD4+CD25+Foxp3+ nTregs are potent suppressors of immune responses. Thus, the administration of ex vivo-expanded Tregs to transplant recipients or in certain autoimmune diseases is considered an attractive approach to reducing dependence on immunosuppressive drugs and to promote or restore tolerance [1–5]. Indeed, the potential of Treg therapy has been shown in clinical trials of GvHD [6–8], type 1 diabetes [9], and a pilot trial for liver transplantation [10]. Current protocols use anti-CD3/CD28 mAb to expand polyclonal Tregs for therapy. However, these Tregs can lose their suppressive potential as a result of unstable Foxp3 expression, and repeated, frequent administration may be required [11, 12]. Furthermore, such ex vivo-expanded Tregs may convert into immune effector T cells [13, 14]. Efforts are being made to devise protocols for generation of a large number of Tregs that retain their suppressive potential. The immunosuppressive serine/threonine protein kinase inhibitor rapamycin has been shown to enhance long-term persistence of the Treg phenotype in culture [15, 16] and to promote conversion of conventional T cells into iTregs [17]. However, instability of iTreg and the loss of Foxp3 expression can render them to become pathogenic Th cells [18]. Thus, it is important to develop methods that increase the in vivo stability and durability of nTreg phenotype and function, while maximizing their ex vivo expansion.
Perisinusoidal HSCs that constitute ∼10% of the entire liver cell population become activated to a potent fibrogenic and contractile myofibroblast-like phenotype during liver injury [19]. They perform several other critical functions, and several laboratories, including our own, have provided strong evidence for a prominent role of HSCs in hepatic inflammation, immune responses, and acute liver injury [20, 21]. HSCs are highly responsive to Gram-negative bacterial endotoxin (LPS) and modulate the immunologic microenvironment of the liver by producing numerous cytokines and chemokines [20, 21]. Thus, LPS–HSC interactions become important in hepatic inflammation and immune regulation in both liver disease and liver transplantation when portal and circulating LPS levels are elevated [22]. We showed previously that LPS-stimulated HSCs (LPS/HSC) are unique in their ability to expand allogeneic but not syngeneic nTregs in a contact-dependent manner and at the same time, enhance their in vivo stability and suppressor function [23]. In this study, we investigated mechanisms of LPS/HSC-induced nTreg expansion and enhanced function. We found that in addition to cell–cell contact, LPS-induced IDO1 in HSCs and consequent AhR signaling in nTregs are also critical to their expansion and superior immunosuppressive functions.
MATERIALS AND METHODS
Unless indicated otherwise, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Animals
Experimental protocols were approved by the Animal Care and Use Committees of the University of Cincinnati and Cincinnati VA Medical Center, in accordance with the U.S. National Institutes of Health guidelines. B6, BALB/c (H-2d), and IDO1−/− (B6.129-Ido1tm1Alm/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA).
HSC isolation
HSCs were isolated essentially as described previously [23, 24]. In brief, livers were perfused in situ via the inferior vena cava with 30–40 ml Ca2+-free HBSS and digested by further perfusion with 30–40 ml HBSS containing collagenase type IV (0.25 mg/ml; Worthington Biochemical, Lakewood, NJ, USA) and protease (0.50 mg/ml; Sigma-Aldrich). The cells were dispersed, and the suspension was filtered through 100 μm nylon mesh. Following removal of hepatocytes and cell debris by low-speed centrifugation (50 g; 2 min), HSCs were purified by Histodenz density gradient centrifugation and suspended in DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin, 10% v/v FBS, and 10% v/v horse serum. Cells were seeded in gelatin-coated (0.1% in PBS) plates at a density of 0.5 × 106/cm2. Loosely adherent HSCs were aspirated 20 min later and reseeded in new, 6- or 96-well, flat-bottom plates. Cells showing 95–98% purity, as assessed by desmin immunostaining and vitamin A autofluorescence (see Fig. 1) and by FACS analysis (LSRFortessa; BD Biosciences, San Jose, CA, USA) using anti-CD68 (KCs), anti-CD11b (KCs and DCs), and anti-CD11c (DCs and NK cells) antibodies (AbD Serotec, Bio-Rad Laboratories, Hercules, CA, USA; and BioLegend, San Diego, CA, USA) [23, 24], were used.
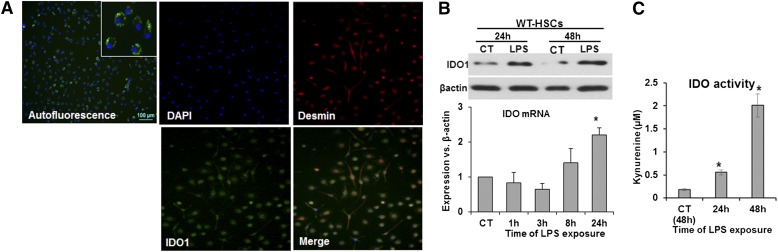
Figure 1. LPS increases expression of IDO in HSCs.
(A, upper left) Vitamin A autofluorescence showing highly pure HSCs. (Middle and right) IDO1 coexpression (DAPI: blue; IDO1: green) and desmin (red) and in B6 mouse HSCs on d 3 of culture, as assessed by immunohistochemistry. (B and C) B6 mouse HSCs were washed and stimulated with 100 ng/ml LPS for the indicated times. (B) Expression of IDO1 mRNA and protein. CT, Control. (C) IDO1 enzyme activity as measured by l-kynurenine in the culture supernatant. *P < 0.05.
Immunofluorescence microscopy
To determine IDO expression, HSCs cultured on coverslips were fixed with 2% paraformaldehyde in PBS and permeabilized using 0.1% Triton X-100. Nonspecific binding was blocked with 2% BSA and cells incubated overnight with rat anti-mouse IDO1 antibody (BioLegend) and rabbit polyclonal anti-desmin antibody (Abcam, Cambridge, MA, USA). Goat anti-rat (Alexa Fluor 488; Cell Signaling Technology, Danvers, MA, USA) and goat anti-rabbit (Alexa Fluor 594; Thermo Fisher Scientific, Waltham, MA, USA) secondary antibodies were used to stain IDO1 and desmin, respectively, whereas nuclei were stained with DAPI (Vector Laboratories, Burlingame, CA, USA).
Measurement of IDO1 activity
IDO1 activity was measured by determining l-kynurenine concentration in culture supernatants, as described [25]. In brief, cells were cultured in DMEM containing 0.6 mM l-tryptophan (0.08 mM in basic medium) for 24–48 h, with or without LPS (100 ng/ml); 160 µl of the culture supernatants was mixed with 10 µl 30% trichloroacetic acid and incubated at 50°C for 30 min. After centrifugation (at 600 g for 10 min), 100 µl of the supernatant was mixed with freshly prepared Ehrlich's reagent [1.2% 4-(dimethylamino)benzaldehyde in glacial acetic acid] and incubated for 10 min, and the absorbance was determined at 492 nm. A linear curve developed with standard l-kynurenine was used to calculate l-kynurenine concentration in the medium.
T cell isolation and purification
A single-cell suspension of splenocytes of BALB/c mice was prepared using RBC lysing buffer (Lonza, Walkersville, MD, USA). Conventional or effector CD4+CD25− T cells and CD4+CD25+ cells were isolated using a MACS kit, according to the manufacturer’s protocols (Miltenyi Biotec, Auburn, CA, USA). To isolate CD4+CD25+ T cells, the splenocytes were labeled with non-CD4+ T cell cocktail antibody (catalog no. 130-091-041; Miltenyi Biotec) and loaded onto LS separation columns (catalog no. 130-042-401; Miltenyi Biotec), followed by CD25 MicroBead-based positive selection of CD4+CD25+ T cells. CD4+ T cells were purified by negative selection using LS columns. Purity of individual cell populations, as determined by flow cytometry, was >95% [23].
