Abstract
Burn wound healing complications, such as graft failure or infection, are a major source of morbidity and mortality in burn patients. The mechanisms by which local burn injury alters epidermal barrier function in autologous donor skin and surrounding burn margin are largely undefined. We hypothesized that defects in the epidermal cholinergic system may impair epidermal barrier function and innate immune responses. The objective was to identify alterations in the epidermal cholinergic pathway, and their downstream targets, associated with inflammation and cell death. We established that protein levels, but not gene expression, of the α7 nicotinic acetylcholine receptor (CHRNA7) were significantly reduced in both donor and burn margin skin. Furthermore, the gene and protein levels of an endogenous allosteric modulator of CHRNA7, secreted mammalian Ly-6/urokinase-type plasminogen activator receptor-related protein-1 (SLURP1) and acetylcholine were significantly elevated in donor and burn margin skin. As downstream proteins of inflammatory and cell death targets of nAChR activation, we found significant elevations in epidermal High Mobility Group Box Protein 1 (HMGB1) and caspase 3 in donor and burn margin skin. Lastly, we employed a novel in vitro keratinocyte burn model to establish that burn injury influences the gene expression of these cholinergic mediators and their downstream targets. These results indicate that defects in cholinergic mediators and inflammatory/apoptotic molecules in donor and burn margin skin may directly contribute to graft failure or infection in burn patients.
Keywords: burn injury, acetylcholine, SLURP1, caspase 3, HMGB1, nicotinic receptors, skin
Introduction
Burn injury continues to be a significant cause of trauma-related morbidity and mortality, which is frequently related to a burn wound infection or secondary infectious complications (1). Burn-mediated changes in epithelial permeability and inflammatory responses are a hallmark of severe thermal injury of the skin. Patients with deep second degree burns and/or third degree burns often require skin grafts because of the pervasive destruction of the underlying tissue (2, 3). Skin grafts promote accelerated healing of burn wounds, limit scar contracture, minimize invasive infection, and prevent excessive fluid shifts. Autologous grafts taken from distal, unburned skin may display large-scale deterioration and/or functional deficits after grafting, leading to complete graft failure. Significant strides have been made in burn wound care to augment graft survival, including enhanced topical treatments, and prevention and management of infection (4, 5). However, the mechanisms that lead to aberrant inflammatory responses in donor and burn margin skin are poorly understood. In order to develop better therapeutics for burn injury, the mechanisms by which burn injury disrupt the normal inflammatory balance need to be elucidated to improve autologous skin prior to grafting and to minimize graft failure and/or infection. In the skin, the epidermal cholinergic pathway has been found to directly modulate the local inflammatory/antimicrobial response and barrier permeability function (6–8). Furthermore, acetylcholine (ACh) has been shown to be imperative for wound epithelialization by regulating keratinocyte motility and adhesion (9). No studies, however, have examined whether defects in the epidermal cholinergic pathway exist in the context of burn injury.
The skin serves as a major target for the non-neuronal cholinergic anti-inflammatory pathway mediating epidermal innate immunity and permeability barrier function. Keratinocytes provide the foundation for the non-neuronal cholinergic system in the epidermis. The keratinocyte-acetylcholine axis is comprised of enzymes for Ach synthesis (choline acetyltransferase; CHAT) and degradation (acetylcholinesterase; ACHE), which maintain epidermal homeostasis (10). ACh induces epidermal cellular processes through muscarinic ACh receptors and nicotinic ACh receptors, as classified by their respective agonists, muscarine and nicotine. Different nAChR subtypes exist in the differentiated epidermal layers to promote specific keratinocyte functions important for barrier function and wound healing. The cholinergic control mechanisms of keratinocyte migration and differentiation by ACh via the CHRNA7 have been well-described (10, 11). Endogenous cholinergic peptides, such as secreted mammalian Ly-6/urokinase-type plasminogen activator receptor-related proteins (SLURPs), selectively facilitate ACh-dependent keratinocyte locomotion (12), with SLURP1 acting predominantly through CHRNA7 (13). Additionally, SLURP1 has been shown to alter inflammatory pathways in keratinocytes, where SLURP1 deficiencies are associated with hyper-inflammatory conditions (14). SLURP1 can further activate apoptosis in keratinocytes by augmenting caspase 3 activity (9, 14–16).
With regard to inflammation, the neuronal cholinergic anti-inflammatory pathway was originally found to control inflammation by modulating CHRNA7 on cytokine producing cells during infection and tissue damage (17, 18). Our lab identified a novel mechanism for immunomodulation via keratinocyte CHRNA7, whereby its activation enhanced bacterial survival and impaired innate immune responses during bacterial skin infection (19). Activation of keratinocyte CHRNA7 has been shown to block inflammatory cytokine induction and High Mobility Group Box Protein 1 (HMGB1) release (8, 20); HMGB1 is a nuclear factor generally released from necrotic cells (20, 21). In addition, HMGB1 is associated with burn wound progression and zones of necrosis (22).
We previously determined that both the burn margin and autologous donor skin of mice and humans exhibit several defects in epidermal barrier function related to trans-epidermal water loss, epidermal structural proteins, epidermal lipids, antimicrobial peptides (AMPs) (23). Previous studies from our lab also established that the activation of the non-neuronal epidermal cholinergic pathway directly impairs epidermal barrier function and AMP responses (6–8). Together, these data suggest that alterations in the epidermal cholinergic pathway may account for the impaired barrier function and immune responses we observed in donor skin, and may have detrimental consequences on burn wound healing. Although several studies have assessed the role of the neuronal cholinergic pathway (e.g. vagal nerve stimulation) on burn-induced pathology, no studies have assessed whether burn injury may promote defects in epidermal cholinergic mediators as a potential mechanism for burn wound complications (i.e. infection or graft failure). In the present study, we analyzed cholinergic mediators, HMGB1, and caspase 3, in donor and burn margin skin from human burn patients, and compared these results to skin from unburned controls.
MATERIALS AND METHODS
Sample Collection
All protocols were approved by the Institutional Review Board at Loyola University Chicago, Health Sciences Campus. Patients admitted to the Burn Intensive Care Unit (BICU) were excluded from the study under the following criteria: age < 18 years, pre-existing skin disease, previous transplant recipient, pre-existing clinically-evident infection, history of disseminated cancer, major traumatic injury <4 months prior to the burn injury, and/or pre-existing immunodeficiency. The percent total body surface area (% TBSA) of burn, and fluid resuscitation have been previously described (24), and are summarized in Table 1. The following clinical characteristics and outcomes were obtained from the electronic medical records and were entered into a database: age, gender, burn injury mechanism, % TBSA, inhalation injury, wound infection, graft failure, pneumonia, urinary tract infection, sepsis and/or multisystem organ dysfunction (MODS), and mortality. In the operating room, discarded skin samples were obtained from burn patients undergoing routine excision/debridement and skin grafting. The burn margin (partial thickness) was retrieved from an area of skin adjacent to the excised burn wound that appeared grossly normal. Following burn wound excision, a 5-10 mm boundary of grossly normal appearing skin was excised concurrently with the wound. The actual wound was debrided down to viable tissue to promote an optimal wound healing response in the patients, thus generating viable tissue along the burn margin that was consequently excised. Although an individual burn patient may have required several surgeries (see Table 1), and thus provided multiple samples to this study, none of the patients necessitated repeat use of a particular donor site. Per standard surgical protocol, donor skin (partial thickness) was taken from a site distal to the original injury (autograft site). Control skin samples were obtained from patients undergoing elective surgeries (such as breast reduction or panniculectomy). Skin samples were either snap-frozen in liquid nitrogen, placed in Trizol® (Life Technologies), or embedded in optimal cutting temperature (OCT) (Tissue-Tek® O.C.T. ™ Compound) prior to tissue processing. For the subsequent experiments described, the N for each group was variable due to the amount of sample obtained at the operation, and thus, it was not feasible to use every sample for every assay. Sub-groups of the patient samples were utilized at the time of each assay performance and were randomly selected for individual assays while quantities were available.
