Abstract
The adult adrenal cortex is organized into concentric zones, each specialized to produce distinct steroid hormones. Cellular composition of the cortex is highly dynamic and subject to diverse signaling controls. Cortical homeostasis and regeneration rely on centripetal migration of steroidogenic cells from the outer to the inner cortex, which is accompanied by direct conversion of zona glomerulosa (zG) into zona fasciculata (zF) cells. Given the important impact of tissue structure and growth on steroidogenic function, it is essential to understand the mechanisms governing adrenal zonation and homeostasis. Towards this end, we review the distinctions between each zone by highlighting their morphological and ultra-structural features, discuss key signaling pathways influencing zonal identity, and evaluate current evidence for long-term self-renewing stem cells in the adult cortex. Finally, we review data supporting zG-to-zF transdifferentiation/direct conversion as a major mechanism of adult cortical renewal.
1. Introduction
The adult adrenal cortex is a major site of steroid hormone production in mammals. It is composed of concentric zones of steroidogenic cells surrounding the chromaffin cells of the adrenal medulla (Gallo-Payet and Battista, 2014; Yates et al., 2013). Each zone of the cortex produces distinct steroid hormones that affect a variety of physiological functions. The outer layer, the zona glomerulosa (zG) makes up about 15% of the cortex and produces aldosterone, a mineralocorticoid whose major function is to regulate intravascular volume through sodium retention and thereby controls blood pressure. Aldosterone excess in pathophysiological conditions such as primary aldosteronism can cause irreversible cardiovascular damage and ultimately lead to multi-system dysfunction (Galati et al., 2013; Magill, 2014). The inner layer, the zona fasciculata (zF), roughly eight times the size of the zG, synthesizes glucocorticoids, which have diverse effects on immunity, metabolism, development and behavior. In humans, some non-human primates (e.g., rhesus macaques, marmosets), ferrets and the spiny mouse, a third layer, the zona reticularis (zR) lies between the zF and the medulla and produces androgens (Pihlajoki et al., 2015). While traditional laboratory mice lack a true zR, a temporary zone, designated the X-zone, has been identified and is believed to be a remnant of the fetal adrenal cortex (Morohashi and Zubair, 2011).
Embryonic development of the adrenal gland is relatively well understood (Xing et al., 2015). At E9.0 in the mouse, a group of cells in the coelomic epithelium become committed to the adrenogonadal lineage by expressing Steroidogenic factor 1 (Sf1). These cells then delaminate into the underlying mesenchyme and form the adrenogonadal primordium (AGP). At E10.5, a subset of AGP cells marked by Sf1-Fetal Adrenal Enhancer (FAdE) enhancer activity separates out to form the fetal adrenal anlagen. At around E12.5, neural crest cells migrate into the fetal adrenal and become precursors of the medulla. The fetal cortex starts to regress at E14.5 as the definitive cortex emerges beneath the newly formed capsule. Lineage tracing studies have shown that the definitive cortex arises from the fetal cortex and later on gives rise to the adult cortex (Wood et al., 2013; Zubair et al., 2008).
Proper control of steroidogenic function in the adult adrenal cortex relies not only on appropriate endocrine signaling but also on the integrity of tissue structure and homeostasis (Gallo-Payet and Battista, 2014). Disruption of zonation and homeostasis has been implicated in many adrenal diseases such as primary aldosteronism, cortisol-producing adenomas, primary pigmented nodular adrenocortical disease (PPNAD), congenital adrenal hyper- and hypoplasia and adrenocortical carcinoma (Walczak and Hammer, 2014). However, the cellular and molecular mechanisms that maintain normal tissue homeostasis in the adult cortex remain poorly understood. Hence, this review highlights our current knowledge of adult adrenocortical homeostasis and zonation, with an emphasis on 1) adrenal morphology and ultrastructure, 2) signaling pathways important for control of zonation, 3) evidence for adrenocortical stem cells and 4) transdifferentiation/direct conversion between differentiated cells.
2. Adrenal Zonation: Morphology and Ultrastructure
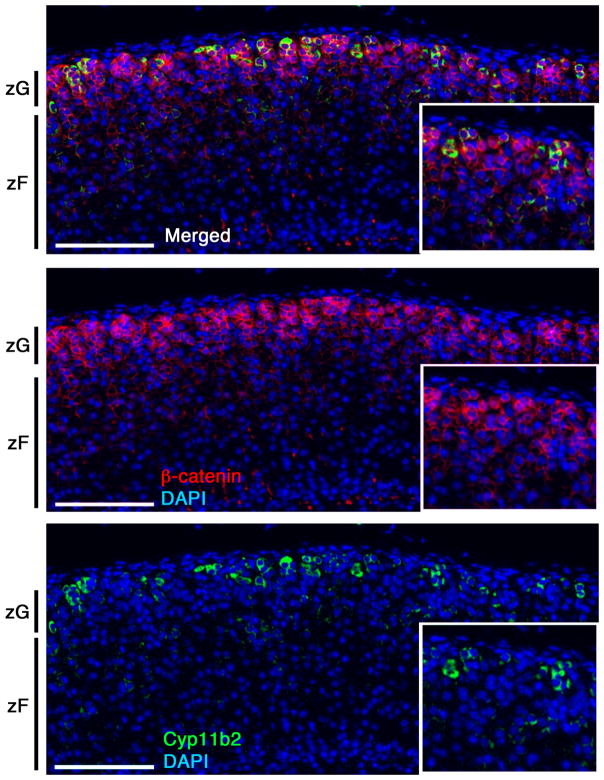
The adrenal cortex is an epithelial tissue enveloped in a mesenchymal capsule. As part of an epithelial structure, adrenocortical cells express epithelial markers such as laminin I and cytokeratins, markers of the basement membrane such as type IV collagen and a diverse array of laminin-associated integrin subunits (i.e., alpha 3, beta 1) (Campbell et al., 2003; Otis et al., 2007; Virtanen et al., 2003; Miettinen et al., 1985). However, in contrast to classical epithelial tissues (e.g., as found in the intestine) adrenocortical cells do not express the epithelial cell marker E-cadherin, but instead express N-cadherin, generally thought to be a neuronal marker (Tsuchiya et al., 2006). Morphologically, the cortical zones demonstrate clear differences in their cellular structure and organization. For instance, cells in the zG are arranged in discrete cellular clusters, referred to as glomeruli, which are surrounded by basement membrane proteins and a capillary network extending from the capsule (Otis et al., 2007). Cells in each glomerulus are densely packed, possess little cytoplasm and present with apposition of large membrane domains. Electron microscopic analysis reveals the presence of narrow gap junctions and a limited number of lipid droplets and mitochondria with lamelliform cristae. In addition, rough endoplasmic reticulum is more abundant than the smooth endoplasmic reticulum (Black et al., 1979; Friend and Gilula, 1972; Nussdorfer, 1980). Notably, the structure of the zG is highly conserved among many species (Nussdorfer, 1980). Along with a morphological identity, zG cells possess a particular molecular signature and can be identified by the presence of patches of Cyp11b2 (Aldosterone Synthase, AS)-expressing cells (Fig. 1) (Walczak et al., 2014) and by the expression of Disabled homolog 2 (Dab2; Romero et al., 2007), Protein delta homolog 1 (Dlk1; Halder et al., 1998) and β-catenin (Fig. 1 and 3), (as discussed below) (Eberhart and Argani, 2001; Walczak et al., 2014). Given the strong association between the region of β-catenin positivity and the morphologically identifiable zG, it is tempting to speculate that β-catenin may promote a transcriptional program that leads to the distinct zG morphology.
Figure 1. Cyp11b2 is expressed in a patchy fashion in the zG.
Immunofluorescent staining of paraffin sections from a wild-type adrenal cortex. The expression pattern of Cyp11b2 is restricted to the putative zG region, marked by β-catenin. However, not all β-catenin positive cells also express Cyp11b2, indicating that Wnt/β-catenin signaling is likely necessary but not sufficient for the synthesis of aldosterone. Insets are magnified details of the larger image. Scale bar: 100μm.