Coculture of HSCs with allogeneic CD4+ T cells
HSCs were treated with 270 μM gadolinium trichloride for 24 h to block the activity of contaminating KCs, if any, then washed, and stimulated with LPS (100 ng/ml) for 24 h. The cells were then washed and cocultured in fresh medium with CSFE-labeled, purified Tregs or conventional CD4+ T cells (1:10) in the presence of polymyxin B (300 ng/ml) to block the direct effect of any residual LPS on CD4+ T cells [23]. At the end of coculture, T cells were aspirated (HSCs remained firmly attached to the plate), and their proliferation was measured by CFSE dilution assay by gating on CD4+CD25+ T cells using FACSCanto (BD Biosciences). Data were analyzed with FlowJo 9.02 software.
IP and Western blotting
T cell homogenates, prepared in RIPA lysis buffer (Santa Cruz Biotechnology, Dallas, TX, USA), were first subjected to a preclearing step using protein A/G agarose (50 µl/ml; Pierce Biotechnology, Rockford, IL, USA). The homogenates were incubated with 5 µg/ml IP antibody [rat IgG2a anti-Foxp3 (eBioscience, San Diego, CA, USA) or control mouse IgG1 anti-AhR (Pierce Biotechnology)] overnight with constant gentle mixing and then for 2 h with protein A/G agarose (25 µl/ml). Following centrifugation, the protein A/G agarose slurry from each sample was washed twice with ice-cold RIPA lysis buffer and then with PBS, resuspended in Laemelli sample buffer, boiled, and centrifuged (14,000 rpm/1 min). The proteins were separated via SDS-PAGE and transferred onto polyvinylidene difluoride membranes. After blocking with 5% nonfat dry milk in PBS–Tween 20, the membranes were incubated with primary antibodies [(mouse anti-p300/CBP; mouse anti-Foxp3 and mouse anti-ubiquitin (eBioscience); and mouse anti-AhR (Pierce Biotechnology)]. Proteins were detected using ECL reagent (GE Life Sciences, Buckinghamshire, United Kingdom).
mRNA extraction and qRT-PCR
Cellular RNA was extracted using TRIzol reagent (Thermo Fisher Scientific). cDNA was prepared using reverse transcription kits (Thermo Fisher Scientific) and expression of various genes determined using the SYBR Green kit and a 7300 real-time PCR machine (Thermo Fisher Scientific). Primer sequences are shown in Table 1.
TABLE 1.
qRT-PCR primer sequences
| Primer sequences | ||
|---|---|---|
| AhR | F | 5′-AGGTGCCTGCTGGATAATTC-3′ |
| R | 5′-CCGTCCTTCCCTTTCTTGTT-3′ | |
| β-actin | F | 5′-AGAGGGAAATCGTGCGTGAC-3′ |
| R | 5′-CAATAGTGATGACCTGGCCGT-3′ | |
| CTLA4 | F | 5′-CCTGGTCACTGCTGTTTCTT-3′ |
| R | 5′-TCACATTCTGGCTCTGTTGG-3′ | |
| Cyp1b1 | F | 5′-TGGCCCTTTCCTCCTATCT-3′ |
| R | 5′-ACTGACACAACCTGCGTATC-3′ | |
| Ebi3 | F | 5′-GCTCAGGACCTCACAGATTATG-3′ |
| R | 5′-TCAAGGATCCAGTCCCTCTT-3′ | |
| Foxp3 | F | 5′-GGCCCTTCTCCAGGACAGA-3′ |
| R | 5′-GGCATGGGCATCCACAGT-3′ | |
| IDO | F | 5′-ACTGTGTCCTGGCAAACTGGAAG-3′ |
| R | 5′-AAGCTGCGATTTCCACCAATAGAG-3′ | |
| IFN-γ | F | 5′-CTCTTCCTCATGGCTGTTTCT-3′ |
| R | 5′-TTCTTCCACATCTATGCCACTT-3′ | |
| IL-1β | F | 5′-GGTGTGTGACGTTCCCATTA-3′ |
| R | 5′-ATTGAGGTGGAGAGCTTTCAG-3′ | |
| IL-12a | F | 5′-CCTCCTCACACAGATAGGAAAC-3′ |
| R | 5′-GAGATGAGATGTGATGGGAGAAC-3′ | |
| IL-33 | F | 5′-CCTACTCCCTCAGCTTTCTTTC-3′ |
| R | 5′-GCAGGGTAAAGACAGTGGAATA-3′ | |
| LAG3 | F | 5′-CTGTCTGTCTGTCTGTCTCTCT-3′ |
| R | 5′-GTCCTCCCTCATCTCCTCTATG-3′ | |
| TNF-α | F | 5′-CCCAGGTATATGGGCTCATACC-3′ |
| R | 5′-GCCGATTTGCTATCTCATACCAGG-3′ | |
F, Forward; R, reverse.
Quantification of cytokines
Cell-free supernatants were prepared to measure cytokine concentrations using mouse 20-plex Luminex beadset (BioSource International, San Diego, CA, USA) or ELISA kits from eBioscience.
Statistical analysis
Results are expressed as means ± sd. Three independent HSC–nTreg coculture experiments, each in duplicate, were performed. Flow cytometry-based CSFE dilution assay was performed 3 times. Immunoblot analysis and protein–protein interactions were carried out 2 times from Treg pooled from 3 independent HSC–Treg cocultures. qRT-PCR and ELISA were performed using duplicate samples from 3 independent experiments. Statistical significance was determined using SigmaPlot 11 (Systat Software, San Jose, CA, USA) and one-way ANOVA and Student’s t test. A P < 0.05 was considered statistically significant.
RESULTS
LPS stimulates IDO expression/activity in HSCs
The HSC preparation was highly pure, as assessed by vitamin A autofluorescence and desmin staining (Fig. 1A, upper left and right, respectively). As IDO1 is a critical regulator of T cell survival and function, we determined its expression and activity in LPS-treated HSCs. Dual immunostaining demonstrated constitutive expression of IDO1 by the desmin-positive HSCs (Fig. 1A); LPS stimulation increased IDO1 mRNA expression, which was apparent at 8 h and significant at 24 h (Fig. 1B). LPS-induced IDO1 protein expression also increased strongly at 24 h and further at 48 h (Fig. 1B). Incubation of HSCs with l-tryptophan showed modest IDO1 enzymatic activity; LPS induced a significant and robust increase in IDO1 activity at 24 and 48 h, respectively (Fig. 1C). These results indicate that LPS enhances the potential immunoregulatory function of HSCs by increasing IDO1 activity.
IDO1-mediated AhR signaling regulates nTreg expansion
Next, we assessed whether HSC-expressed IDO1 is involved in nTreg expansion and function using cells purified as shown in Fig. 2A. As reported previously [23], allogeneic nTreg expansion increased strongly upon coculture with LPS-prestimulated HSCs (LPS/HSC; Fig. 2B and C). Non-Tregs, such as conventional CD4 T cells, do not respond the same way as Tregs to unstimulated or LPS-pretreated HSCs; in fact, HSCs suppress activation of conventional T cells and induce their apoptosis [23]. LPS/HSC-induced nTreg proliferation was strongly attenuated upon pharmacological inhibition of IDO1 by 1MT (Fig. 2B) and when HSCs from IDO1−/− mice were used (Fig. 2C). These results suggest that in addition to the cell contact [23], HSC-expressed IDO1-mediated metabolism of l-tryptophan also plays an essential role in nTreg expansion.