Table 1.
Patient demographics and clinical information for control and burn subjects. CS= control skin; DS= donor skin; BM= burn margin; N/A= not applicable; TBSA= Total body surface area.
| Sample ID# | Specimen Subtype | Specimen Site | Specimen Collection (Days Post-burn) | Burn Type | Age | Gender | Race | % TBSA |
|---|---|---|---|---|---|---|---|---|
| Control 1 | CS | Abdomen | N/A | N/A | 62 | F | black | N/A |
| Control 2 | CS | Abdomen | N/A | N/A | 68 | M | hispanic | N/A |
| Control 5 | CS | Chest | N/A | N/A | 44 | F | hispanic | N/A |
| Control 7 | CS | Chest | N/A | N/A | 48 | F | black | N/A |
| Control 8 | CS | Chest | N/A | N/A | 57 | F | white | N/A |
| Control 9 | CS | Chest | N/A | N/A | 66 | F | white | N/A |
| Control 10 | CS | Abdomen | N/A | N/A | 44 | M | white | N/A |
| Control 11 | CS | Abdomen | N/A | N/A | 46 | M | white | N/A |
| Control 12 | CS | Chest | N/A | N/A | 52 | F | white | N/A |
| Control 14 | CS | Chest | N/A | N/A | 50 | M | white | N/A |
| Control 15 | CS | Abdomen | N/A | N/A | 33 | M | white | N/A |
| Burn 3N | DS | Leg/thigh | 19 | Flame | 36 | male | black | 31.0% |
| *Burn 5N | DS | Thigh | 1 | Steam | 53 | female | white | 2.4% |
| *Burn 7N | DS | Thigh | 3 | Steam | 53 | female | white | 2.4% |
| Burn 8N | DS | Thigh | 3 | Flame | 57 | male | black | 26.2% |
| Burn 9N | DS | Thigh | 6 | Flame | 42 | female | black | 1.0% |
| Burn 10N | DS | Leg | 7 | Flame | 21 | male | black | 10.0% |
| Burn 11N | DS | Thigh | 6 | Flame | 36 | male | white | 22.0% |
| Burn 13N | DS | Thigh | 5 | Flame | 57 | male | black | 26.2% |
| Burn 42N | DS | Thigh | 2 | Flame | 59 | female | hispanic | 35.0% |
| Burn 43N | DS | Thigh | 3 | Scald | 43 | female | black | 3.8% |
| Burn 44N | DS | Thigh | 2 | Flame | 37 | male | black | 11.0% |
| Burn 49N | DS | Back | 4 | Flame | 59 | female | black | 2.0% |
| Burn 50N | DS | Thigh | 7 | Flame | 39 | male | white | 20.0% |
| Burn 53N | DS | Thigh | 2 | Flame | 28 | male | white | 2.9% |
| Burn 59N | DS | Thigh | 3 | Flame | 24 | female | white | 6.5% |
| *Burn 74N | DS | Shoulder | 5 | Scald | 53 | male | hispanic | 52.0% |
| *Burn 75N | DS | Abdomen | 6 | Scald | 20 | female | black | 35.0% |
| *Burn 76N | DS | Shoulder | 10 | Scald | 53 | male | hispanic | 52.0% |
| *Burn 77N | DS | Arm | 10 | Scald | 20 | female | black | 35.0% |
| Burn 78N | DS | Thigh | 10 | Flame | 30 | male | hispanic | 17.0% |
| Burn 79N | DS | Thigh | 4 | Flame | 54 | female | white | 14.0% |
| Burn 80N | DS | Thigh | 6 | Flame | 54 | male | hispanic | 32.0% |
| Burn 81N | DS | Thigh | 3 | Flame | 47 | male | white | 11.4% |
| Burn 3M | BM | Leg | 19 | Flame | 36 | male | black | 31.0% |
| *Burn 5M | BM | Hand | 1 | Steam | 53 | female | white | 2.4% |
| *Burn 7M | BM | Hand | 3 | Steam | 53 | female | white | 2.4% |
| Burn 8M | BM | Knee/Leg | 3 | Flame | 57 | male | black | 26.2% |
| Burn 10M | BM | Back | 7 | Flame | 21 | male | black | 10.0% |
| Burn 11N | BM | Hand | 6 | Flame | 36 | male | white | 22.0% |
| Burn 13N | BM | Leg | 5 | Flame | 57 | male | black | 26.2% |
| Burn 42M | BM | Buttock | 2 | Flame | 59 | female | hispanic | 35.0% |
| Burn 43M | BM | Thigh | 3 | Scald | 43 | female | black | 3.8% |
| Burn 44M | BM | Chest | 2 | Flame | 37 | male | black | 11.0% |
| Burn 49M | BM | Back | 4 | Flame | 59 | female | black | 2.0% |
| Burn 50M | BM | Thigh | 7 | Flame | 39 | male | white | 20.0% |
| Burn 53M | BM | Calf | 2 | Flame | 28 | male | white | 2.9% |
| Burn 59M | BM | Arm | 3 | Flame | 24 | female | white | 6.5% |
| *Burn 74M | BM | Hand | 5 | Scald | 53 | male | hispanic | 52.0% |
| *Burn 75M | BM | Thigh | 6 | Scald | 20 | female | black | 35.0% |
| *Burn 76M | BM | Arm | 10 | Scald | 53 | male | hispanic | 52.0% |
| *Burn 77M | BM | Arm | 10 | Scald | 20 | female | black | 35.0% |
| Burn 79M | BM | Arm | 4 | Flame | 54 | female | white | 14.0% |
| Burn 81M | BM | Chest | 3 | Flame | 47 | male | white | 11.4% |
Note: Burn samples 5/7, 74/76 and 75/77 are from the same patient. Each patient had two surgeries, which are listed separately.
Quantitative PCR (qPCR)
Skin samples were homogenized in Trizol (Life Technologies), and RNA was extracted per the manufacturer’s instructions. RNA was reverse transcribed using iScript (Bio-Rad) according to the manufacturer’s protocol, and cDNA samples were used to analyze the expression of CHRNA7 (Hs01063373_m1), ACHE (Hs01085739_q1), CHAT (Hs00252848_m1), SLURP1 (Hs04189088_g1), Caspase 3 (Hs00234387_m1), HMGB1 (Hs01923466_g1) (Life Technologies). Quantitative real-time PCR was performed using the TaqMan Gene Expression pre-mix (Life Technologies) and the Applied Biosystems 7000 Sequence Detection System (Foster City, CA). To normalize the mRNA expression levels, the relative expression of β-2-microglobulin (B2MG, Hs99999907_m1) was analyzed in parallel. Results were analyzed using the 2−ΔΔCt method. Fold-change relative to the control group was calculated (default level set at 1). All analyses were performed in duplicate.