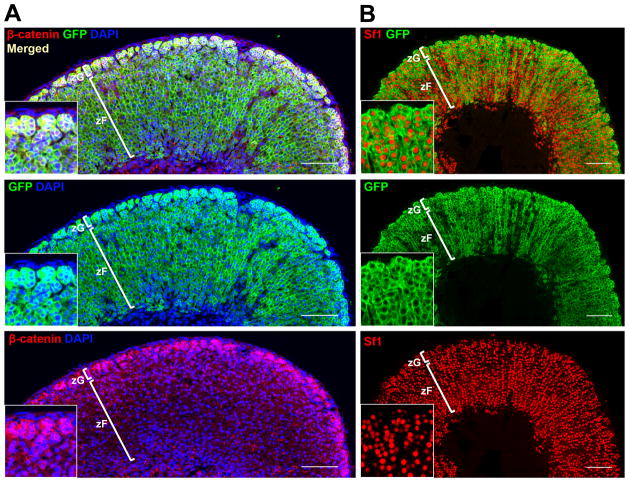
Figure 3. Cyp11b2+ lineage contributes to the entire zG and the majority of the zF by ~3 months of age.
Representative confocal images showing co-immunofluorescent staining of paraffin sections of adrenals from female AS-Cre/+; R26R-mTmG mice. The entire zG and the majority of the zF became labeled within 3 months. A, GFP expression completely overlaps with β-catenin staining in the zG. B, Sf1 staining reveals all steroidogenic cells in the zG and the majority of cells in the zF are marked by GFP. Insets are magnified details of the larger image. Scale bar: 100μm.
The cells in the zF, which produce glucocorticoids in response to the adrenocorticotropic hormone (ACTH), have strikingly different morphological and ultra-structural features. zF cells are organized in cord-like structures flanked by fenestrated blood vessels, which facilitate the rapid exchange of hormones between the circulation and steroidogenic cells. Cells in the zF are larger and less densely packed than those in the zG and possess a well developed smooth endoplasmic reticulum, large gap junctions, many lipid droplets, and mitochondria characterized by tubule-vesicular cristae (Black et al., 1979; Nussdorfer, 1980). zF cells are commonly identified by the expression of Cyp11b1 (11-beta hydroxylase, 11β-OH; Gomez-Sanchez et al., 2014) and Akr1b7 (aldo-keto reductase family 1, member B7) (Aigueperse et al., 1999).
The cells of the zR produce dehydroepiandrostenedione (DHEA) and the sulfated derivative DHEA-S during fetal life and upon adrenarche in humans, some non-human primates, ferrets and the spiny mouse (Pihlajoki et al., 2015). zR cells display similar characteristics to zF cells, though they tend to exhibit less lipid droplets, more lysosomes and lipofuscin pigment granules (Rhodin, 1971). The X-zone in mice represents a transient, fetal-derived region enriched in eosinophilic cells located between the zF and the cortical-medullary boundary (Morohashi and Zubair, 2011). The cells of the X-zone are smaller than zF cells, with varied degrees of cytoplasmic density and display diverse mitochondrial shapes endowed mainly with tubular cristae (Sato, 1968). The X-zone seems to be linked to catabolism of progesterone (Hershkovitz et al., 2007).
The existence of morphologically different zones in the adrenal cortex, without the presence of physical barriers between them, suggests the presence of molecular cues that tightly control the identity of each zone. In the following section we will review data that implicate several signaling pathways in the regulation of zonation.
3. Signaling Pathways and Zonation
Over the past 15 years, significant advances have led to an increased understanding of how Angiotensin II (AngII), potassium ions (K+) and ACTH, as well as their corresponding downstream signaling pathways, contribute to zonation. Important progress has also been made regarding the role of the canonical Wnt/β-catenin signaling pathway in maintaining proper zonation. In addition, several studies have shown that tight regulation of the ACTH/cyclic adenosine monophosphate (cAMP) and Wnt/β-catenin signaling pathways is required in order to preserve the morphological and functional boundaries between the neighboring zones of the cortex (Table 1). The importance of these pathways in controlling normal adrenocortical homeostasis and zonation is underscored by the consequences of somatic gain-of-function mutations, including those that give rise to aldosterone producing adenomas (APAs) (e.g., mutations in K+ channels and components of the Wnt/β-catenin signaling pathway) and PPNAD (e.g., mutations in PRKAR1A, the gene encoding the cAMP-dependent protein kinase type I-alpha regulatory subunit) (Berthon et al., 2015; Boulkroun et al., 2015). The following sections highlight the role these various factors and pathways play in regulation of zonation during homeostasis and disease.
Table 1. Disruption of cortical zonation.
Constitutive activation of Wnt/β-catenin and ACTH/cAMP signaling results in disruption of functional zonation and affects cell identity.
| Wnt/β-catenin signaling | ACTH/cAMP signaling | |||
|---|---|---|---|---|
|
|
||||
| Normal Function | • Mineralocorticoid Synthesis | Heikkilä, 2002 | • Glucocorticoid Synthesis | Pulichino, 2003 |
| • CYP11B2, AT1R, NURR1, NUR77 | Kim, 2008 | • Cyp11b1, Mc2r, Mrap | Karpac, 2005 | |
| • Orthotopic zG | Berthon, 2014 | • Orthotopic zF | Chida, 2007 | |
| Constitutive Activation | ↑ Mineralocorticoid Synthesis | Berthon, 2014 | ↑ Glucocorticoid Synthesis | |
| ↓ Cyp11b1, Akr1b7 | Berthon, 2010 | • Expression of Cyp17 | Sahut-Barnola, 2010 | |
| Ectopic zG cells in zF | Walczak, 2014 | • Zonation defects | ||
3.1. Regulation of zonation in the zG
The main stimuli responsible for modulating zG size are AngII and plasma K+ levels (Gallo-Payet and Battista, 2014). AngII is an octapeptide, derived from serial proteolytic cleavage of angiotensinogen and Angiotensin I, which acutely regulates blood pressure through direct effects on vascular tone. As reviewed elsewhere, AngII also indirectly regulates blood pressure through its acute and chronic effects on the adrenal cortex, which include stimulating the synthesis of aldosterone through the transcriptional regulation of Cyp11b2 and trophic effects on the zG (as reviewed in Bollag, 2014). Additional studies have shown that AngII, a low sodium diet or high potassium intake stimulates proliferation of zG cells and induces expansion of the zone (Deane et al., 1948; McEwan et al., 1999; Nishimoto et al., 2014; Shelton and Jones, 1971; McNeill, 2005). Conversely, zG size rapidly decreases upon sustained sodium loading (Shelton and Jones, 1971).
Most of AngII’s effects in the adrenal gland are mediated by the Angiotensin II receptor type 1 (AT1R), a G-protein coupled receptor enriched in the zG. AT1R is responsible for the transactivation of several downstream mediators, including phospholipase C via calcium/calmodulin-dependent protein kinase I and diacylglycerol/protein kinase C (PKC) (Breault et al., 1996; Wang et al., 1997, and reviewed in Mehta and Griendling, 2007). The use of specific AT1R blockers, such as losartan and candesartan, has confirmed a role for AT1R in aldosterone synthesis, zG cell proliferation and width (Davies et al., 2008; McEwan et al., 1999, 1996). The activity of AT1R is counter-balanced by the type-2 angiotensin receptor (AT2R), expressed throughout the cortex and enriched in the adrenal medulla, which promotes cell death and cell differentiation (Breault et al., 1998; Lu et al., 1995 and reviewed in Steckelings et al., 2010).