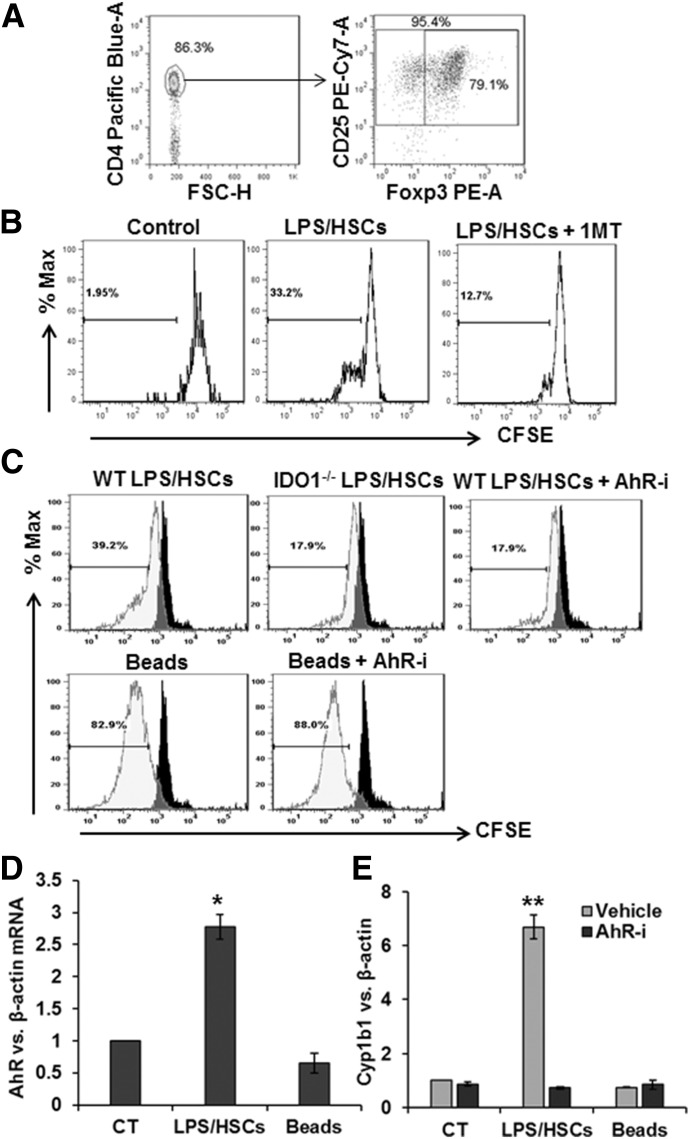
Figure 2. LPS/HSC-induced Treg expansion is mediated by HSC-expressed IDO1 and AhR signaling in nTreg.
(A) Purity of MACS-sorted Tregs, as determined by flow cytometry. Isolated cells were stained for surface molecules with anti-CD4 and anti-CD25 antibodies, and intracellular staining was performed for Foxp3. More than 95% of the cells were CD4+CD25+ T cells, and ∼80% of the total CD4+CD25+ T cells expressed Foxp3. FSC, Forward-scatter; A, area; H, height. (B) LPS-prestimulated B6 HSCs were incubated with CFSE-labeled BALB/c splenic Tregs at a 1:10 ratio, without or with 200 µM 1MT. After 5 d, Treg proliferation was determined via CFSE dilution assay. (C) HSCs from B6 WT or IDO−/− mice were cultured in serum-free medium containing 0 or 100 ng/ml LPS for 24 h. Cells were then washed and incubated with purified allogeneic (BALB/c) splenic Tregs at a 1:10 ratio, without or with 3 µM AhR inhibitor (AhR-i) CH-223191. For comparison, Tregs were cultured in medium containing anti-CD3/CD28 beads. After 5 d culture, Tregs were aspirated, washed, and assessed for their expansion by flow using CFSE dilution assay. (D) AhR and (E) Cyp1b1 mRNA expression in nTregs was determined by qRT-PCR assay. *P < 0.05; **P < 0.005 vs. control.
Although IDO1 is known to regulate Treg expansion [26], molecular mechanisms underlying this effect are not completely understood. As l-kynurenine is a natural ligand for AhR [27], and activation of AhR regulates differentiation and proliferation of several cell types [28], we investigated whether l-kynurenine-dependent AhR activation might be a mechanism of HSC-induced nTreg expansion. Indeed, an AhR-specific inhibitor (CH-223191) strongly inhibited LPS/HSC-induced but not anti-CD3/CD28-induced proliferation of nTregs (Fig. 2C).
We then asked whether LPS/HSCs modulate AhR expression in nTregs, which aspirated from coculture with HSCs that are highly pure, as HSCs remain firmly attached to the culture plate. A robust increase in AhR expression was observed in nTregs cocultured with LPS/HSCs (Fig. 2D). In contrast, anti-CD3/CD28 beads did not stimulate AhR expression, demonstrating AhR independence of anti-CD3/CD28-induced nTreg expansion (Fig. 2C and D). Furthermore, LPS/HSC but not anti-CD3/CD28 increased the expression of the AhR target gene Cyp1b1 in nTregs, an effect that was blocked by CH-223191 (Fig. 2E). Together, these data suggest that IDO1-mediated AhR activation in nTreg is required for their expansion by LPS/HSC.
As the magnitude of Foxp3 expression is a determinant of Treg function [1–5, 11–13], whether LPS/HSCs also alter the Foxp3 expression in cocultured nTregs was determined. As shown in Fig. 3, LPS/HSC increased Foxp3 mRNA, as well as protein expression in nTregs, and intracellular staining demonstrated expression of Foxp3 in the proliferating cells (Fig. 3C). The up-regulation of LPS/HSC-induced Foxp3 expression was markedly attenuated by 1MT, indicating that both expansion of nTregs and increase in their Foxp3 expression are dependent on HSC-expressed IDO1.
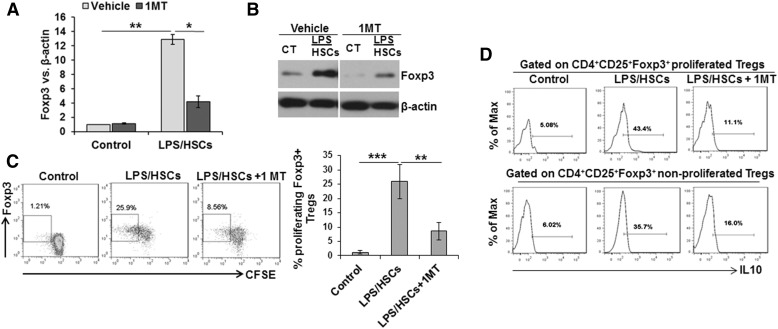
Figure 3. Inhibition of LPS/HSC-induced Foxp3 expression in nTreg by 1MT.
B6 mouse HSCs were incubated in serum-free medium containing 0 or 100 ng/ml LPS for 24 h. Cells were then washed and incubated with bulk BALB/c splenic CD4+ T cells at a 1:10 ratio, without or with 200 µM 1MT. After 5 d of coculture, T cells were aspirated and nTregs purified. Foxp3 mRNA (A) and protein (B) expression was determined via qRT-PCR and Western blot procedures, respectively. (C) Representative dot plot shows CFSE-based proliferation of Foxp3+ Tregs gated on CD4+CD25+ T cells and its inhibition by 1MT. Bar graph shows percent proliferating Foxp3+ Tregs upon coculture with LPS/HSCs compared with control. *P < 0.05; **P < 0.005; ***P < 0.0005. (D) Histogram shows intracellular IL-10 in Foxp3+ Tregs at the end of 5 d coculture with LPS/HSCs in expanded and nonexpanded populations.
To examine whether LPS/HSC-expanded Tregs are superior compared with nonexpanded cells, we determined intracellular IL-10 in the 2 populations. Although IL-10 expression was greater in expanded than in nonexpanded cells, the increase was robust in both populations compared with control Tregs and was reduced in the presence of 1MT (Fig. 3D). These results indicate that Tregs that have not yet proliferated were also modulated by LPS/HSCs and might be similarly superior immunosuppressors as the expanded Tregs.