Western Blot Analysis
Skin specimens were flash frozen in liquid nitrogen and then pulverized using a liquid nitrogen cooled biopulverizer (Biospec). Powdered tissues were dissolved in RIPA buffer containing 10u/mL Collagenase (Sigma-Aldrich) and were incubated for 1 hour at 37°C. Homogenates were sonicated (Fischer Scientific) on ice for 10 sec and centrifuged at 17,000xG for 5 minutes. Supernatants were transferred to new tubes, and the HALT ® phosphatase and protease inhibitor cocktail (Pierce Biotechnology) was added to the samples. Protein samples were heated at 90°C for 5 minutes prior to separation in a 4–20% gradient minigel (Bio Rad). Proteins in gels were transferred to PVDF membranes and were immunoblotted with the following antibodies: rabbit polyclonal anti-human β2 microblobulin at 1:1000 (Pierce Biotechnology), anti-human CHRNA7 (56 kD band; abcam, #24644) at 1:500, SLURP1 (22 kD band; R&D, #MC6401) at 1:10, and HMGB1 (29 kD band; abcam, #18256) at 1:1000. Goat anti–rabbit HRP, goat anti–mouse HRP, Goat anti–rat HRP (Vector Laboratories) at 1:2500 were used as secondary antibodies. Visualization of the immunoreactive bands was performed by enhanced chemiluminescence (Pierce Biotechnology), and blots were imaged using ChemiDoc MP (Bio-Rad). Band densitometry was analyzed using ImageLab software version 4.0 (Bio-Rad).
Immunohistochemistry (IHC)
Standard IHC was performed on human skin using primary antibodies for CHRNA7 (abcam, #24644) at 1:200, SLURP1 (R&D, #MC6401) at 1:2, ACHE (abcam, #97298) at 1:200, HMGB1 (abcam, #18256) at 1:300, and Cleaved Caspase 3 (CC3) (Cell signaling, #9664) at 1:200. Appropriate secondary antibodies were selected according to primary antibody species and were previously described (15, 23). In brief, tissue samples collected at the time of the operation were mounted in OCT medium. Samples were then sectioned at 8μm using a cryostat, fixed in paraformaldehyde, incubated in blocking buffer, and incubated overnight at 4°C with the appropriate dilution of primary antibody in blocking buffer. The following day, sections were washed and incubated at room temperature with appropriate secondary antibodies conjugated to Cy3 or Alexa-fluor 456. Nuclei were stained using ProLong® Antifade Gold with DAPI (Life Technologies). Photos were taken on an Evos Digital Inverted Microscope using a 20× objective and were analyzed in a blinded manner. IHC was performed for each sample in duplicate.
Enzyme-Linked Immunosorbent Assay and Acetylcholine Detection
HMGB1 (Biotang) levels in skin homogenates were determined by ELISA according to the manufacturer’s recommended protocol. Acetylcholine levels were measured using the Amplex® Red Acetylcholine/Acetylcholinesterase Assay Kit (A12217, Molecular Probes) using the manufacturer’s protocol with slight modification – the addition of 1mM of physostigmine (25) to inhibit native ACHE before ACh measurement. Fluorescence was measured using a fluorescence microplate reader (POLARstar Omega, BMG Labtech) and an excitation range of 530–560nm and emission detection at 590nm. The level of Ach was normalized to the dry weight of the skin homogenate samples (a portion of each homogenate was lyophilized and the weight was measured). These analyses were performed in duplicate.
Keratinocyte in vitro burn model
Normal human epidermal keratinocytes (NHEKs) were obtained from the laboratory of Dr. Mitchell Denning (LUC), and were grown in EpiLife® medium (Gibco, REF MEPI500CA) containing EpiLife Defined Growth Supplement (Gibco, REF S-012-04), L-glutamine (HyClone Laboratory), 100 U/ml of penicillin, and 100 μg/ml of streptomycin. Cells were incubated at 37 °C under standard tissue culture conditions in culture dishes or plates coated with the Coating Matrix Kit (Life Technologies). Cultures were maintained for up to 5 passages. When cells reached 90–100% confluence, the media was removed, and a predetermined area of the cells were burned using a disposable cautery device at ~1500°F (Cardinal Health #65410-181). A sham burn was performed using the cautery device at ambient temperatures (no heat; device in the “off” position). Cells treated with the sham burn were used as controls for these experiments, rather than distal cells in the wells that were subjected to burn injury, because we previously determined that burn injury in humans elicits skin barrier defects in distal unburned skin (24). Thus, unburned control cells were deemed a more appropriate cohort for these studies.
Our in vitro NHEK model was designed to replicate a human 15% TBSA burn injury. This assay was performed in only half of the culture plate well to allow collection of cells from the “margin” and “distal” sites. Margin cells were defined as cells ~10mm from the burn site, and distal cells were from all other locations. In pilot experiments, we initially assessed the cautery-mediated burn injury between 1 vs. 2 vs. 3 sites in the well after 24 hours. We did not observe any gross differences in the development of injury zones surrounding the burn sites, or a significant differences in gene expression of our target genes between 1 vs. 2 vs. 3 sites. Burn times of either 1, 2 or 5 seconds were also assessed; however, a burn of >1 second resulted in excessive cell detachment from the plate. Thus, the cautery device was held in contact with the surface of the cell monolayer for 1 second at 3 separate burn sites within the well using the cautery tip (for either burn or sham injury) to achieve a burn area of ~15%, which represents a moderate to severe burn injury in humans. We initially analyzed 6 and 12 hour time-points, but the zones of injury were not very distinct at these time-points. The 24 hour end time was chosen after initial experiments demonstrated cell detachment of >95% at 48 hours, but demonstrated well-defined zones surrounding the burn site. After 24 hours, cells were collected from the distal and margin areas by scraping cells from each defined site. Released cells were collected and centrifuged down for each site. Margin and distal site collection was rotated in order to ensure that the cell populations were not interspersed. More specifically, margin cells were first scraped and collected, then distal cells were scraped and collected in the first well. In the second well, distal cells were first scraped and collected, then margin cells were scraped and collected. This pattern of alternating margin and distal cell collection was repeated for the remaining 4 wells per treatment group. Experiments were performed in 6 wells per group and repeated 3 times. Cells were then processed for RNA isolation and qPCR analysis, as described above.
Statistical Analysis
All quantitative data are described as mean ± standard error of the mean (SEM). For analysis of the human samples, comparisons between control vs. margin and control vs. donor were performed using a Mann-Whitney test. We did not use a one-way ANOVA, as we were not comparing donor vs. margin. For analysis of the in vitro model data, a two-way ANOVA with a Bonferroni post-test was used. For all evaluations, a p-value ≤0.05 was considered statistically significant. Data analyses were performed using GraphPad Prism (GraphPad Software v5, La Jolla, CA, USA).
RESULTS
Clinical Assessment and Patient Demographics
Donor and burn margin specimens were evaluated from 43 patients admitted to Loyola’s burn intensive care unit (BICU) between 2010–2012. Patient characteristics that represent the general population treated in the Loyola BICU have been previously described (24). In brief, patient ages ranged from 20 to 87 years (mean 47 years, median 46 years). The burn injuries were between 1% to 52% TBSA (mean 18%, median 12.5%). Clinical outcomes for the 43 patients included were as follows: 28% (12 patients) developed pneumonia, 42% (18 patients) developed a wound infection of the donor or burn site, 25% (11 patients) were treated for blood culture positive sepsis, and 11% (3 patients) succumbed to their illness. Control samples were obtained from healthy patients with no chronic diseases or skin disorders, with a mean age of 49 years.