The canonical Wnt/β-catenin signaling pathway has also been proposed to regulate zG proliferation and to be a main determinant of zG identity (Berthon et al., 2010; Drelon et al., 2014). β-catenin is the essential transducer of canonical Wnt signaling and its nuclear and cytoplasmic accumulation is a marker of an active signaling cascade (Clevers and Nusse, 2012). The cytoplasmic amount of β-catenin is regulated by a destruction complex composed of the serine/threonine kinases casein kinase 1 (CK1) and glycogen synthase kinase 3 (GSK3) and the proteins adenomatous polyposis coli (APC), Axin and Wilms’ tumor gene on X chromosome (WTX). When Wnt ligands bind to the Frizzled and LRP5/6 receptors, Axin is recruited to the plasma membrane and relieves the destruction of β-catenin protein. β-catenin can then accumulate in the cytoplasm and translocate into the nucleus, where it acts as a transcriptional co-activator for members of the Tcf/Lef family of transcription factors.
Tcf/Lef transcription reporter activity as well as strong cytoplasmic and nuclear β-catenin staining have been used to establish that canonical Wnt/β-catenin signaling is active in the outer region of the adrenal cortex, beneath the capsule, overlapping with the morphologically distinct zG (Fig. 1) (Berthon et al., 2010; Kim et al., 2008; Walczak et al., 2014). Exactly what triggers Wnt signaling activity in the zG is still not completely understood. Nineteen Wnt ligands exist, of which six have been identified in the mouse adrenal (Drelon et al., 2014). Of these, Wnt4 is specifically expressed in the zG region of the adult adrenal and is required for adrenal homeostasis (Heikkilä et al., 2002). The expression patterns of the other ligands, including Wnt5a, Wnt5b, Wnt2b, Wnt9a, and Wnt11, have only been described in the fetal adrenal, where they are expressed in the capsular/subcapsular region. Recently, Rspo3 (encoding R-spondin 3) has been recognized as an essential Wnt ligand that signals from the capsule to the zG to activate canonical Wnt/β-catenin signaling and maintain zonation throughout life (Vidal et al., 2016).
Canonical Wnt/β-catenin signaling has also been linked to the steroidogenic activity of the zG. Human cell culture studies showed β-catenin directly binds to the AT1R enhancer to drive AT1R expression and indirectly activates CYP11B2 transcription via the transcriptional regulation of the orphan nuclear receptors NUR77 and NURR1 (Berthon et al., 2014). Additionally, in mice, constitutive activation of β-catenin or inactivation of APC leads to hyperaldosteronism (Berthon et al., 2010; Bhandaru et al., 2009), while deletion of either β-catenin or Wnt4 results in decreased Cyp11b2 transcripts and lower aldosterone production (Heikkilä et al., 2002; Kim et al., 2008). Together, these data indicate that Wnt/β-catenin signaling plays an important role in regulating aldosterone production, perhaps through an interaction with AngII-dependent signaling. Interestingly, however, in mice, while the entire zG is Wnt-responsive, only a subset of zG cells express Cyp11b2 (Fig. 1) (Berthon et al., 2010; Kim et al., 2008; Walczak et al., 2014), implying that Wnt/β-catenin signaling may be necessary but not sufficient for aldosterone production.
Given the well-documented contribution of Wnt/β-catenin signaling to the proliferative activity of several tissues (Masckauchán et al., 2005; Pei et al., 2012), this pathway may also play a role in the regulation of zG cell proliferation, thereby affecting the overall size of the zone. Indeed, recent studies have shown that loss of Rspo3 (leading to a decrease in Wnt/β-catenin signaling) results in decreased zG cell proliferation and overall adrenal size (Vidal et al., 2016), while stabilization of β-catenin in the cortex stimulates cell proliferation (Berthon et al., 2010). Whether the response of zG cells to physiological cues, such as AngII and high K+ levels, is mediated by Wnt/β-catenin signaling remains to be fully investigated. Therefore, it remains an interesting possibility that Wnt/β-catenin signaling may mediate the proliferative response of zG cells to physiological cues. It should be noted, however, that short-term treatment with AngII suppresses Wnt/β-catenin signaling (Berthon et al., 2014), suggesting the presence of a complex interplay (e.g., feedback loop) between endocrine and paracrine factors in the regulation of zG homeostasis and aldosterone production.
While inactivation of Wnt signaling impaired steroidogenesis and proliferation, stabilization of β-catenin or inactivation of the Wnt inhibitor Sfrp2 disrupted zonation leading to ectopic zG cells in the region normally occupied by zF cells (Berthon et al., 2010, 2014). While the cellular mechanism(s) underlying this effect are unknown, the presence of zG cells within the zF may either arise from (1) the failure of zG cells to transdifferentiate/directly convert into zF cells (see section 4.2), or from (2) the reverse transdifferentiation (or de-differentiation) of zF cells into zG cells. The decreased amounts of Cyp11b1 and Akr1b7 transcripts in adrenals with stabilized β-catenin are consistent with both of these scenarios (Berthon et al., 2010). However, it has also been suggested that β-catenin can directly antagonize zF function. Activation of Wnt signaling in a mouse zF-like cell line, ATC7-L, causes reduced transcription of the zF-specific steroidogenic genes Cyp11b1 and Mc2r (Walczak et al., 2014). This results from β-catenin titrating Sf1 away from its target genes and sustaining the expression of the secreted factor coiled-coil domain containing 80 (Ccdc80), a putative zF inhibitor (Walczak et al., 2014). Altogether, these data imply that Wnt/β-catenin signaling can influence zonation by promoting the zG identity and antagonizing the zF identity.
Sustained activity of the Wnt/β-catenin signaling pathway is the most common alteration in APAs (about 70% of the tumors) (Berthon et al., 2014) and β-catenin activating mutations represent the second leading genetic mutation associated with this disease (about 5% of the tumors) (Åkerström et al., 2016). APAs carrying activating mutations in β-catenin exhibit a bimodal score for CYP11B2 expression, displaying either low or very high levels of the protein in the nodules (Åkerström et al., 2016). Since this study did not report clinical or genetic stratification associated with the different levels of CYP11B2 expression, variation may be explained by the β-catenin activating mutations arising from different cell populations or during different stages of the disease. Consistent with these hypotheses, β-catenin mutations can also be found in non-functioning adenomas and in carcinomas (Tissier et al., 2005), suggesting that the timing of the mutational event in the oncogenic process along with additional putative genetic hits may be critical in the outcome of the disease. Altogether, these data imply that canonical Wnt/β-catenin signaling plays a critical role in establishing and regulating zG steroidogenic activity and suppressing zF activity, both in homeostasis and disease.
3.2. Regulation of zonation in the zF
Steroidogenesis and cell proliferation in the zF are under the control of the ACTH/cAMP signaling pathway. This signaling cascade is initiated by ACTH, which acts on the melanocortin 2 G-protein coupled receptor (MC2R) and the MC2R accessory protein (MRAP) (Lefkowitz et al., 1970; Lehoux et al., 1998; Metherell et al., 2005). MC2R and MRAP expression are also regulated by the ACTH/cAMP pathway (Xing et al., 2010). The occupancy of the receptor triggers the release of the alpha subunit of the stimulatory G protein and the conversion of ATP into cAMP by adenylyl cyclase (Kim et al., 2005, 2007). These events ultimately result in the activation of the catalytic subunit of PKA and the subsequent phosphorylation of cytoplasmic and nuclear targets, which include the cAMP-responsive element modulator (CREM) and the cAMP-responsive element binding protein (CREB) transcription factors (Peri et al., 2001).
A large amount of evidence suggests that the cAMP signaling pathway is primarily active in the zF. While several molecular components of the cAMP cascade are present in both the zF and the zG (Côté et al., 2001; Gorrigan et al., 2011), Cyp11b1, the enzyme that catalyzes the final step in the biosynthesis of cortisol and corticosterone, is a target of ACTH and selectively expressed in the zF (Freedman et al., 2013; Gomez-Sanchez et al., 2014). Additionally, the aldo-keto reductase Akr1b7, which is selectively expressed in the zF, is also a target of cAMP signaling (Aigueperse et al., 1999; Berthon et al., 2010; Sahut-Barnola et al., 2010). Together, these data suggest that the cAMP signaling pathway is primarily active in the zF.