LPS/HSCs induce phosphorylation of Rb protein in nTregs via AhR
Phosphorylation of Rb protein has been shown to enhance cell-cycle progression from the G1 to S phase [29], whereas interaction between p300/CBP and Rb protein have been implicated in AhR-mediated cell-cycle regulation [30–32]. Furthermore, p300 is potentially critical for the survival and suppressive function of Tregs in vitro and in vivo [33]. We found that LPS/HSCs increased phosphorylation of Rb protein in nTregs, and this effect was markedly reduced in the presence of the AhR inhibitor (Fig. 4A). To elucidate whether the interaction between p300 and AhR regulated nTreg expansion, IP assay was performed. However, interaction between AhR and p300 was identical in control nTregs and nTregs cocultured with LPS/HSCs (in the absence and presence of AhR inhibitor; Fig. 4B), ruling out a role for this interaction in LPS/HSC-induced nTreg expansion.
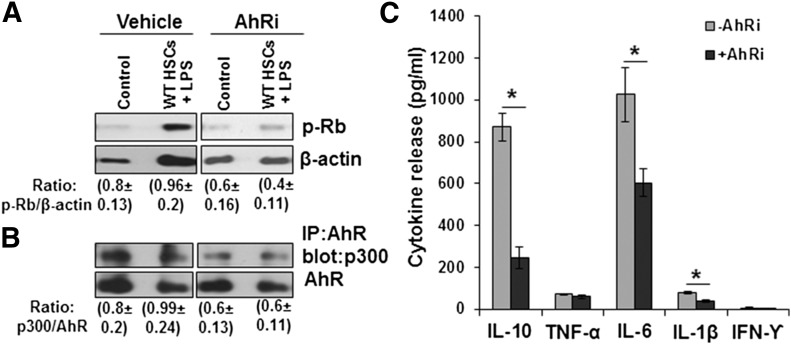
Figure 4. LPS/HSCs induce phosphorylation of Rb protein in nTregs in an AhR-dependent manner.
B6 mouse HSCs were cultured in serum-free medium containing 0 or 100 ng/ml LPS for 24 h. HSCs were then washed and incubated with BALB/c splenic nTregs at a 1:10 ratio, without or with 3 µM AhR inhibitor CH-223191. After 5 d culture, nTregs were aspirated, washed, and assessed for their expression of (A) phosphorylated Rb (p-Rb) protein in the cell lysates or (B) for p300 expression following IP with anti-AhR antibody. (C) The supernatants from the LPS/HSC–nTreg ± CH-223191 cocultures were assessed for indicated cytokines by ELISA. *P < 0.05 vs. control (−AhRi).
The role of AhR in cytokine expression by LPS/HSC–nTreg coculture
Recent work from Gandhi and coworkers [20, 21, 34] has established that HSCs play an important role in hepatic inflammation and immunoregulation by producing numerous cytokines and chemokines. We have also reported bidirectional interaction between nTregs and HSCs in releasing several cytokines; LPS-induced TNF-α, IL-1β, and IL-6 synthesis by HSCs is inhibited, whereas that of IL-10 is increased upon coculture with Tregs [23]. Here, we found that the already lower release of TNF-α, IL-1β, and IL-6 by LPS/HSC–nTreg cocultures compared with LPS/HSC alone [23], as well as that of IL-10, was attenuated by the AhR inhibitor (Fig. 4C). Concentrations of IFN-γ, IL-4, and IL-17, the cytokines associated with Tregs converted into pathogenic cells [18], were below the lower limit of detection, indicating that nTregs retain their immunosuppressive phenotype.
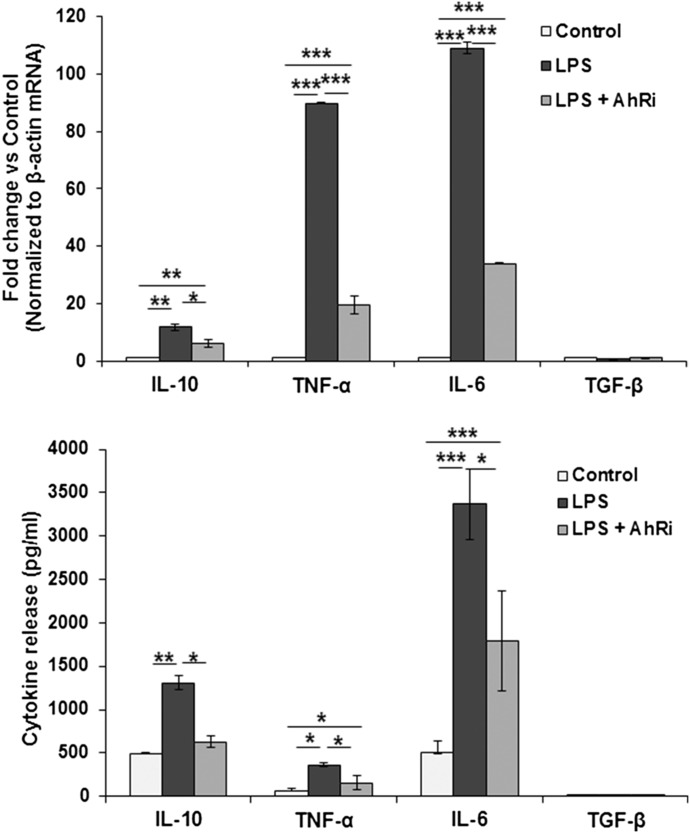
We also determined whether LPS-induced cytokine synthesis is influenced by AhR signaling in a separate experiment. As shown in Fig. 5, AhR inhibition reduced the mRNA expression and protein release of IL-10, TNF-α, and IL-6; HSCs express TGF-β at a very low level, and its expression or release was not affected by LPS stimulation.
Figure 5. HSCs (0.5 × 106/cm2) in 6-well plates were incubated in serum-free condition with 100 ng/ml LPS ± 3 µM AhR inhibitor CH-223191 (added 1 h before LPS).
After 24 h (A), cellular mRNA expression of cytokines was determined, and (B) cytokines in culture supernatant were measured by ELISA. *P < 0.05; **P < 0.005; ***P < 0.0005.