CHRNA7 is reduced, while SLURP1 is increased in donor and burn margin skin
We previously determined that AMP and pro-inflammatory cytokine levels were elevated in donor and burn margin skin (23, 24) and we have demonstrated that activation of nicotinic receptors reduces cutaneous AMP responses during skin infection (8). To determine if burn injury may be escalating inflammation by altering epidermal cholinergic activation, we first assessed CHRNA7 abundance. Although no differences in the CHRNA7 gene (were observed between controls and burn cohorts (Fig. 1A), we did observe a >70% reduction in protein levels of CHRNA7 when analyzing the 56 kDa band, the predicted molecular weight of CHRNA7. (Fig. 1B) in donor and burn margin skin relative to controls, which was statistically significant (p<0.025) (Fig. 1C). We also observed a 48kD band that was primarily present only in burn margin, and an 80kD band that was uniformly expressed within each cohort at levels similar to CHRNA7. These bands likely represent alternative splice-variants, or products of post-translational modification of CHRNA7, as this has been previously described (9, 26, 27). We further confirmed that the reduction in CHRNA7 in donor and burn margin skin was primarily within the epidermis, as most of the staining was confined to the upper layers of the epidermis (e.g. stratum corneum) (Fig. 1D). SLURP1 is an allosteric modulator of the CHRNA7 (28). Therefore, we next assessed whether SLURP1 levels were altered after burn injury. We found that SLURP1 gene expression was significantly elevated in donor skin (p<0.025), but there was no change in the burn margin relative to controls (Fig. 2A). We next assessed SLURP1 protein levels and observed a robust increase in both donor and burn margin skin (Fig. 2B), which was statistically significant (p<0.025) compared to control samples (Fig. 2C).
Fig. 1. Gene expression and protein levels of CHRNA7 in skin from control and burn patients.
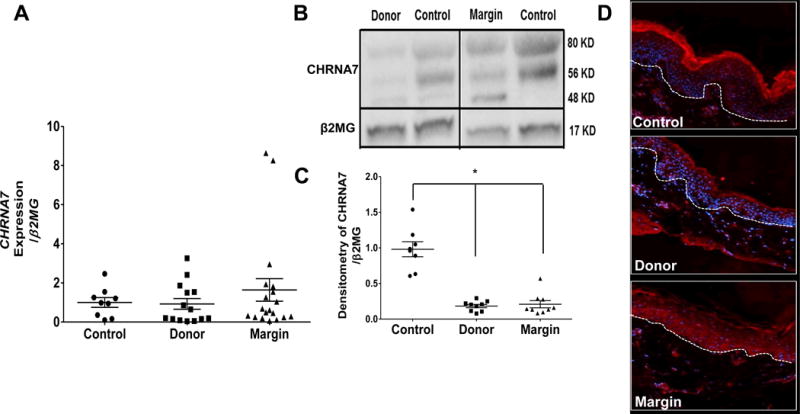
(A) Gene expression of CHRNA7 in control, donor, and burn margin skin; n=9–19/group. (B–C) Protein levels of CHRNA7 in control, donor, and burn margin skin by Western Blot analysis; n=8–9/group. *p< 0.001 vs. control using Mann-Whitney U test. (D) Protein levels of CHRNA7 by IHC; Red: CHRNA7; Blue: DAPI; the white dashed line designates the epidermal-dermal junction; n=10/group. Magnification 20×.
Fig. 2. Gene expression and protein levels of SLURP1 in skin from control and burn patients.
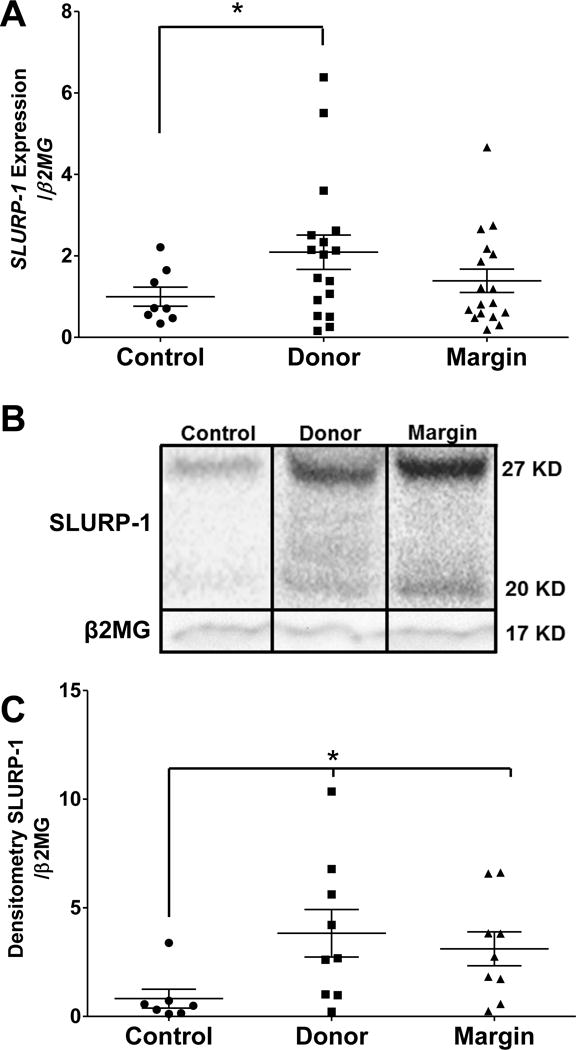
(A) Gene expression of SLURP1 in control, donor, and burn margin skin; n=8–17/group. (B–C) Protein levels of SLURP1 in control, donor, and burn margin skin by Western Blot analysis; n=7–9/group. *p< 0.025 vs. control using Mann-Whitney U test.
ACh production is increased in donor and burn margin skin
Because SLURP1 preferentially binds CHRNA7 in the presence of its natural ligand, ACh (15), we next determined the abundance of ACh and related enzymes in skin samples from human subjects, controls and burn injured. We observed that ACh levels were significantly augmented in both donor and burn margin skin (p<0.025) relative to controls (Fig. 3A). We next assessed the levels of the enzymes responsible for ACh synthesis (CHAT) and ACh degradation (ACHE). Although we were unable to detect CHAT gene expression in our skin samples (data not shown), we did observe that the gene expression of ACHE was significantly reduced in both donor skin and burn margin (p<0.025) (Fig. 3B). We further determined that ACHE protein abundance in the epidermis was diminished in donor and burn margin skin (Fig. 3C), whereas the controls exhibited ACHE staining primarily in the differentiated epidermis (e.g. stratum corneum).
Fig. 3. Acetylcholine (ACh) levels and Acetylcholinesterase (ACHE) gene expression in skin from control and burn patients.