In a physiological context, ACTH/cAMP signaling also controls the size of the zF without disrupting its functional and morphological boundaries with the zG or the X-zone. This is evident in humans, where mutations in genes encoding MC2R and MRAP are associated with glucocorticoid insufficiency, leading to adrenals with a hypoplastic zF but a morphologically intact zG and normal aldosterone production (Clark and Weber, 1998). Moreover, mice lacking T-box transcription factor (Tpit), encoding a transcription factor essential for the expression of the ACTH precursor proopiomelanocortin (POMC), develop severe adrenocortical hypoplasia mainly affecting the zF (Pulichino et al., 2003). Similarly, deletion of Pomc leads to overall reduced adrenal mass associated with impaired proliferative activity and hypoplasia of the zF (Karpac et al., 2005). Inactivation of the Mc2r gene also produces hypoplasia of the zF (Chida et al., 2007). In addition, this same effect is seen following suppression of the zF using dexamethasone (Freedman et al., 2013; Thomas et al., 2004). Overall, these results point to a role for physiological levels of ACTH/cAMP signaling in supporting adrenal mass and proliferation, with a primary effect on the zF.
On the other hand, dysregulated cAMP signaling in a pathological context, can also result in disruption of zonation. This emerges from studies on constitutive activation of cAMP signaling in the adrenal cortex, obtained with genetic deletion of the regulatory subunit 1α of PKA (Prkar1a). Adrenals with constitutive cAMP signaling present with large eosinophilic cells at the cortical-medullary boundary that express Cyp17, a mouse adrenal fetal marker, as well as markers of the zF and the X-zone (Akr1b7 and 20-alpha-hydroxylase, respectively) (Sahut-Barnola et al., 2010). These atypical cells were shown to expand in a centrifugal direction and to occupy most of the cortex in aged mice, thereby disrupting the concentric organization of the cortical zones. These data show that regulation of cAMP signaling is essential for the maintenance of proper structural and functional zonation, and suggest that disruption of this regulation may lead to destabilized zonal identity and altered cell differentiation.
4. Mechanisms Underlying Adrenocortical Cell Renewal in the Adult
Classically, highly regenerative tissues maintain cellular homeostasis by utilizing an active stem/progenitor cell compartment, or by replication of pre-existing differentiated cells. In some tissues where mature cell types are post-mitotic, such as the gastrointestinal tract, the epidermis, and the hematopoietic system, tissue homeostasis relies heavily on the capacity of adult stem cells to self-renew and give rise to differentiated cell lineages (Barker et al., 2012; Blanpain and Fuchs, 2009; Oguro et al., 2013). In other tissues, however, such as the liver, normal homeostasis is largely achieved through direct replication of mature differentiated cells, whereas stem cell-like activities only emerge upon injury (Miyajima et al., 2014).
The adult adrenal cortex is a moderately proliferative tissue. Cells in the outer cortex including the zG and the outer zF exhibit a higher proliferation rate than the inner cortex, and cell death is predominantly observed at the cortical-medullary boundary under physiological conditions (Kataoka et al., 1996; Mitani et al., 1999). Adrenocortical turnover is accompanied by the centripetal migration of cells from the outer to the inner cortex. A number of groups have attempted to measure the speed of centripetal migration in rodents using pulse-labeling techniques. Zajicek et al. reported that the time for a cell to traverse the entire cortex in rats is around 104 days (Zajicek et al., 1986). While Chang et al. estimated the speed of migration to be 13–20 μm/day in mice (Chang et al., 2013), which translates to 12–19 days to traverse the entire cortex (assuming an average cortical thickness of ~250 μm). However, label-retention studies used to measure the rate of tissue renewal in mice revealed that complete turnover took 60 days in the zG, and 120 days in the zF (Kataoka et al., 1996), much slower than would be inferred using the speed of migration. The discrepancy between these data may reflect an important feature of the adrenal, namely that not all cells participate in centripetal migration to the same degree. That is, some cells may migrate at a much lower speed or exhibit long-term tissue residence. Nonetheless, the speed of centripetal migration is a good proxy for overall tissue dynamics; while the prolonged turnover time of each zone may reflect the behavior of long-lived cortical cells.
4.1. Evidence for Adrenocortical Stem Cells
While the full extent of the proliferative potential of differentiated zG and zF cells has not been thoroughly investigated, it has been proposed that the adult adrenal contain tissue-resident stem cells in the peripheral cortex capable of unlimited self-renewal and differentiation into steroidogenic cell types (Pihlajoki et al., 2015; Walczak and Hammer, 2014). This notion is supported by the observation that enucleated rat adrenal glands can fully regenerate from the remaining capsular and subcapsular cells (Greep and Deane, 1949), consistent with a remarkable degree of proliferative and differentiation potential, at least under extreme regenerative stress. During normal tissue maintenance, the constant centripetal migration of cells implies that new cells are generated at the outer cortex, and have the potential to differentiate into the cells of the zG and zF. Collectively, these data point to a remarkable ability of the outer cortex to support long-term cortical renewal, assuming the turnover rate is constant. However, the degree to which ongoing cortical renewal is derived from adult stem cell activity versus proliferation of differentiated cortical cells remains to be elucidated.
The search for adult tissue stem cells is often aided by the presence of an easily identifiable niche with distinct anatomical and molecular features (Rezza et al., 2014). An increased understanding of the signaling network in the peripheral cortex has led to the identification of putative adrenal stem cell markers. One popular model contends that stem cells are located in the capsule as non-steroidogenic mesenchymal cells (Kim et al., 2009; Walczak and Hammer, 2014). The first direct evidence supporting this idea came from King et al. in 2009. Using genetic lineage tracing studies, the investigators effectively demonstrated capsular cells marked by Gli1 expression can give rise to all steroidogenic cell types in the adult cortex (King et al., 2009). Gli1 along with two other family members, Gli2 and Gli3, are transcription factors whose activities are controlled by the Hedgehog (Hh) paracrine signaling pathway. Hh signaling has well studied functions throughout vertebrate and invertebrate development and plays important roles in the maintenance and regeneration of numerous adult tissues (McMahon et al., 2003). Sonic hedgehog (Shh) is the only ligand expressed in the adrenal cortex, and its onset of expression at E11.5 together with other pathway components, such as the membrane receptors Patched1 (Ptch1) and Smoothened (Smo), suggest this pathway regulates adrenal organogenesis as well as adult homeostasis, potentially through regulating the activity of Gli1+ capsular stem cells (King et al., 2009). This hypothesis has been partly substantiated by three studies examining the effect of genetic ablation of Shh. Collectively, their data showed that loss of Shh expression from the subcapsular/zG region during embryonic development leads to decreased proliferation in the capsule and adrenal hypoplasia that persists into adulthood (Ching and Vilain, 2009; Huang et al., 2010; King et al., 2009). Even though the role of Shh signaling in adult homeostasis has not been formally tested, the long-lasting effect of Shh ablation at fetal stages points to its important role in establishing and/or maintaining a key population of cells needed for continuous cortical renewal. Interestingly, while the Gli1+ cell lineage is long lasting in the adult cortex, its contribution to cortical renewal is relatively infrequent (King et al., 2009). This suggests that while Shh signaling is absolutely required for organogenesis, it may play a less crucial role once the adult cortex has formed. Cellular sources apart from the Gli1+ lineage must be in place to support continuous adult cortical renewal.