HSC/ IDO1 promotes acetylation of Foxp3 in nTregs
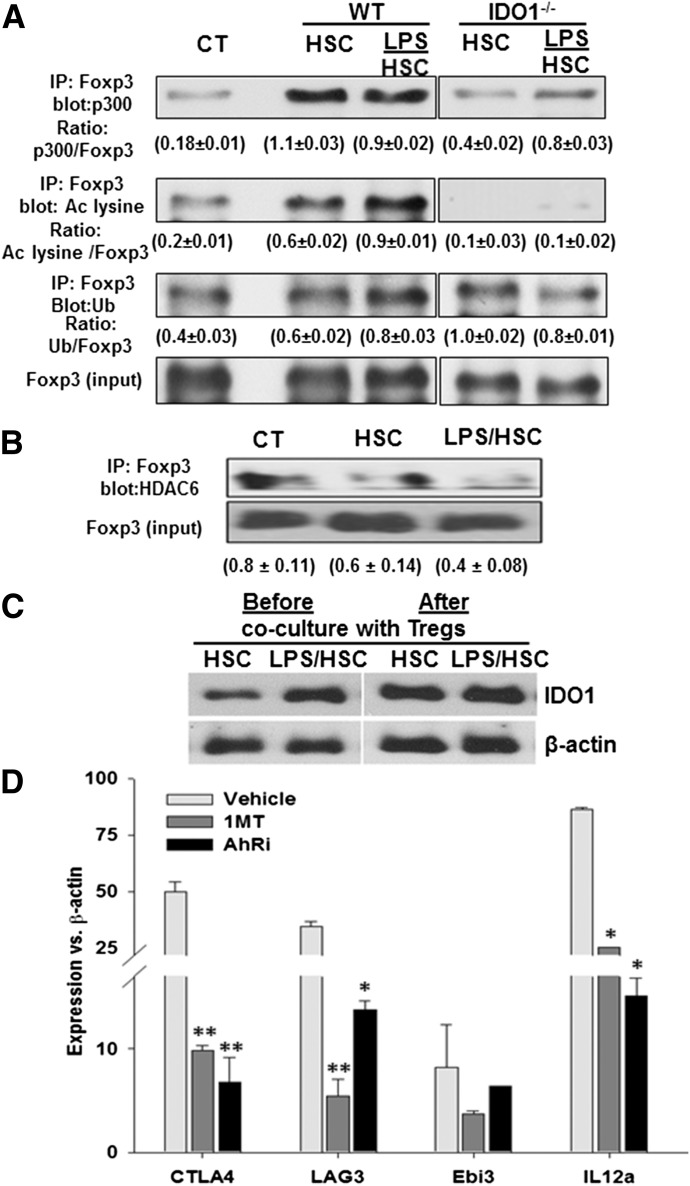
High and stable expression of Foxp3 is a critical requirement for the sustained, suppressive function of Tregs, and loss of Foxp3 leads to their conversion to pathogenic cells [18]. Hyperacetylation of Foxp3 protein by interaction with lysine acetyltransferases prevents its polyubiquitination and proteosomal degradation and maintains a higher level of expression [35, 36]. As HSC-expanded nTregs are stable in vivo and in vitro [23], we examined whether acetylation of lysine residues of the Foxp3 protein is a mechanism underlying their stability. An IP assay showed that unstimulated and LPS-prestimulated WT HSCs strongly increased interaction of protein acetyltransferase p300 with Foxp3, leading to the acetylation of lysine residues of Foxp3 in nTregs (Fig. 6A). Although HSCs also appeared to increase Foxp3 ubiquitination, the magnitude of Foxp3 acetylation was much greater (0.3 ± 0.1 and 0.45 ± 0.12 for HSCs vs. control and LPS/HSCs vs. control, respectively) compared with ubiquitination (0.15 ± 0.09 and 0.2 ± 0.11, respectively, for HSCs vs. control and LPS/HSCs vs. control). These data indicate significantly superior stabilization of Foxp3 in HSC- or LPS/HSC-expanded Tregs. This effect was IDO1 dependent, as HSCs derived from IDO−/− mice were inferior in stimulating interaction between p300 and Foxp3 protein and unable to acetylate the Foxp3 protein.
Figure 6. HSCs stabilize Foxp3 via acetylation (Ac) and increase expression of immunosuppressive molecules in nTregs in an IDO-dependent manner.
(A) B6 WT or IDO−/− mouse HSCs were cultured in serum-free medium containing 0 or 100 ng/ml LPS for 24 h. Cells were then washed and incubated with purified BALB/c splenic nTregs at a 1:10 ratio. After 5 d, nTregs were aspirated for determination of the indicated molecules by Western blot analysis. Ub, Ubiquitination. (B) Association of Foxp3 with HDAC6 in Tregs. (C) Western blots show IDO expression in control or LPS-stimulated HSCs before and after 5 d coculture with nTregs. (D) Following incubation with LPS, HSCs were washed and cocultured with nTregs in the absence or presence of 200 µM 1MT for 5 d. nTregs were then aspirated and their expression of the indicated molecules determined via qRT-PCR. The mRNA expression was normalized to that of β-actin. Fold change over control is shown. *P < 0.05; **P < 0.005 vs. vehicle.
HDACs, including HDAC6-mediated control Foxp3+ Treg generation and function, have been demonstrated. The targeting of HDAC6 has been shown to enhance Treg expansion and its suppressive function by favoring Foxp3 acetylation and stability through increased acetylation [37]. We found markedly reduced interaction of HDAC6 with Foxp3 in Tregs cocultured with LPS/HSCs (Fig. 6B), confirming the stability of Tregs.
With the consideration of similar IDO1-dependent Foxp3 acetylation in nTregs by unstimulated and LPS-prestimulated HSCs, we measured HSC expression of IDO1 at the end of coculture (i.e., d 5). Interestingly, unlike after 24 h stimulation with LPS, when IDO1 expression in HSCs was greater than in unstimulated HSCs, unstimulated and LPS-prestimulated HSCs expressed similarly increased levels of IDO1 following coculture with nTregs (Fig. 6C). These data and our earlier observation—showing down-regulation of the secretion of inflammatory cytokines by HSCs in the presence of nTregs [21]—support the concept of reverse signaling by Tregs [38].
As HSC-expanded nTregs display superior, suppressive potential than control or anti-CD3/CD28-expanded nTregs [23], we examined the expression of immunosuppressive molecules by nTregs and its dependence on HSC-expressed IDO1. Levels of CTLA4, LAG3, and Ebi3 and IL-12a (that transcribe the IL-27β and IL-12α chains, respectively, of the immunosuppressive cytokine IL-35) all increased in nTregs cocultured with LPS/HSCs. These effects were IDO1 and AhR dependent, as demonstrated by their inhibition with 1MT and CH-223191 (Fig. 6D).
Cryopreserved HSCs induce similar nTreg expansion and impart suppressive potential as quiescent HSCs
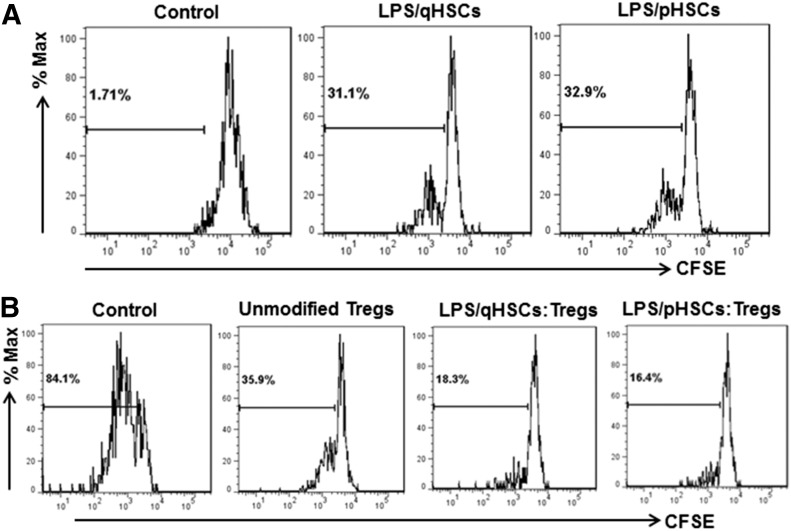
The above results are derived from experiments using primary culture of HSCs. Although ex vivo, HSC-expanded nTregs are potentially important for therapeutic use, a major limitation is the availability of human livers to obtain HSCs. However, HSCs can be cryopreserved and are ready to use when required. Therefore, we compared primary-cultured and cryopreserved (frozen after passage 2) mouse HSCs for their ability to expand nTregs and the suppressive potential of the expanded nTregs. As shown in Fig. 7A, LPS-pretreated, freshly isolated, as well as cryopreserved, HSCs increased the proliferation of nTregs similarly. When their suppressive potential was assessed, nTregs, expanded by both HSC phenotypes, were found to suppress the proliferation of effector CD4+ T cells similarly (Fig. 7B).
Figure 7. Expansion of nTregs and their immunosuppressive activity following coculture with primary-cultured and highly activated HSCs.