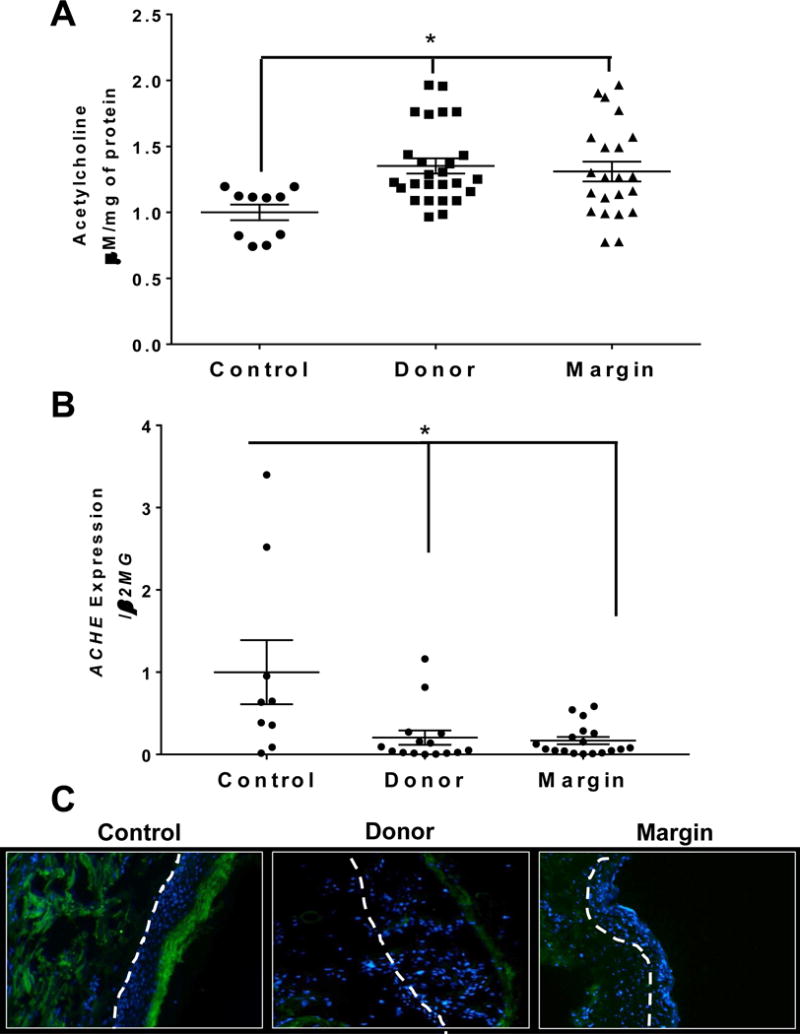
(A) Quantification of ACh levels in control, donor, and burn margin skin; n=10–18/group. *p< 0.025 vs. control using Mann-Whitney U test. (B) Gene expression of ACHE in control, donor, and burn margin skin; n=10–20/group. *p<0.025 vs. control using Mann-Whitney U test. (C) Protein levels of ACHE by IHC staining; Green: ACHE; Blue: DAPI; the white dashed line designates the epidermal-dermal junction; n=10/group. Magnification 20×.
HMGB1 and Caspase 3 are elevated in distal sites
We previously determined that burn injury increases IL-8 gene expression and protein production in both donor and burn margin skin, and increases IL6 gene expression in burn margin (24). HMGB1 is another important inflammatory marker and indicator of cellular necrosis that is regulated by the cholinergic anti-inflammatory pathway, and higher levels are associated with greater mortality in sepsis models (20, 21). Although we did not observe any changes in HMGB1 gene expression between our control and burn cohorts (Fig. 4A), we did identify a significant increase in HMGB1 protein levels by ELISA (p<0.025 and p<0.001) (Fig. 4B) in both donor and burn margin skin, respectively. We further determined that the increase in HMGB1 protein was confined to the epidermis based on IHC analysis (Fig. 4C). Because HMGB1 released from keratinocytes was previously shown to correlate with cleaved caspase 3 (CC3) in the skin (29), we next assessed the gene and protein levels of caspase 3, a key mediator in cellular apoptosis. We determined that CASP3 gene expression was significantly elevated in donor and burn margin skin relative to controls (p<0.025) (Fig. 5A). However, we observed that CC3 was increased in the more differentiated layers of the epidermis (e.g. stratum corneum) in donor skin, as compared to controls, whereas the margin skin exhibited an increase in CC3 primarily in the dermis (Fig. 5B).
Fig. 4. Gene expression and protein levels of HMGB1 in skin from control and burn patients.
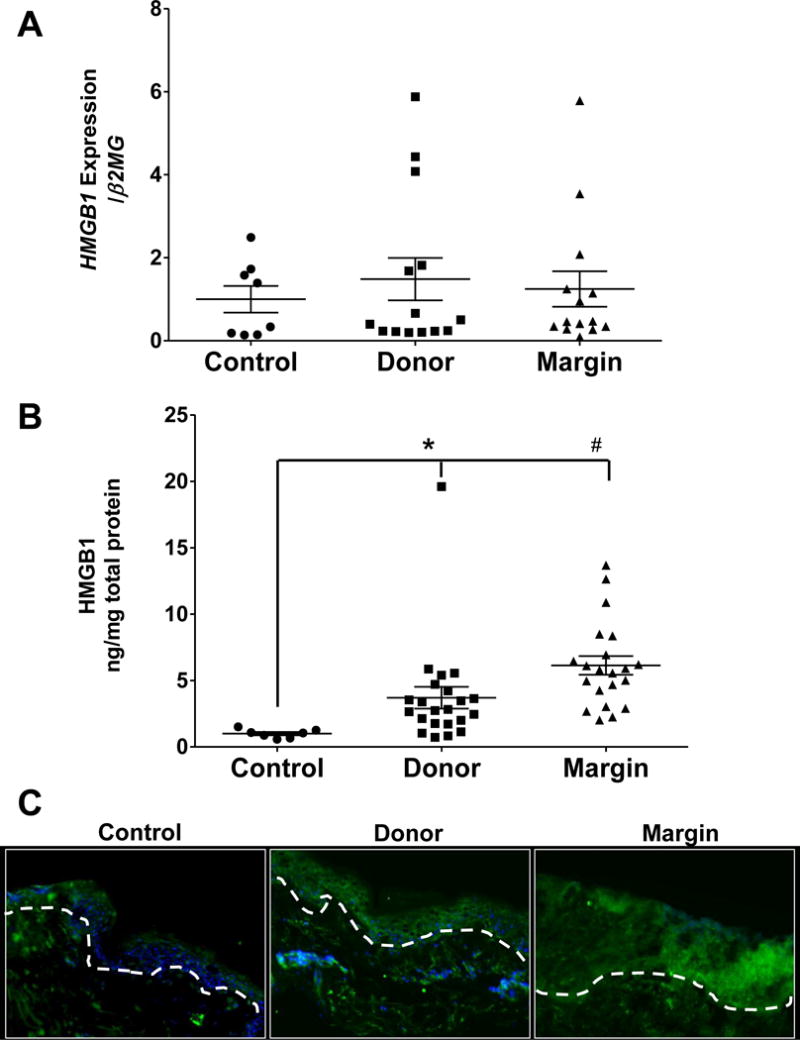
(A) Gene expression of HMGB1 in control, donor, and burn margin skin; n=8–14/group. (B) Protein levels of HMGB1in control, donor, and burn margin skin by ELISA analysis; n=7–22/group. *p<0.025 and #p<0.001 vs. control using Mann-Whitney U test. (C) Protein levels of HMGB1 by IHC staining; Green: HMGB1; Blue: DAPI; the white dashed line designates the epidermal-dermal junction; n=10/group. Magnification 20×.
Fig. 5. Gene expression and protein levels of Caspase 3 in skin from control and burn patients.