Another proposed marker of stem cell identity in the capsule is Wilms tumor suppressor gene 1 (Wt1). Wt1 is a transcription factor with many functions in tissue growth and homeostasis (Chau et al., 2011; Hohenstein and Hastie, 2006). It is known that formation of the AGP requires Wt1 partly through its role in regulating Sf1 expression (Val et al., 2007; Wilhelm and Englert, 2002). In 2013, Bandiera et al. demonstrated cortical cells can arise from Wt1-expressing cells in the adult capsule. However, lineage-marked cell patches are present at low frequency, suggesting relatively little contribution of Wt1+ capsular cells during adult tissue maintenance (Bandiera et al., 2013). Furthermore, it has been proposed that Tcf21/Pod1, a transcriptional modulator of Sf1 (Simon and Hammer, 2012), marks cells in the adrenal capsule that exhibit clonogenic activity during development. However, in the adult they seem to exclusively contribute to a mesenchymal lineage of non-endothelial stromal cells (Wood et al., 2013). Together, these lineage-tracing studies have identified mesenchymal cells in the adrenal capsule that can give rise to either steroidogenic cells or non-endothelial stromal cells in the cortex (Fig. 2A). However, capsular contribution to cortical cell mass appears to be rather limited in the adult.
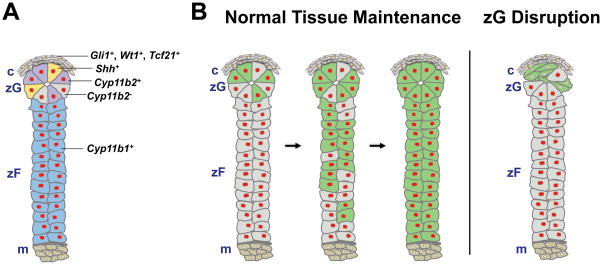
Figure 2. Cellular mechanisms underlying adrenocortical renewal in the adult.
A, Schematic showing location of proposed markers for clonogenic capsular cells (Gli1+, WT1+, Tcf21+; grey with brown nuclei), putative TA progenitors (Shh+; yellow), aldosterone-producing zG cells (Cyp11b2+; purple), non-aldosterone-producing zG cells (Cyp11b2−, can be identified with pan-zG markers such as At1r, β-catenin; grey with red nuclei), and zF cells (Cyp11b1+; blue). Red nuclei denote Sf1+ cells. B, Left panel, schematic showing progression of lineage marking in AS-Cre/+; R26R-mTmG mice during normal tissue maintenance. The Cyp11b2+ lineage gives rise to the entire cortex through transdifferentiation/direct conversion and centripetal migration. Right panel, schematic showing disruption of the zG following Sf1 deletion in AS-Cre/+; Sf1 fl/fl; R26R-mTmG mice. In these mice Cyp11b2− cells give rise to a normal zF. Green cells denote lineage-marked Cyp11b2+ cells and their descendants and red nuclei denote Sf1+ cells. m, medulla.
Given that the adult cortex maintains a steady rate of centripetal migration (at least during the time points examined), new cells need to be continuously generated to replenish displaced cells. There is a clear discrepancy between the infrequent capsular contribution and the continuous need for new cortical cells, suggesting that other cell populations may function to maintain adult homeostasis. It has been proposed that Shh-expressing cells in the subcapsular/zG region serve as transit-amplifying (TA) progenitors to sustain cortical renewal (Laufer et al., 2012). The authors performed short-term lineage tracing to show that the Shh+ lineage can give rise to Cyp11b2+ and Cyp11b1+ cells during adult homeostasis, but it is unclear whether these cells do indeed sustain long-term cortical renewal (King et al., 2009). In addition, Shh-expressing cells are not more proliferative than other cells in the subcapsular region, which is inconsistent with their proposed identity as TA cells (Walczak et al., 2014). Thus, it remains to be demonstrated whether the adrenal cortex utilizes the classical stem cell-TA progenitor lineage hierarchy to maintain adult homeostasis and if not, what cellular mechanisms support the majority of adult cortical cell turnover.
4.2. Role of Transdifferentiation/Direct Conversion
The centripetal migration model implies that mature aldosterone-producing zG cells have the ability to convert into zF cells as they cross the zonal boundary. Using a Cyp11b2 (AS)-Cre mouse model, Cyp11b2-expressing zG cells were permanently marked by GFP (Green Fluorescent Protein) expression and lineage-traced (Freedman et al., 2013). Remarkably, the entire zG and the majority of the zF became labeled within 3 months (Figs. 3 and 2B), suggesting that all cortical cells are descendants from the Cyp11b2+ cell lineage during normal tissue maintenance. The extensive overlap between the GFP-marked population and the steroidogenic cortex has been further confirmed by co-staining with Sf1 and β-catenin (Fig. 3) as well as confocal 3D reconstructions (unpublished data). These data challenge the view that the adult adrenal cortex contains a strict lineage hierarchy of undifferentiated progenitors giving rise to mature cell types. It is also theoretically possible that mature cortical cells could dedifferentiate into immature progenitors, which could then give rise to mature zG and zF cells, however, there is no direct evidence for such a phenomenon. Given the robust degree of GFP marking throughout the entire cortex in the AS-Cre model, it is unlikely that Cyp11b2+ lineage contribution to cortical renewal represents a minor event, such as mature cells dedifferentiating into progenitors. Hence, we propose that mature zG to zF transdifferentiation/direct conversion represents a major cellular mechanism of adult adrenocortical turnover during normal homeostasis. However, it remains unclear to what extent zG cells alone can sustain long-term cortical renewal, or to what degree zG cells rely on replenishment from the capsule. To address the self-renewing potential of mature zG cells, an inducible mouse model, such as AS-CreER, will greatly facilitate further investigation.
The idea that undifferentiated progenitors exist in the subcapsular cortex is partly based on the observation of heterogeneous Cyp11b2 expression in the zG region (Fig 1. Guasti et al., 2011; Walczak et al., 2014). Accordingly, Shh-expressing cells are thought to represent a unique non-Cyp11b2-expressing population in the zG, given the minimal overlap between the two markers (King et al., 2009; Walczak et al., 2014). However, many other markers such as At1r, Dab2, and β-catenin, identify the morphological zG as a homogeneous population (Fig. 3A; Berthon et al., 2010; Huang et al., 2013; Walczak et al., 2014). Given the wide range of aldosterone output in response to varying physiological demands, it is possible that at any given time only a subset of zG cells produce aldosterone. Total cortical labeling in the AS-Cre model, therefore, can be interpreted as a cumulative readout of all Cyp11b2-expressing cells, and their progeny, prior to the time of observation (Fig. 2B). Interestingly, Shh expression has also been reported to respond to endocrine input. In the rat, levels of Shh transcript are markedly down-regulated upon low sodium diet and up-regulated when AngII signaling is blocked (Guasti et al., 2013), suggesting that Shh expression, in the opposite direction of Cyp11b2, is dynamically regulated by the physiological demand for aldosterone. Hence, it remains to be demonstrated whether Shh expression truly marks a unique population in the zG, or rather represents a dynamic gene expression state that is complementary to the Cyp11b2-expressing state. In fact, the evidence supporting Shh-expressing cells as unique progenitors is based on the fact that the Shh+ lineage gives rise to Cyp11b2+ and Cyp11b1+ cells. However, as presented above, the Cyp11b2+ lineage behaves in the same way. Furthermore, because Cyp11b2+ cells can give rise to the zF, the ability of a cell to give rise to multiple cortical cell types can no longer be used as a fail-safe criteria to define bona fide, undifferentiated, stem/progenitor cells in the adrenal cortex, as mature zG cells can also give rise to other zonal cell types. The question of which cell population provides the major source of cortical replenishment remains to be further elucidated.
While during normal tissue maintenance all cortical cells descend from the Cyp11b2+ lineage, analysis of mice having undergone AS-Cre mediated Sf1 deletion revealed that the zF can also be derived from a separate (Cyp11b2−) cellular origin (Freedman et al., 2013) (Fig. 2B). In this model, deletion of Sf1 using AS-Cre led to zG disruption (Fig. 2B), which could no longer give rise to lineage-marked zF cells. Despite this, an entirely normal zF was still evident. Based on this observation, we previously proposed that an “alternative pathway” for zF formation might exist (Freedman et al., 2013), though we have yet to define such a mechanism. Upon further reflection, given the heterogeneous, and presumed dynamic, nature of Cyp11b2 expression in the zG it is also conceivable that under extreme conditions (where selective pressure to generate the zF is high), Cyp11b2− zG cells transit into the zF before Cyp11b2 can be expressed, thereby escaping Sf1-deletion. Whether or not putative progenitor/stem cells in the capsule are activated under these stressed conditions remains to be established. Testing this hypothesis would require combining models of zG-specific gene ablation and lineage-tracers that mark putative capsular stem cell populations.