(A) B6 mouse HSCs (qHSC; d 2 of culture) or passaged HSCs (pHSC; subcultured twice and stored frozen) were pretreated with 100 ng/ml LPS for 24 h. Cells were then washed and cocultured with BALB/c nTregs at a 1:10 ratio for 5 d. Proliferation of nTregs was determined by CFSE dilution assay. (B) CD4+ T cells were incubated with control nTregs or nTregs expanded by quiescent or passaged HSCs (CD4:nTreg ratio of 1:10). CD4+ T cell proliferation was measured by CFSE dilution assay.
DISCUSSION
We reported previously that the Helios+ nTregs, expanded in coculture with control or LPS-prestimulated, allogeneic HSCs, are superior immune suppressors and more stable in vivo than anti-CD3/CD28-expanded nTregs [23]. As LPS levels are elevated following liver transplantation (until graft function is restored) [22], it is plausible that an important mechanism of liver allograft tolerance might involve LPS/HSC-induced nTreg modulation. Whereas MHC II that is up-regulated by LPS is required for HSC-induced nTreg expansion [23], the present study demonstrates that LPS-mediated, increased activity of IDO1 in HSCs also plays an important role in nTreg expansion, stability, and potency.
IDO1, an immunoregulatory enzyme that catabolizes l-tryptophan into l-kynurenine, is expressed by or induced in APCs, including DCs [26]. As reduced availability of l-tryptophan suppresses survival/expansion of conventional T cells but not Tregs [39, 40], HSCs may play an important role in immunologic tolerance. DCs and other APCs have been shown to induce the expression of Foxp3 in naïve CD4+CD25− T cells via IDO1 activity [26]. In addition, APCs expressing IDO1, in conjunction with TCR stimuli, have been shown to activate Foxp3+ Tregs directly and promote their immunosuppressive function [26]. We found that increased IDO1 in LPS-stimulated HSCs was associated not only with expansion but also with a strong increase in Foxp3 expression in cocultured, alloreactive nTregs. Furthermore, 1MT inhibited LPS/HSC-induced nTreg expansion and also reduced Foxp3 expression in cocultured nTregs. These findings emphasize that HSC-expressed IDO1 may be very critical to the liver’s immunologic tolerance.
It is apparent that the mechanisms of HSC-expressed, IDO1-dependent expansion of nTregs may involve a role of l-kynurenine, a natural ligand for an intracellular signaling molecule AhR [27]. The ligand–AhR complex binds to specific response elements close to the promoters of target genes following translocation to the nucleus; CH-223191 has been shown to block 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated nuclear translocation and DNA binding of AhR [41]. Of the genes under regulation of AhR signaling, Cyp1a1 and Cyp1b1 have been well characterized [42]. Thus, increased expression of AhR in nTregs cocultured with LPS/HSC and inhibition of LPS/HSC-induced expansion, as well as increased expression of Cyp1b1 in nTregs by CH-223191, suggest that HSC-induced AhR signaling is required for nTreg expansion and function.
Our previous work demonstrated a strong reciprocal interaction between LPS/HSCs and nTregs in producing inflammatory cytokines; HSCs produced significantly greater amounts of IL-1β, IL-6, TNF-α, and IL-10 upon stimulation with LPS, and nTregs inhibited production of all but IL-10, which increased further in coculture [23]. As IL-6 has been shown to protect suppression of conventional CD4+ T cells by Tregs [43], inhibition of LPS-induced IL-6 production in HSCs by Tregs may be a component of the mechanism of tolerance. Interestingly, CH-223191 strongly attenuated the secretion of IL-10, IL-1β, and IL-6 and to a lesser extent, that of TNF-α released in the LPS/HSC–Treg coculture. Whereas the role of AhR in IL-10 expression has been shown, its involvement in IL-1β, IL-6, and TNF-α synthesis was unexpected. As HSCs express AhR [34], and LPS-induced cytokine synthesis in HSCs was found to be regulated by p38-MAPK and NF-κB activation [44], it is possible that a cross-talk between AhR and MAPK/NF-κB signaling may play an important role in cytokine production by HSCs. Indeed, in monoculture, CH-223191 inhibited LPS-induced mRNA expression and the release of IL-10, TNF-α, and IL-6 by HSCs (Fig. 5). These data are supported by the reports showing the interaction of AhR with NF-κB through the p65/RelA subunit in human breast cancer MCF-7 cells [45]. Furthermore, activation of protein kinase B/AKT, ERK1, MAPK, and Rac-1 is lower in AhR null cells, and its involvement in JNK–MAPK activation has already been postulated.
As a result of the adverse short- and long-term effects of pharmacologic immunosuppressive agents, adoptive Treg therapy is considered potentially important to treat chronic, immune-mediated inflammatory disorders, including transplant rejection. However, in vivo instability of infused Tregs, as well as their conversion to effector T cells under inflammatory conditions, is a potential limitation of this approach. Our data show that LPS/HSCs impart stability to cocultured nTregs by increasing Foxp3 expression and its acetylation in an IDO1-dependent manner. The HSC/IDO–nTreg/AhR axis was also found to be an integral part of the superior suppressive function of nTregs, as evidenced by the inhibition of nTreg-expressed CTLA4, LAG3, as well as Ebi3 and IL-12a by both 1MT and CH-223191. Furthermore, strongly increased IL-10 expression by LPS/HSC-expanded and nonexpanded Tregs indicates that the as-yet non expanded Tregs are also modulated to be superior in their immunosuppressive function. An interesting aspect of the bidirectional interactions between the 2 cell types is that nTregs increase IDO1 expression in HSCs during coculture. As HSCs were found to interact closely with nTregs following cold-ischemic storage and reperfusion [23] and the presence of >50% of Foxp3+ Tregs in close proximity to HSCs following liver allograft transplantation in mice [21], it can be postulated that LPS- and/or nTreg-induced increases in IDO1 in HSCs may be important mechanisms underlying the liver’s immunologic tolerance.
Purified HSCs were shown to cause Foxp3 induction in naïve CD4+ T cells in the presence of exogenous TGF-β and required HSC-released all-trans retinoic acid [46, 47]. HSCs, as well as nTregs used in the present investigation, were highly pure, and Tregs expanded by HSCs are exclusively nTregs and not iTregs, as assessed by their Helios expression [23]. Furthermore, expression of TGF-β in HSCs is very low, and it is not stimulated by LPS [34, 48] (Fig. 5). Together, these data exemplify the importance of HSCs in the liver’s immunologic tolerance by inducing Treg formation [46, 47], as well as by expanding nTregs with superior suppressor function (ref.[ 23] and the present study). The ability of HSCs to suppress CD4+CD25− effector T cells and also cause their apoptosis [21, 49] separates them from KCs, endothelial cells, and hepatocytes that induce their proliferation [50]. Another point to note is that KCs lose their ability to expand Tregs upon stimulation with LPS, and their suppressor function is also reduced, which may be a result of IL-6 released by LPS-stimulated KCs [43, 50]. In contrast, nTregs inhibit IL-6 release by LPS/HSCs, and LPS/HSCs cause increased expansion of nTregs compared with unstimulated HSCs [23].
HSCs express MHC II at a very low level, which is up-regulated upon stimulation with LPS [23] or IFN-γ [46, 49, 51]. LPS/HSC-induced expansion of allogeneic nTregs was found to be dependent on MHC II, and soluble mediators alone from control or LPS-stimulated HSCs failed to expand nTregs [23]. However, the results of the present study indicate that both stimuli act in concert to cause nTreg expansion. Whereas the HSC/IDO1-induced AhR signaling is critical, which signal(s) are generated by HSC-MHC II in nTregs to cause nTreg expansion remain to be determined. In this regard, MHC II–TCR ligation has been shown to induce several signaling pathways, including MAPKs (MEK1/2 and ERK1/2), Ca2+ mobilization, protein kinase C, tyrosine kinases, and NFATs [52]. Furthermore, evidence indicates that MHC II-mediated self-antigen presentation by DCs induces Treg proliferation (ref. [53] and references therein), and such a pathway could very well be involved in HSC-induced Treg expansion.