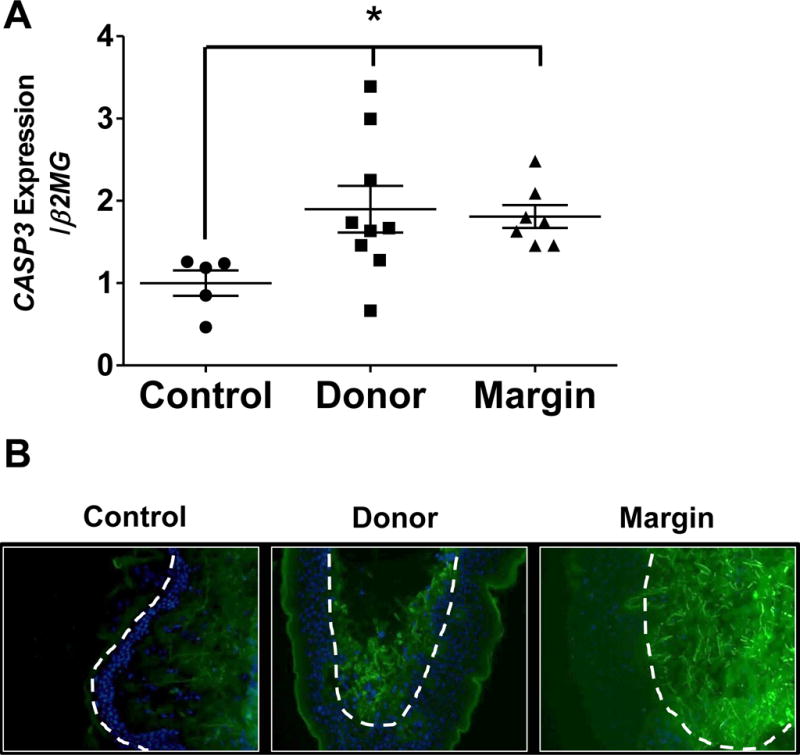
(A) Gene expression of CASP3 in control, donor, and burn margin skin; n=5–9/group. *p<0.025 vs. control using Mann-Whitney U test. (B) Protein levels of cleaved caspase 3 (CC3) by IHC staining; Green: CC3; Blue: DAPI; the white dashed line designates the epidermal-dermal junction; n=10/group. Magnification 20×.
Keratinocytes exhibit alterations in CHRNA7, SLURP1, CASP3, HMGB1, and ACHE gene expression in an in vitro burn injury model
To assess whether human keratinocytes subjected to burn injury would exhibit defects in the gene expression of cholinergic mediators, HMGB1 and caspase 3, we developed an in vitro thermal burn model. Keratinocytes were subjected to sham or thermal injury using a cautery device, and the thermal injury resulted in a burned area from direct contact with the cautery device, a burn margin in the adjacent area, and the remaining area was termed “distal” (analogous to a human autograft donor site) (Fig. 6A). Compared to sham-injured keratinocytes, burn-injured keratinocytes demonstrated a significant decrease in CHRNA7 gene expression in distal cells vs. controls (p< 0.001) (Fig. 6B). In parallel, SLURP1 gene expression was significantly higher in burn margin cells relative to controls (p<0.025) (Fig. 6C). Both CHRNA7 and SLURP1 gene expression patterns were similar to what we observed in human skin after burn injury (Fig. 1A and 2A). No significant changes in ACHE gene expression were observed with distal cells or burn margin cells relative to controls (Fig. 6D), as compared to the significant increase observed in human skin after burn injury (Fig. 3A). HMGB1 gene expression in burn margin cells was significantly reduced relative to controls (Fig. 6E), whereas no significant changes in HMGB1 gene expression were observed in our human skin samples (Fig. 4A). However, CASP3 gene expression was significantly lower in both distal cells and burn margin cells (p< 0.001 and p<0.025 respectively) (Fig. 6F), which did not correlate with the changes in CASP3 gene expression observed in our human skin samples (Fig. 5A).
Fig. 6. Gene expression of CHRNA7, SLURP1, Caspase 3, HMGB1, and ACHE in keratinocytes subjected to in vitro thermal injury.
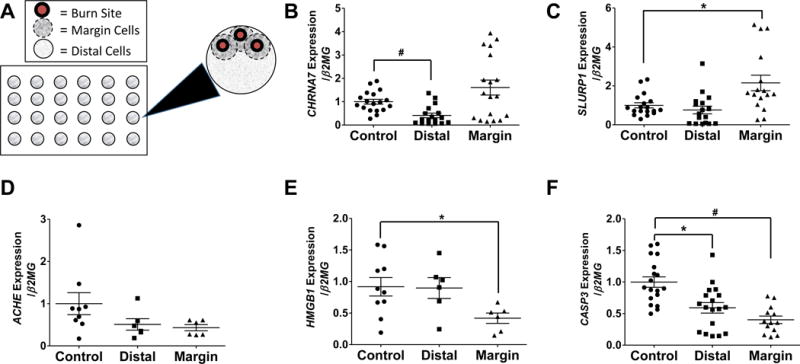
(A) Schematic of the in vitro burn model in a 24 well plate. The large black arrow indicates inset outlining the individual cell regions within each well. Burn cells are shown as red; margin cells are shown inside the dashed line; distal cells are shown outside the dashed line. Gene expression of (B) CHRNA7 (n=12–18/group), (C) SLURP1 (n=12–18/group), (D) HMGB1 (n=6–10/group), (E) CASP3 (n=12–18/group), and (F) ACHE (n=6–10/group), in sham-injured (control) or burn-injured (distal or margin) keratinocyte monolayers after 24 hours; n=12–18/group. *p<0.025 and #p<0.001 using Mann-Whitney U test.
DISCUSSION
Burn wound healing complications at both the donor site and/or the primary burn site augment morbidity and mortality in burn subjects, and are frequently associated with graft failure or a cutaneous infection (1, 4, 5). The current study established that epidermal CHRNA7 production was reduced in both donor and burn margin skin, while SLURP1 and ACh/ACHE production were elevated. These alterations in cholinergic mediators paralleled an increase in two targets of cholinergic activation, HMGB1 and caspase 3, which are indicators of cellular necrosis and apoptosis, respectively. These results were largely recapitulated at the level of CHRNA7 and SLURP1 gene expression in an in vitro keratinocyte model of burn injury, but more divergent changes were observed with ACHE, HMGB1 and CASP3. Together, these data establish that burn injury can promote significant changes in epidermal cholinergic mediators and their downstream targets associated with cell death pathways, which likely contribute to impaired burn wound healing and graft failure/infection. The cholinergic pathway would be of particular interest and a target for topical therapies, since burn injury and other pathologies (e.g., skin cancer reconstruction, diabetes, venous insufficiency, etc.) necessitate skin grafting procedures.
One unique aspect of the epidermal cholinergic pathway is that it can be targeted topically using exogenous nAChR-selective agonists or antagonists to modulate permeability barrier function and/or inflammation (6–8). These agents can be used on potential donor skin prior to grafting, in order to improve barrier function deficiencies. Alternatively, these agents may be useful as a topical adjunct therapy after skin grafting to minimize pathologic inflammation at the burn wound site.