5. Concluding Remarks
Being a remarkably regenerative and multifunctional organ, the adult adrenal cortex presents a number of fascinating questions for developmental biologists as well as endocrine researchers. 1) How does it achieve such precise zonation of closely related yet notably different steroidogenic cells? 2) How is the control of zonation so sensitive and responsive to dynamic physiological demands? 3) What pathological conditions might disturb its regulation circuitry and give rise to clinically observed diseases? In the recent decade, researchers have begun to address these questions by applying knowledge of endocrine and paracrine signaling pathways as well as adult tissue stem cell biology. Two pathways have been identified that directly influence zonal identity, the canonical Wnt pathway for the zG, and cAMP signaling for the zF. Many insights have been gained regarding the connection between these pathway activities and steroidogenesis; however, it still remains largely unclear how zonation is established, maintained, and dynamically controlled. Wnt signaling modulators are being pursued as prime candidates for the dynamic control over zonation. It has also been proposed that cAMP signaling may directly or indirectly antagonize Wnt signaling activity (Drelon et al., 2014).
The notion that adrenocortical renewal relies on resident tissue stem cells is a longstanding hypothesis that has fueled a series of lineage-tracing studies in an attempt to identify such cells. Collectively, these studies have demonstrated that mesenchymal cells in the capsule are able to give rise to the steroidogenic lineage, yet the inductive signal remains unknown. In all cases identified so far, however, lineage contribution from the capsule is rather limited during adult homeostasis. Results from our lab have shown transdifferentiation/direct conversion of Cyp11b2+ zG cells to zF cells can account for turnover of the whole-cortex throughout adult life. Current data highlight an essential role of Sf1 in mediating transdifferentiation of zG cells, yet it still remains an open question what mechanisms govern the migration and conversion of cells across the zG/zF boundary. Future studies on these topics will facilitate our understanding of zonation and the diseases that arise from its disruption.
Highlights.
The adrenal cortex contains concentric zones without anatomical barriers.
The different zones are distinguishable by morphology and steroidogenic activity.
Wnt/β-catenin and ACTH/cAMP signaling pathways are essential for zonation.
The capsule contains progenitor cells with limited activity in adult maintenance.
Direct zG/zF conversion represents the major mechanism of adult tissue maintenance.
Acknowledgments
We thank members of the Breault laboratory and J. Majzoub, W. Engeland, and P. Barrett for helpful discussions. This research was supported by R01 DK 084056, an EFF Award, a PES Research Award and Career Development Award and an HSCI Junior Faculty Award to D.T.B., the Timothy Murphy Fund, IDDRC grant P30 HD18655 and HDDC P30DK034854.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigueperse C, Martinez A, Lefrançois-Martinez AM, Veyssière G, Jean CI. Cyclic AMP regulates expression of the gene coding for a mouse vas deferens protein related to the aldo-keto reductase superfamily in human and murine adrenocortical cells. J Endocrinol. 1999;160:147–154. doi: 10.1677/joe.0.1600147. [DOI] [PubMed] [Google Scholar]
- Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6:19546. doi: 10.1038/srep19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera R, Vidal VPI, Motamedi FJ, Clarkson M, Sahut-Barnola I, von Gise A, Pu WT, Hohenstein P, Martinez A, Schedl A. WT1 Maintains Adrenal-Gonadal Primordium Identity and Marks a Population of AGP-like Progenitors within the Adrenal Gland. Dev Cell. 2013;27:5–18. doi: 10.1016/j.devcel.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–460. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Berthon A, Drelon C, Ragazzon B, Boulkroun S, Tissier F, Amar L, Samson-Couterie B, Zennaro MC, Plouin PF, Skah S, Plateroti M, Lefèbvre H, Sahut-Barnola I, Batisse-Lignier M, Assié G, Lefrançois-Martinez AM, Bertherat J, Martinez A, Val P. WNT/β-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet. 2014;23:889–905. doi: 10.1093/hmg/ddt484. [DOI] [PubMed] [Google Scholar]
- Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, Taketo MM, Tissier F, Bertherat J, Lefrançois-Martinez AM, Martinez A, Val P. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19:1561–1576. doi: 10.1093/hmg/ddq029. [DOI] [PubMed] [Google Scholar]
- Berthon AS, Szarek E, Stratakis CA. PRKACA: the catalytic subunit of protein kinase A and adrenocortical tumors. Front Cell Dev Biol. 2015;3:26. doi: 10.3389/fcell.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandaru M, Kempe DS, Rotte A, Rexhepaj R, Kuhl D, Lang F. Hyperaldosteronism, hypervolemia, and increased blood pressure in mice expressing defective APC. Am J Physiol Regul Integr Comp Physiol. 2009;297:R571–575. doi: 10.1152/ajpregu.00070.2009. [DOI] [PubMed] [Google Scholar]
- Black VH, Robbins D, McNamara N, Huima T. A correlated thin-section and freeze-fracture analysis of guinea pig adrenocortical cells. Am J Anat. 1979;156:453–503. doi: 10.1002/aja.1001560404. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag WB. Regulation of aldosterone synthesis and secretion. Compr Physiol. 2014;4:1017– 1055. doi: 10.1002/cphy.c130037. [DOI] [PubMed] [Google Scholar]
- Boulkroun S, Fernandes-Rosa FL, Zennaro MC. Molecular and Cellular Mechanisms of Aldosterone Producing Adenoma Development. Front Endocrinol. 2015;6:95. doi: 10.3389/fendo.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breault L, Chamoux E, Lehoux JG, Gallo-Payet N. The role of angiotensin II in human adrenal gland development. Endocr Res. 1998;24:953–954. doi: 10.3109/07435809809032714. [DOI] [PubMed] [Google Scholar]
- Breault L, Lehoux JG, Gallo-Payet N. Angiotensin II receptors in the human adrenal gland. Endocr Res. 1996;22:355–361. doi: 10.1080/07435809609043718. [DOI] [PubMed] [Google Scholar]
- Campbell S, Otis M, Côté M, Gallo-Payet N, Payet MD. Connection between integrins and cell activation in rat adrenal glomerulosa cells: a role for Arg-Gly-Asp peptide in the activation of the p42/p44(mapk) pathway and intracellular calcium. Endocrinology. 2003;144:1486–1495. doi: 10.1210/en.2002-220903. [DOI] [PubMed] [Google Scholar]
- Chang SP, Morrison HD, Nilsson F, Kenyon CJ, West JD, Morley SD. Cell Proliferation, Movement and Differentiation during Maintenance of the Adult Mouse Adrenal Cortex. PLoS ONE. 2013;8:e81865. doi: 10.1371/journal.pone.0081865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau YY, Brownstein D, Mjoseng H, Lee WC, Buza-Vidas N, Nerlov C, Jacobsen SE, Perry P, Berry R, Thornburn A, Sexton D, Morton N, Hohenstein P, Freyer E, Samuel K, van’t Hof R, Hastie N. Acute multiple organ failure in adult mice deleted for the developmental regulator Wt1. PLoS Genet. 2011;7:e1002404. doi: 10.1371/journal.pgen.1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, Suzuki H, Mitani F, Ogishima T, Shimizu C, Kotaki H, Kakuta S, Sudo K, Koike T, Kubo M, Iwakura Y. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci U S A. 2007;104:18205–18210. doi: 10.1073/pnas.0706953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. genesis. 2009;47:628–637. doi: 10.1002/dvg.20532. [DOI] [PubMed] [Google Scholar]
- Clark AJ, Weber A. Adrenocorticotropin insensitivity syndromes. Endocr Rev. 1998;19:828–843. doi: 10.1210/edrv.19.6.0351. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Côté M, Guillon G, Payet MD, Gallo-Payet N. Expression and regulation of adenylyl cyclase isoforms in the human adrenal gland. J Clin Endocrinol Metab. 2001;86:4495–4503. doi: 10.1210/jcem.86.9.7837. [DOI] [PubMed] [Google Scholar]
- Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane HW, Shaw JH, Greep RO. The effect of altered sodium or potassium intake on the width and cytochemistry of the zona glomerulosa of the rat’s adrenal cortex. Endocrinology. 1948;43:133–153. doi: 10.1210/endo-43-3-133. [DOI] [PubMed] [Google Scholar]
- Drelon C, Berthon A, Mathieu M, Martinez A, Val P. Adrenal cortex tissue homeostasis and zonation: A WNT perspective. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Eberhart CG, Argani P. Wnt signaling in human development: beta-catenin nuclear translocation in fetal lung, kidney, placenta, capillaries, adrenal, and cartilage. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc. 2001;4:351–357. doi: 10.1007/s10024001-0037-y. [DOI] [PubMed] [Google Scholar]
- Freedman BD, Kempna PB, Carlone DL, Shah MS, Guagliardo NA, Barrett PQ, Gomez-Sanchez CE, Majzoub JA, Breault DT. Adrenocortical Zonation Results from Lineage Conversion of Differentiated Zona Glomerulosa Cells. Dev Cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DS, Gilula NB. A distinctive cell contact in the rat adrenal cortex. J Cell Biol. 1972;53:148–163. doi: 10.1083/jcb.53.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati SJ, Hopkins SM, Cheesman KC, Zhuk RA, Levine AC. Primary aldosteronism: emerging trends. Trends Endocrinol Metab. 2013;24:421–430. doi: 10.1016/j.tem.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N, Battista M-C. Steroidogenesis-Adrenal Cell Signal Transduction. In: Terjung R, editor. Comprehensive Physiology. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2014. pp. 889–964. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383:111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrigan RJ, Guasti L, King P, Clark AJ, Chan LF. Localisation of the melanocortin-2receptor and its accessory proteins in the developing and adult adrenal gland. J Mol Endocrinol. 2011;46:227–232. doi: 10.1530/JME-11-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greep RO, Deane HW. Histological, cytochemical and physiological observations on the regeneration of the rat’s adrenal gland following enucleation. Endocrinology. 1949;45:42–56. doi: 10.1210/endo-45-1-42. [DOI] [PubMed] [Google Scholar]
- Guasti L, Cavlan D, Cogger K, Banu Z, Shakur A, Latif S, King PJ. Dlk1 Up-Regulates Gli1 Expression in Male Rat Adrenal Capsule Cells Through the Activation of β1 Integrin and ERK1/2. Endocrinology. 2013;154:4675–4684. doi: 10.1210/en.2013-1211. [DOI] [PubMed] [Google Scholar]
- Guasti L, Paul A, Laufer E, King P. Localization of Sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex. Mol Cell Endocrinol. 2011;336:117–122. doi: 10.1016/j.mce.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Takemori H, Hatano O, Nonaka Y, Wada A, Okamoto M. Cloning of a membrane-spanning protein with epidermal growth factor-like repeat motifs from adrenal glomerulosa cells. Endocrinology. 1998;139:3316–3328. doi: 10.1210/endo.139.7.6081. [DOI] [PubMed] [Google Scholar]
- Heikkilä M, Peltoketo H, Leppäluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alphahydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11 deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148:976–988. doi: 10.1210/en.2006-1100. [DOI] [PubMed] [Google Scholar]
- Hohenstein P, Hastie ND. The many facets of the Wilms’ tumour gene, WT1. Hum Mol Genet. 2006;15(Spec No 2):R196–201. doi: 10.1093/hmg/ddl196. [DOI] [PubMed] [Google Scholar]
- Huang CCJ, Miyagawa S, Matsumaru D, Parker KL, Yao HHC. Progenitor Cell Expansion and Organ Size of Mouse Adrenal Is Regulated by Sonic Hedgehog. Endocrinology. 2010;151:1119–1128. doi: 10.1210/en.2009-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Ohno N, Terada N, Saitoh Y, Chen J, Ohno S. Immunohistochemical detection of angiotensin II receptors in mouse cerebellum and adrenal gland using “in vivo cryotechnique. Histochem Cell Biol. 2013;140:477–490. doi: 10.1007/s00418-013-1084-y. [DOI] [PubMed] [Google Scholar]
- Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology. 2005;146:2555–2562. doi: 10.1210/en.2004. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Ikehara Y, Hattori T. Cell proliferation and renewal of mouse adrenal cortex. J Anat. 1996;188( Pt 2):375–381. [PMC free article] [PubMed] [Google Scholar]
- Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, Scheys JO, Simon DP, Trovato A, Yang WH, Hammer GD. In Search of Adrenocortical Stem and Progenitor Cells. Endocr Rev. 2009;30:241–263. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Dev Camb Engl. 2008;135:2593–2602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- Kim C, Cheng CY, Saldanha SA, Taylor SS. PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell. 2007;130:1032–1043. doi: 10.1016/j.cell.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Kim C, Xuong NH, Taylor SS. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science. 2005;307:690–696. doi: 10.1126/science.1104607. [DOI] [PubMed] [Google Scholar]
- King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer E, Kesper D, Vortkamp A, King P. Sonic hedgehog signaling during adrenal development. Mol Cell Endocrinol. 2012;351:19–27. doi: 10.1016/j.mce.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Roth J, Pricer W, Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970;65:745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux JG, Fleury A, Ducharme L. The acute and chronic effects of adrenocorticotropin on the levels of messenger ribonucleic acid and protein of steroidogenic enzymes in rat adrenal in vivo. Endocrinology. 1998;139:3913–3922. doi: 10.1210/endo.139.9.6196. [DOI] [PubMed] [Google Scholar]
- Lu X, Grove KL, Zhang W, Speth RC. Pharmacological characterization of angiotensin II AT(2) receptor subtype heterogeneity in the rat adrenal cortex and medulla. Endocrine. 1995;3:255–261. doi: 10.1007/BF03021402. [DOI] [PubMed] [Google Scholar]
- Magill SB. Pathophysiology, Diagnosis, and Treatment of Mineralocorticoid Disorders. In: Terjung R, editor. Comprehensive Physiology. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2014. pp. 1083–1119. [DOI] [PubMed] [Google Scholar]
- Masckauchán TNH, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/betacatenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- McEwan PE, Lindop GB, Kenyon CJ. Control of cell proliferation in the rat adrenal gland in vivo by the renin-angiotensin system. Am J Physiol. 1996;271:E192–198. doi: 10.1152/ajpendo.1996.271.1.E192. [DOI] [PubMed] [Google Scholar]
- McEwan PE, Vinson GP, Kenyon CJ. Control of adrenal cell proliferation by AT1 receptors in response to angiotensin II and low-sodium diet. Am J Physiol. 1999;276:E303–309. doi: 10.1152/ajpendo.1999.276.2.E303. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- McNeill H. Distribution of extracellular signal-regulated protein kinases 1 and 2 in the rat adrenal and their activation by angiotensin II. J Endocrinol. 2005;187:149–157. doi: 10.1677/joe.1.06347. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJL. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Lehto VP, Virtanen I. Immunofluorescence microscopic evaluation of the intermediate filament expression of the adrenal cortex and medulla and their tumors. Am J Pathol. 1985;118:360–366. [PMC free article] [PubMed] [Google Scholar]
- Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. Development of functional zonation in the rat adrenal cortex. Endocrinology. 1999;140:3342–3353. doi: 10.1210/endo.140.7.6859. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Tanaka M, Itoh T. Stem/Progenitor Cells in Liver Development, Homeostasis, Regeneration, and Reprogramming. Cell Stem Cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Morohashi K, Zubair M. The fetal and adult adrenal cortex. Mol Cell Endocrinol. 2011;336:193–197. doi: 10.1016/j.mce.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Harris RBS, Rainey WE, Seki T. Sodium deficiency regulates rat adrenal zona glomerulosa gene expression. Endocrinology. 2014;155:1363–1372. doi: 10.1210/en.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussdorfer GG. Cytophysiology of the adrenal zona glomerulosa. Int Rev Cytol. 1980;64:307–368. doi: 10.1016/s0074-7696(08)60240-5. [DOI] [PubMed] [Google Scholar]
- Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis M, Campbell S, Payet MD, Gallo-Payet N. Expression of extracellular matrix proteins and integrins in rat adrenal gland: importance for ACTH-associated functions. J Endocrinol. 2007;193:331–347. doi: 10.1677/JOE-07-0055. [DOI] [PubMed] [Google Scholar]
- Pei Y, Brun SN, Markant SL, Lento W, Gibson P, Taketo MM, Giovannini M, Gilbertson RJ, Wechsler-Reya RJ. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Dev Camb Engl. 2012;139:1724–1733. doi: 10.1242/dev.050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri A, Luciani P, Conforti B, Baglioni-Peri S, Cioppi F, Crescioli C, Ferruzzi P, Gelmini S, Arnaldi G, Nesi G, Serio M, Mantero F, Mannelli M. Variable expression of the transcription factors cAMP response element-binding protein and inducible cAMP early repressor in the normal adrenal cortex and in adrenocortical adenomas and carcinomas. J Clin Endocrinol Metab. 2001;86:5443–5449. doi: 10.1210/jcem.86.11.8042. [DOI] [PubMed] [Google Scholar]
- Pihlajoki M, Dorner J, Cochran RS, Heikinheimo M, Wilson DB. Adrenocortical Zonation, Renewal, and Remodeling. Front Endocrinol. 2015:6. doi: 10.3389/fendo.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulichino AM, Vallette-Kasic S, Couture C, Gauthier Y, Brue T, David M, Malpuech G, Deal C, Van Vliet G, De Vroede M, Riepe FG, Partsch CJ, Sippell WG, Berberoglu M, Atasay B, Drouin J. Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes Dev. 2003;17:711–716. doi: 10.1101/gad.1065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza A, Sennett R, Rendl M. Current Topics in Developmental Biology. Elsevier; 2014. Adult Stem Cell Niches; pp. 333–372. [DOI] [PubMed] [Google Scholar]
- Rhodin JA. The ultrastructure of the adrenal cortex of the rat under normal and experimental conditions. J Ultrastruct Res. 1971;34:23–71. doi: 10.1016/s0022-5320(71)90004-9. [DOI] [PubMed] [Google Scholar]
- Romero DG, Yanes LL, de Rodriguez AF, Plonczynski MW, Welsh BL, Reckelhoff JF, Gomez-Sanchez EP, Gomez-Sanchez CE. Disabled-2 is expressed in adrenal zona glomerulosa and is involved in aldosterone secretion. Endocrinology. 2007;148:2644–2652. doi: 10.1210/en.2006-1509. [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Lalli E. Impact of ACTH Signaling on Transcriptional Regulation of Steroidogenic Genes. Front Endocrinol. 2016;7:24. doi: 10.3389/fendo.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahut-Barnola I, de Joussineau C, Val P, Lambert-Langlais S, Damon C, Lefrançois-Martinez AM, Pointud JC, Marceau G, Sapin V, Tissier F, Ragazzon B, Bertherat J, Kirschner LS, Stratakis CA, Martinez A. Cushing’s syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet. 2010;6:e1000980. doi: 10.1371/journal.pgen.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. The fine structure of the mouse adrenal X zone. Z Für Zellforsch Mikrosk Anat Vienna Austria 1948. 1968;87:315–329. doi: 10.1007/BF00333683. [DOI] [PubMed] [Google Scholar]
- Shelton JH, Jones AL. The fine structure of the mouse adrenal cortex and the ultrastructural changes in the zona glomerulosa with low and high sodium diets. Anat Rec. 1971;170:147–181. doi: 10.1002/ar.1091700204. [DOI] [PubMed] [Google Scholar]
- Simon DP, Hammer GD. Adrenocortical stem and progenitor cells: Implications for adrenocortical carcinoma. Mol Cell Endocrinol. 2012;351:2–11. doi: 10.1016/j.mce.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelings UM, Rompe F, Kaschina E, Namsolleck P, Grzesiak A, Funke-Kaiser H, Bader M, Unger T. The past, present and future of angiotensin II type 2 receptor stimulation. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2010;11:67–73. doi: 10.1177/1470320309347791. [DOI] [PubMed] [Google Scholar]
- Thomas M, Keramidas M, Monchaux E, Feige JJ. Dual hormonal regulation of endocrine tissue mass and vasculature by adrenocorticotropin in the adrenal cortex. Endocrinology. 2004;145:4320–4329. doi: 10.1210/en.2004-0179. [DOI] [PubMed] [Google Scholar]
- Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagneré AM, René-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, Perret C, Bertherat J. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- Tsuchiya B, Sato Y, Kameya T, Okayasu I, Mukai K. Differential expression of Ncadherin and E-cadherin in normal human tissues. Arch Histol Cytol. 2006;69:135–145. doi: 10.1679/aohc.69.135. [DOI] [PubMed] [Google Scholar]
- Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Dev Camb Engl. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- Vidal V, Sacco S, Rocha AS, da Silva F, Panzolini C, Dumontet T, Doan TMP, Shan J, Rak-Raszewska A, Bird T, Vainio S, Martinez A, Schedl A. The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes Dev. 2016;30:1389–1394. doi: 10.1101/gad.277756.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I, Korhonen M, Petäjäniemi N, Karhunen T, Thornell LE, Sorokin LM, Konttinen YT. Laminin isoforms in fetal and adult human adrenal cortex. J Clin Endocrinol Metab. 2003;88:4960–4966. doi: 10.1210/jc.2003-030418. [DOI] [PubMed] [Google Scholar]
- Walczak EM, Hammer GD. Regulation of the adrenocortical stem cell niche: implications for disease. Nat Rev Endocrinol. 2014;11:14–28. doi: 10.1038/nrendo.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak EM, Kuick R, Finco I, Bohin N, Hrycaj SM, Wellik DM, Hammer GD. Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol Endocrinol Baltim Md. 2014;28:1471–1486. doi: 10.1210/me.2014-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DH, Qiu J, Hu Z, Du Y. Regulation of type 1 angiotensin II receptor in adrenal gland: role of alpha1-adrenoreceptor. Hypertens Dallas Tex 1979. 1997;30:345–350. doi: 10.1161/01.hyp.30.3.345. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16:1839–1851. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Acharya A, Finco I, Swonger JM, Elston MJ, Tallquist MD, Hammer GD. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140:4522–4532. doi: 10.1242/dev.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Lerario AM, Rainey W, Hammer GD. Development of Adrenal Cortex Zonation. Endocrinol Metab Clin North Am. 2015;44:243–274. doi: 10.1016/j.ecl.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Parker CR, Edwards M, Rainey WE. ACTH is a potent regulator of gene expression in human adrenal cells. J Mol Endocrinol. 2010;45:59–68. doi: 10.1677/JME-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates R, Katugampola H, Cavlan D, Cogger K, Meimaridou E, Hughes C, Metherell L, Guasti L, King P. Current Topics in Developmental Biology. Elsevier; 2013. Adrenocortical Development, Maintenance, and Disease; pp. 239–312. [DOI] [PubMed] [Google Scholar]
- Zajicek G, Ariel I, Arber N. The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol. 1986;111:477–482. doi: 10.1677/joe.0.1110477. [DOI] [PubMed] [Google Scholar]
- Zubair M, Parker KL, Morohashi K-i. Developmental Links between the Fetal and Adult Zones of the Adrenal Cortex Revealed by Lineage Tracing. Mol Cell Biol. 2008;28:7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]