Finally, our data demonstrate that passaged and cryopreserved HSCs are not only similarly effective in expanding nTregs as HSCs in primary culture, but the nTregs expanded by both phenotypes elicit similarly superior T cell suppression. In these and our previously reported [23] experiments, HSCs and Tregs were cocultured at a 1:10 ratio. After 5 d of coculture of 0.1 × 106 HSCs with 1 × 106 Tregs, ∼8 × 106 Tregs were generated, as determined by cell counting. Clinically, e.g., in treatment of GvHD, 1 × 105 Tregs/kg body weight of ex vivo-expanded human Tregs are adoptively transferred [6]. From our experience, 1 g liver yields ∼2 × 106 HSCs. Therefore, a human liver (1.2–1.5 kg) can be estimated to yield 2.5 × 109 HSCs. As HSCs proliferate extensively upon coculture, an exponentially large number can be cryopreserved and made available to obtain an adequate number of Tregs for clinical use. It is important to note that IDO-expressing DCs also impart superior immunosuppressive potential to Tregs [26, 54]. Although a direct comparison between HSCs and DCs in providing Tregs with superior suppressive function is highly interesting and awaits future investigation, the limitation concerning the unavailability of the liver to isolate HSCs when required to obtain HSC-expanded Tregs can be overcome by using cryopreserved HSCs as an important source for Treg expansion for a therapeutic purpose.
AUTHORSHIP
C.R.G. developed the concept, designed the experiments, interpreted the results, and wrote the manuscript. S.K. designed and performed the experiments, interpreted the results, and helped write the manuscript. J.W. and A.W.T. provided expertise in evaluating and interpreting the results and writing the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. VA Merit Review Award 1IO1BX001174-01 and U.S. National Institutes of Health National Institute of Allergy and Infectious Diseases Grant PO1AIO81678. The authors thank Dr. Senad Divanovic for providing IDO−/− mice for preliminary experiments and Dr. Anil Dangi for valuable technical contribution.
Glossary
- 1MT
1-methyltryptophan
- AhR
aryl hydrocarbon receptor
- B6
C57BL/6 (H-2b)
- CBP
cAMP response element-binding protein
- Cyp1a1/Cyp1b1
cytochrome P450 family 1, subfamily A/B, member 1
- DC
dendritic cell
- Ebi
EBV-induced gene
- Foxp3
forkhead box p3
- GvHD
graft-versus-host disease
- HDAC
histone deacetylase
- HSC
hepatic stellate cell
- IP
immunoprecipitation
- iTreg
inducible regulatory T cell
- KC
Kupffer cell
- LAG
lymphocyte activation gene
- MHC II
MHC class II
- nTreg
naturally occurring regulatory T cell
- qRT-PCR
quantitative RT-PCR
- Rb
retinoblastoma
- RIPA
radioimmunoprecipitation assay
- Treg
regulatory T cell
- WT
wild-type
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Wood K. J., Sakaguchi S. (2003) Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 3, 199–210. [DOI] [PubMed] [Google Scholar]
- 2.Mottet C., Golshayan D. (2007) CD4+CD25+Foxp3+ regulatory T cells: from basic research to potential therapeutic use. Swiss Med. Wkly. 137, 625–634. [DOI] [PubMed] [Google Scholar]
- 3.Prinz I., Koenecke C. (2012) Therapeutic potential of induced and natural FoxP3(+) regulatory T cells for the treatment of graft-versus-host disease. Arch. Immunol. Ther. Exp. (Warsz.) 60, 183–190. [DOI] [PubMed] [Google Scholar]
- 4.Perdigoto A. L., Chatenoud L., Bluestone J. A., Herold K. C. (2016) Inducing and administering Tregs to treat human disease. Front. Immunol. 6, 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allan S. E., Broady R., Gregori S., Himmel M. E., Locke N., Roncarolo M. G., Bacchetta R., Levings M. K. (2008) CD4+ T-regulatory cells: toward therapy for human diseases. Immunol. Rev. 223, 391–421. [DOI] [PubMed] [Google Scholar]
- 6.Trzonkowski P., Bieniaszewska M., Juścińska J., Dobyszuk A., Krzystyniak A., Marek N., Myśliwska J., Hellmann A. (2009) First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin. Immunol. 133, 22–26. [DOI] [PubMed] [Google Scholar]
- 7.Brunstein C. G., Miller J. S., Cao Q., McKenna D. H., Hippen K. L., Curtsinger J., Defor T., Levine B. L., June C. H., Rubinstein P., McGlave P. B., Blazar B. R., Wagner J. E. (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117, 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Ianni M., Falzetti F., Carotti A., Terenzi A., Castellino F., Bonifacio E., Del Papa B., Zei T., Ostini R. I., Cecchini D., Aloisi T., Perruccio K., Ruggeri L., Balucani C., Pierini A., Sportoletti P., Aristei C., Falini B., Reisner Y., Velardi A., Aversa F., Martelli M. F. (2011) Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928. [DOI] [PubMed] [Google Scholar]
- 9.Bluestone J. A., Buckner J. H., Fitch M., Gitelman S. E., Gupta S., Hellerstein M. K., Herold K. C., Lares A., Lee M. R., Li K., Liu W., Long S. A., Masiello L. M., Nguyen V., Putnam A. L., Rieck M., Sayre P. H., Tang Q. (2015) Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 7, 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S., Yamashita K., Goto R., Zaitsu M., Nagatsu A., Oura T., Watanabe M., Aoyagi T., Suzuki T., Shimamura T., Kamiyama T., Sato N., Sugita J., Hatanaka K., Bashuda H., Habu S., Demetris A. J., Okumura K. (2016) A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 64, 632–643. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann P., Boeld T. J., Eder R., Huehn J., Floess S., Wieczorek G., Olek S., Dietmaier W., Andreesen R., Edinger M. (2009) Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 39, 1088–1097. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X., Bailey-Bucktrout S. L., Jeker L. T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J. A. (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Hennezel E., Piccirillo C. A. (2012) Functional plasticity in human FOXP3(+) regulatory T cells: implications for cell-based immunotherapy. Hum. Vaccin. Immunother. 8, 1001–1005. [DOI] [PubMed] [Google Scholar]
- 14.Hamann A. (2012) Regulatory T cells stay on course. Immunity 36, 161–163. [DOI] [PubMed] [Google Scholar]
- 15.Battaglia M., Stabilini A., Tresoldi E. (2012) Expanding human T regulatory cells with the mTOR-inhibitor rapamycin. Methods Mol. Biol. 821, 279–293. [DOI] [PubMed] [Google Scholar]
- 16.Hippen K. L., Merkel S. C., Schirm D. K., Nelson C., Tennis N. C., Riley J. L., June C. H., Miller J. S., Wagner J. E., Blazar B. R. (2011) Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am. J. Transplant. 11, 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgoffe G. M., Powell J. D. (2009) mTOR: taking cues from the immune microenvironment. Immunology 127, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J., Zhou X. (2015) Regulatory T cells turn pathogenic. Cell. Mol. Immunol. 12, 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa D., Wallace M. C., Friedman S. L. (2015) Stellate cells and hepatic fibrosis. In Stellate Cells in Health and Disease (Gandhi C. R., Pinzani M., eds.), New York, Elsevier, 41–62. [Google Scholar]
- 20.Gandhi C. R. (2010) Hepatic stellate cells. In Molecular Pathology of Liver Diseases (Monga S. P., ed.), New York, Springer, 53–80. [Google Scholar]
- 21.Gandhi C. R. (2015) Stellate cells in hepatic immunological tolerance. In Stellate Cells in Health and Disease (Gandhi C. R., Pinzani M., eds.), New York, Elsevier, 227–249. [Google Scholar]