The reduction in epidermal CHRNA7 protein production after burn injury likely impairs several aspects of epidermal barrier function and wound healing. Further studies are needed to determine if burn injury promotes the generation of alternative splice-variants or select post-translational modification of CHRNA7 in burn margin vs. donor skin, as we observed a 48kD band that was primarily present only in burn margin, and an 80kD band that was uniformly expressed within each cohort at levels similar to CHRNA7 (9, 26, 27). Activation of CHRNA7 in acute wounds in vitro or in vivo was previously noted to enhance keratinocyte motility and lateral migration by ionic- and kinase-dependent pathways (30) in a dose-dependent fashion. Both ACh and nicotine, which represent the endogenous or exogenous agonists, respectively, for CHRNA7, were found to promote cell-cell adherence migration of epidermal keratinocytes (11). CHRNA7 activation was also shown to promote keratinocyte differentiation, cell cycle progression, and chemotaxis (9, 31), which are all key components of normal tissue repair. Furthermore, keratinocyte nAChRs are activated by their endogenous ligands, SLURPs, in order to stimulate wound-healing (12) via autocrine/paracrine ACh-mediated responses. By acting as positive allosteric modulators, SLURPs augment the physiologic regulation of keratinocyte responses by ACh. SLURP1 predominantly stimulates the migratory locomotion of keratinocytes via CHRNA7-coupled sedentary integrins, whereas SLURP2 signals via CHRNA3- and CHRNA9-coupled migratory integrins (12). SLURP1 was also found to inhibit TNFα release from keratinocytes and macrophages (28). Since SLURP1 preferentially binds CHRNA7 in the presence of the natural ligand ACh (28), SLURP1 could be used topically to selectively modulate CHRNA7 activation by endogenous ACh, as a means to control local inflammation after burn injury. This may have significant advantages over indiscriminate or direct activation by nicotine or acetylcholinesterase (ACE) inhibitors. The observed increase in the SLURP1 protein and ACh/ACHE ratio in both donor and burn margin skin may suggest a potential compensatory mechanism to augment the CHRNA7-mediated inflammatory response after burn injury in the presence of reduced CHRNA7 levels. The in vitro studies in keratinocytes further emphasize that keratinocytes are a major player in the epidermal cholinergic response to burn injury, and suggest that exploitation of the keratinocyte cholinergic pathway may serve as a novel therapeutic target to improve burn injury outcomes.
Both HMGB1 and caspase 3 are downstream targets of cholinergic activation (20), and the observed increase in epidermal HMGB1 and caspase 3 can be attributed to, in part, diminished CHRNA7 activation following burn injury. Cellular necrosis was previously identified as the most robust mechanism of cell death in burn injury progression, as indicated by early, intermediate, and late staining patterns of HMGB1 in a porcine model of burn injury (32). HMGB1 is a nuclear non-histone binding protein which serves as an early marker of cellular necrosis after burn injury, where its appearance in the cytoplasm, accompanied by the loss of nuclear membrane integrity, distinguishes necrotic cells (33). In contrast, apoptosis was detected in cells at the boundary between necrotic and viable tissue by 24 hours post-burn, as indicated by caspase 3 staining (32). Although the donor and burn margin skin was collected from each burn patient at variable times post-burn in the present study, we did identify a relatively uniform increase in HMGB1 protein expression (in skin homogenates) and epidermal protein abundance in skin (by IHC), as well as caspase 3 at the level of gene expression and epidermal protein abundance in skin (by IHC) in burn patients. Because inflammatory cell numbers appeared to remain stable in donor skin by hematoxylin and eosin staining (data not shown), this may suggest that the keratinocytes are notably contributing to the observed increase in HMGB1 and caspase 3 in skin from burn patients. Of note, because we observed epidermal protein abundance of HMGB1 only by immunostaining, this does not represent secreted HMGB1 that would elicit its pro-inflammatory effect through its interaction with cognate receptors on resident or infiltrating immune cells. The mechanism by which burn injury increases epidermal HMGB1, as well as the translocation of keratinocyte-derived HMGB1 from the nucleus to the cytoplasm and extracellular space warrants further investigation. In parallel with our observed increase in caspase 3 in donor and burn margin skin, SLURP1 was also shown to enhance the activity of caspase 3, suggesting a pro-apoptotic role for SLURP1 in the keratinocyte response to burn injury (15).
In summary, the observed decrease in CHRNA7, accompanied by the increase in SLURP1 and the ratio of ACh/ACHE, in both donor and burn margin skin establishes that burn injury promotes dynamic changes in epidermal cholinergic mediators after burn injury (Figure 7). These alterations likely shift the balance of necrotic and apoptotic mediators at the burn site and in autologous donor skin. Such changes would presumably influence burn wound healing outcomes, including the risk for graft failure, delayed wound healing, and scarring and/or infection. Previous studies assessing HMGB1 and Caspase 3 in burn injury (32, 33), along with data presented here, establish a link between the cholinergic pathway and tissue necrosis/apoptosis after burn injury, as indicated by elevated HMGB1 protein levels and elevated caspase 3 gene expression and epidermal protein levels (assessed by IHC) in the same samples exhibiting defects in cholinergic mediators. Further studies using ex vivo donor and burn margin skin, as well as in vitro burn models, are necessary to directly establish a causal effect of impaired epidermal cholinergic activation on tissue inflammation and necrosis/apoptosis after burn injury. In this study, we ensured that the control sites match the general microenvironment of the donor sites, based on the general microenvironment (sebaceous, moist, dry). Although we did not observe significant differences in cholinergic or cell death mediators when comparing TBSA or skin site, we may observe more robust changes if our patient population was expanded to include >52% TBSA and less similar skin sites (e.g., face, scalp, palmar/plantar regions) in terms of skin microenvironments. Ultimately, by employing topical cholinergic agonists, antagonists, or allosteric modulators in these model systems, we hope to identify novel therapeutic targets and elucidate the molecular mechanisms by which keratinocyte CHRNA7 and SLURP1 modulate tissue necrosis/apoptosis in burn patients as a means to improve skin graft survival and wound healing outcomes.
Fig. 7. Burn injury promotes defects in the keratinocyte non-neuronal cholinergic system and elevates necrosis/apoptosis markers at both the primary injury site and donor site.
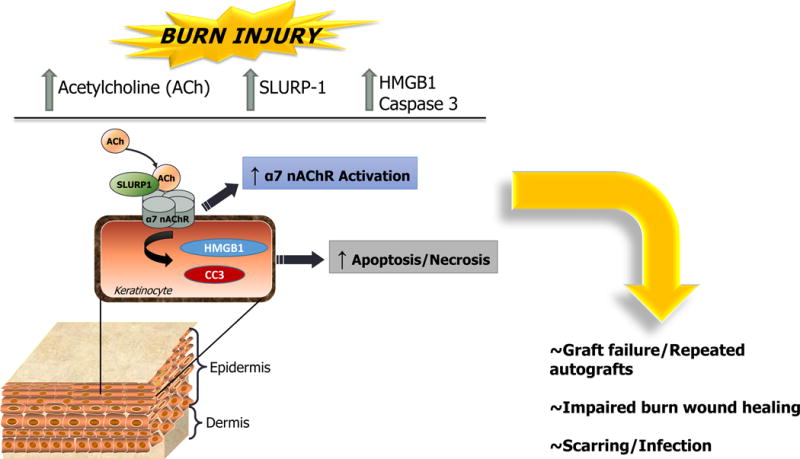
Elevated SLURP-1 and ACh suggest enhanced α7 nAChR activation in donor skin. Elevated HMGB1 in donor skin further indicates tissue necrosis, while elevated cleaved caspase 3 (CC3) in donor skin indicates tissue apoptosis. SLURP-1 may also be influencing epidermal apoptosis and re-epithelialization via activation of pro-apoptotic pathways (e.g. caspase 3). Collectively, these changes in the donor skin suggest that local burn injury may promote tissue damage, necrosis, and/or apoptosis in presumably “normal” tissue.
Acknowledgments
Financial Support: Research reported in this publication was supported by the National Institutes of Health (NIH) grants NIH T32 GM008750 (RLG), NIH R01 AR061497-04 and the Dr. Ralph and Marian C. Falk Medical Research Trust (KAR and RLG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NIH.