- 22.Yokoyama I., Gavaler J. S., Todo S., Miyata T., Van Thiel D. H., Starzl T. E. (1995) Endotoxemia is associated with renal dysfunction in liver transplantation recipients during the first postoperative week. Hepatogastroenterology 42, 205–208. [PMC free article] [PubMed] [Google Scholar]
- 23.Dangi A., Sumpter T. L., Kimura S., Stolz D. B., Murase N., Raimondi G., Vodovotz Y., Huang C., Thomson A. W., Gandhi C. R. (2012) Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J. Immunol. 188, 3667–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumpter T. L., Dangi A., Matta B. M., Huang C., Stolz D. B., Vodovotz Y., Thomson A. W., Gandhi C. R. (2012) Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J. Immunol. 189, 3848–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Däubener W., Wanagat N., Pilz K., Seghrouchni S., Fischer H. G., Hadding U. (1994) A new, simple, bioassay for human IFN-gamma. J. Immunol. Methods 168, 39–47. [DOI] [PubMed] [Google Scholar]
- 26.Munn D. H., Mellor A. L. (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 34, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen L. P., Bradfield C. A. (2008) The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 21, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barouki R., Coumoul X., Fernandez-Salguero P. M. (2007) The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 581, 3608–3615. [DOI] [PubMed] [Google Scholar]
- 29.Harbour J. W., Dean D. C. (2000) Rb function in cell-cycle regulation and apoptosis. Nat. Cell Biol. 2, E65–E67. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi A., Numayama-Tsuruta K., Sogawa K., Fujii-Kuriyama Y. (1997) CBP/p300 functions as a possible transcriptional coactivator of Ah receptor nuclear translocator (Arnt). J. Biochem. 122, 703–710. [DOI] [PubMed] [Google Scholar]
- 31.Ge N. L., Elferink C. J. (1998) A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J. Biol. Chem. 273, 22708–22713. [DOI] [PubMed] [Google Scholar]
- 32.Tohkin M., Fukuhara M., Elizondo G., Tomita S., Gonzalez F. J. (2000) Aryl hydrocarbon receptor is required for p300-mediated induction of DNA synthesis by adenovirus E1A. Mol. Pharmacol. 58, 845–851. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Wang L., Han R., Beier U. H., Akimova T., Bhatti T., Xiao H., Cole P. A., Brindle P. K., Hancock W. W. (2014) Two histone/protein acetyltransferases, CBP and p300, are indispensable for Foxp3+ T-regulatory cell development and function. Mol. Cell. Biol. 34, 3993–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey S. A. K., Dangi A., Tandon A., Gandhi C. R. (2013) The transcriptomic response of rat hepatic stellate cells to endotoxin: implications for hepatic inflammation and immune regulation. PLoS One 8, e82159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Loosdregt J., Vercoulen Y., Guichelaar T., Gent Y. Y., Beekman J. M., van Beekum O., Brenkman A. B., Hijnen D. J., Mutis T., Kalkhoven E., Prakken B. J., Coffer P. J. (2010) Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 115, 965–974. [DOI] [PubMed] [Google Scholar]
- 36.Kwon H. S., Lim H. W., Wu J., Schnölzer M., Verdin E., Ott M. (2012) Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J. Immunol. 188, 2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Zoeten E. F., Wang L., Butler K., Beier U. H., Akimova T., Sai H., Bradner J. E., Mazitschek R., Kozikowski A. P., Matthias P., Hancock W. W. (2011) Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol. Cell. Biol. 31, 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puccetti P., Grohmann U. (2007) IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat. Rev. Immunol. 7, 817–823. [DOI] [PubMed] [Google Scholar]
- 39.Wang C., Tay S. S., Tran G. T., Hodgkinson S. J., Allen R. D., Hall B. M., McCaughan G. W., Sharland A. F., Bishop G. A. (2010) Donor IL-4-treatment induces alternatively activated liver macrophages and IDO-expressing NK cells and promotes rat liver allograft acceptance. Transpl. Immunol. 22, 172–178. [DOI] [PubMed] [Google Scholar]
- 40.Ingelsten M., Gustafsson K., Oltean M., Karlsson-Parra A., Olausson M., Haraldsson B., Nyström J. (2009) Is indoleamine 2,3-dioxygenase important for graft acceptance in highly sensitized patients after combined auxiliary liver-kidney transplantation? Transplantation 88, 911–919. [DOI] [PubMed] [Google Scholar]
- 41.Kim S. H., Henry E. C., Kim D. K., Kim Y. H., Shin K. J., Han M. S., Lee T. G., Kang J. K., Gasiewicz T. A., Ryu S. H., Suh P. G. (2006) Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol. Pharmacol. 69, 1871–1878. [DOI] [PubMed] [Google Scholar]
- 42.Korn T. (2010) How T cells take developmental decisions by using the aryl hydrocarbon receptor to sense the environment. Proc. Natl. Acad. Sci. USA 107, 20597–20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasare C., Medzhitov R. (2003) Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299, 1033–1036. [DOI] [PubMed] [Google Scholar]
- 44.Thirunavukkarasu C., Watkins S., Gandhi C. R. (2006) Mechanisms of endotoxin-induced NO, IL-6, and TNF-alpha production in activated rat hepatic stellate cells: role of p38 MAPK. Hepatology 44, 389–398. [DOI] [PubMed] [Google Scholar]
- 45.Kim D. W., Gazourian L., Quadri S. A., Romieu-Mourez R., Sherr D. H., Sonenshein G. E. (2000) The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 19, 5498–5506. [DOI] [PubMed] [Google Scholar]
- 46.Ichikawa S., Mucida D., Tyznik A. J., Kronenberg M., Cheroutre H. (2011) Hepatic stellate cells function as regulatory bystanders. J. Immunol. 186, 5549–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunham R. M., Thapa M., Velazquez V. M., Elrod E. J., Denning T. L., Pulendran B., Grakoui A. (2013) Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J. Immunol. 190, 2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thirunavukkarasu C., Uemura T., Wang L. F., Watkins S. C., Gandhi C. R. (2005) Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J. Cell. Physiol. 204, 654–665. [DOI] [PubMed] [Google Scholar]
- 49.Yu M. C., Chen C. H., Liang X., Wang L., Gandhi C. R., Fung J. J., Lu L., Qian S. (2004) Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 40, 1312–1321. [DOI] [PubMed] [Google Scholar]
- 50.Wiegard C., Frenzel C., Herkel J., Kallen K. J., Schmitt E., Lohse A. W. (2005) Murine liver antigen presenting cells control suppressor activity of CD4+CD25+ regulatory T cells. Hepatology 42, 193–199. [DOI] [PubMed] [Google Scholar]
- 51.Jiang G., Yang H. R., Wang L., Wildey G. M., Fung J., Qian S., Lu L. (2008) Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation 86, 1492–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarbell K. V., Yamazaki S., Steinman R. M. (2006) The interactions of dendritic cells with antigen-specific, regulatory T cells that suppress autoimmunity. Semin. Immunol. 18, 93–102. [DOI] [PubMed] [Google Scholar]
- 53.Zou T., Caton A. J., Koretzky G. A., Kambayashi T. (2010) Dendritic cells induce regulatory T cell proliferation through antigen-dependent and -independent interactions. J. Immunol. 185, 2790–2799. [DOI] [PubMed] [Google Scholar]
- 54.Sharma M. D., Baban B., Chandler P., Hou D. Y., Singh N., Yagita H., Azuma M., Blazar B. R., Mellor A. L., Munn D. H. (2007) Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Invest. 117, 2570–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]