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1.Fitzwater J, Purdue GF, Hunt JL, O’Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma. 2003;54(5):959–66. doi: 10.1097/01.TA.0000029382.26295.AB. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JW, MacMillan BG, Law E, Kittur DS. Treatment of severe burns with widely meshed skin autograft and meshed skin allograft overlay. J Trauma. 1981;21(6):433–8. [PubMed] [Google Scholar]
- 3.Phillips TJ, Gilchrest BA. Clinical applications of cultured epithelium. Epithelial Cell Biol. 1992;1(1):39–46. [PubMed] [Google Scholar]
- 4.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn Wound Infections. 2006;19(2):403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saffle JR, Davis B, Williams P. Recent outcomes in the treatment of burn injury in the United States: a report from the American Burn Association Patient Registry. J Burn Care Rehabil. 1995;16(3 Pt 1):219–32. doi: 10.1097/00004630-199505000-00002. discussion 288–9. [DOI] [PubMed] [Google Scholar]
- 6.Curtis BJ, Radek Ka. Cholinergic regulation of keratinocyte innate immunity and permeability barrier integrity: new perspectives in epidermal immunity and disease. The Journal of investigative dermatology. 2012;132(1):28–42. doi: 10.1038/jid.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis BJ, Plichta JK, Blatt H, Droho S, Griffin TM, Radek Ka. Nicotinic acetylcholine receptor stimulation impairs epidermal permeability barrier function and recovery and modulates cornified envelope proteins. Life Sciences. 2012;91:1070–1076. doi: 10.1016/j.lfs.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radek KA, Elias PM, Taupenot L, Mahata SK, O’Connor DT, Gallo RL. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host and Microbe. 2010;7:277–289. doi: 10.1016/j.chom.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernyavsky AI, Arredondo J, Marubio LM, Grando Sa. Differential regulation of keratinocyte chemokinesis and chemotaxis through distinct nicotinic receptor subtypes. Journal of cell science. 2004;117(Pt 23):5665–79. doi: 10.1242/jcs.01492. [DOI] [PubMed] [Google Scholar]
- 10.Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101(1):32–6. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- 11.Grando SA, Horton RM, Pereira EF, Diethelm-Okita BM, George PM, Albuquerque EX, Conti-Fine BM. A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J Invest Dermatol. 1995;105(6):774–81. doi: 10.1111/1523-1747.ep12325606. [DOI] [PubMed] [Google Scholar]
- 12.Chernyavsky AI, Kalantari-Dehaghi M, Phillips C, Marchenko S, Grando SA. Novel cholinergic peptides SLURP-1 and -2 regulate epithelialization of cutaneous and oral wounds. Wound Repair Regen. 2012;20(1):103–13. doi: 10.1111/j.1524-475X.2011.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chernyavsky AI, Arredondo J, Galitovskiy V, Qian J, Grando SA. Upregulation of nuclear factor-kappaB expression by SLURP-1 is mediated by alpha7-nicotinic acetylcholine receptor and involves both ionic events and activation of protein kinases. Am J Physiol Cell Physiol. 2010;299(5):C903–11. doi: 10.1152/ajpcell.00216.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, Huber M, Bertrand D, Hohl D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Human Molecular Genetics. 2003;12(22):3017–3024. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- 15.Arredondo J, Chernyavsky AI, Webber RJ, Grando Sa. Biological effects of SLURP-1 on human keratinocytes. Journal of Investigative Dermatology. 2005;125:1236–1241. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalantari-Dehaghi M, Phillips C, Chernyavsky AI, Marchenko S, Grando SA. Novel cholinergic peptides SLURP-1 and -2 regulate epithelialization of cutaneous and oral wound. 2013;20(1):103–113. doi: 10.1111/j.1524-475X.2011.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201(7):1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 19.Radek KA, Elias PM, Taupenot L, Mahata SK, O’Connor DT, Gallo RL. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host Microbe. 2010;7(4):277–89. doi: 10.1016/j.chom.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature medicine. 2004;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 21.Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. The Journal of experimental medicine. 2012;209(3):551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer AJ, McClain SA, Taira BR, Guerriero JL, Zong W. Apoptosis and necrosis in the ischemic zone adjacent to third degree burns. Acad Emerg Med. 2008;15(6):549–54. doi: 10.1111/j.1553-2712.2008.00115.x. [DOI] [PubMed] [Google Scholar]
- 23.Plichta JK, Droho S, Curtis BJ, Patel P, Gamelli RL, Radek KA. Local Burn Injury Impairs Epithelial Permeability and Antimicrobial Peptide Barrier Function in Distal Unburned Skin. Critical Care Medicine. 2014;42(6):e420–31. doi: 10.1097/CCM.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plichta JK, Holmes CJ, Gamelli RL, Radek KA. Local Burn Injury Promotes Defects in the Epidermal Lipid and Antimicrobial Peptide Barriers in Human Autograft Skin and Burn Margin: Implications for Burn Wound Healing and Graft Survival. J Burn Care Res, May. 2016 doi: 10.1097/BCR.0000000000000357. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grando SA, Kist DA, Qi M, Dahl MV. Human Keratinocytes Synthesize, Secrete, and Degrade Acetylcholine. Journal of Investigative Dermatology. 1993;101(1):32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- 26.Dang X, Eliceiri BP, Baird A, Costantini TW. CHRFAM7A: a human-specific alpha7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS. FASEB J. 2015;29(6):2292–302. doi: 10.1096/fj.14-268037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo P, Cardinale A, Shuller H. A new “era” for the alpha7-nAChR. Curr Drug Targets. 2012;13(5):721–5. doi: 10.2174/138945012800398946. [DOI] [PubMed] [Google Scholar]
- 28.Arredondo J, Chernyavsky AI, Webber RJ, Grando SA. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol. 2005;125(6):1236–41. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- 29.Abdulahad DA, Westra J, Reefman E, Zuidersma E, Bijzet J, Limburg PC, Kallenberg CG, Bijl M. High mobility group box1 (HMGB1) in relation to cutaneous inflammation in systemic lupus erythematosus (SLE) Lupus. 2013;22(6):597–606. doi: 10.1177/0961203313483377. [DOI] [PubMed] [Google Scholar]
- 30.Chernyavsky AI, Arredondo J, Qian J, Galitovskiy V, Grando SA. Coupling of ionic events to protein kinase signaling cascades upon activation of alpha7 nicotinic receptor: cooperative regulation of alpha2-integrin expression and Rho kinase activity. J Biol Chem. 2009;284(33):22140–8. doi: 10.1074/jbc.M109.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arredondo J, Nguyen VT, Chernyavsky AI, Bercovich D, Orr-Urtreger A, Kummer W, Lips K, Vetter DE, Grando SA. Central role of alpha7 nicotinic receptor in differentiation of the stratified squamous epithelium. J Cell Biol. 2002;159(2):325–36. doi: 10.1083/jcb.200206096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanier ST, McClain SA, Lin F, Singer AJ, Clark RA. Spatiotemporal progression of cell death in the zone of ischemia surrounding burns. Wound Repair Regen. 2011;19(5):622–32. doi: 10.1111/j.1524-475X.2011.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirth DA, Singer AJ, Clark RA, McClain SA. Histopathologic staining of low temperature cutaneous burns: comparing biomarkers of epithelial and vascular injury reveals utility of HMGB1 and hematoxylin phloxine saffron. Wound Repair Regen. 2012;20(6):918–27. doi: 10.1111/j.1524-475X.2012.00847.x. [DOI] [PubMed] [Google Scholar]


