Abstract
Cytochrome P450 17A1 (CYP17A1) operates at the core of human steroidogenesis, directing precursors into mineralocorticoids, glucocorticoids, or sex steroids. Although the 17α–hydroxylase and 17,20-lyase activities of this dual function enzyme have been investigated extensively, until recently no CYP17A1 structures were available to inform our understanding. Structures of CYP17A1 with a range of steroidal inhibitors and substrates are now available. This review relates functional knowledge of this enzyme to structural features defining the selective differentiation between its various substrates. While both hydroxylase and lyase substrates have similar orientations with respect to the heme, subtle differences in hydrogen bonding between CYP17A1 and the C3 substituent at the opposite end of ligands appear to correlate with differential substrate utilization and product formation. Complementary structural information from solution NMR supports cytochrome b5 allosteric modulation of the lyase reaction, implicating regions involved in ligand access to the otherwise buried active site.
Keywords: Steroidogenesis; cytochrome P450 17A1; X-ray crystallography; 17α–hydroxylase; 17,20-lyase
1. Introduction
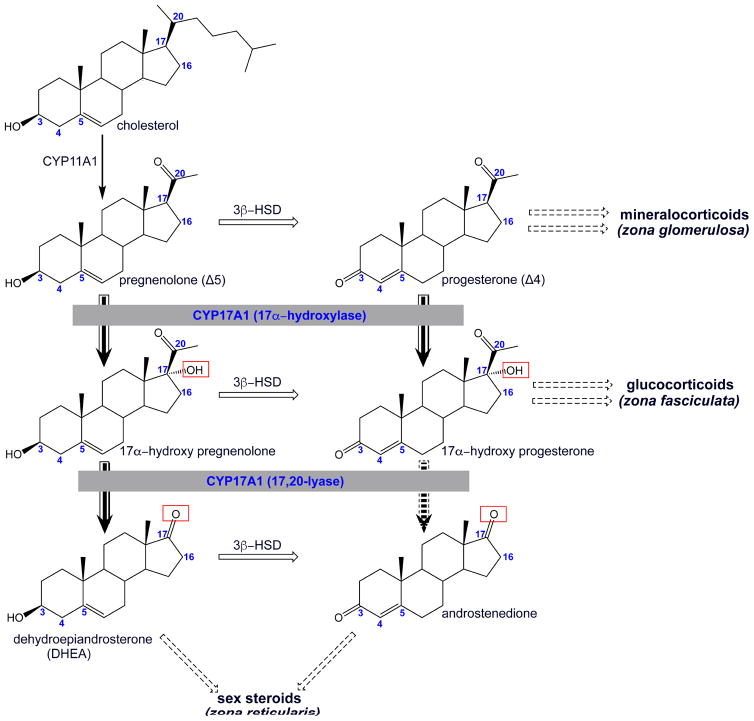
Human gonadal and adrenal steroidogenesis involves a set of six mitochondrial and microsomal cytochrome P450 enzymes (Miller and Auchus, 2011). Of these, cytochrome P450 17A1 (CYP17A1) operates at an intersection between the production of mineralocorticoids, glucocorticoids, and sex steroids (Figure 1). Specifically, CYP17A1 is a dual function enzyme that catalyzes both 17α-hydroxylation and a 17,20-lyase reaction. Canonical 17α-hydroxylase substrates are the Δ5 pregnenolone and the Δ4 progesterone. Both 17α-hydroxy products of this first reaction can dissociate from CYP17A1 and then serve as precursors for glucocorticoid production. The resulting Δ5 17α–hydroxypregnenolone product is also a substrate for the second CYP17A1-mediated reaction. This 17,20-lyase reaction generates the first androgen, the C19 steroid dehydroepiandrosterone (DHEA). In contrast, for human CYP17A1 the Δ4 17α-hydroxyprogesterone is a much less efficient substrate for this lyase reaction, forming little of the corresponding C19 androstenedione (Miller, 2008; Porubek, 2013). As a result, in humans androstenedione is typically generated from DHEA by 3β-hydroxysteroid dehydrogenase rather than directly by CYP17A1. Thus, the fate of 17α-hydroxypregnenolone is determined by the efficiency of the CYP17A1 lyase reaction. Both CYP17A1 reactions are dependent on electron delivery from its redox partner, NADPH-cytochrome P450 reductase (CPR), but the lyase reaction is additionally regulated by the presence of cytochrome b5 (Estrada et al., 2016; Estrada et al., 2014). The combinations of these proteins present in different zones of the human adrenal gland control the steroidogenic outcome. CYP17A1 is not expressed in the outermost zona glomerulosa, thus preventing either reaction so that the Δ5 pregnenolone and Δ4 progesterone are both directed toward mineralocorticoid production, eventually yielding aldosterone. In the middle, zona fasciculata layer of the adrenal cortex, both CYP17A1 and CPR are present, so that the hydroxylated products of pregnenolone and progesterone are directed towards glucocorticoid synthesis, ultimately generating cortisol (Rege et al., 2014). Only in the inner, zona reticularis zone of the adrenal cortex and in gonadal tissues are all three proteins present, such that both hydroxylation and lyase reactions occur to ultimately generate DHEA, the precursor for all subsequent sex steroids. An alternate “backdoor pathway” for androgen production has also been identified in human fetal testis and adults in some pathological conditions. In this pathway, the sequence of other key steroid interconversions is different, but CYP17A1 17-hydroxylation and 17,20-lyase reactions are still required for androgen production (Fukami et al., 2013).
Figure 1.
Schematic representation of the initial steps in the biosynthesis of steroid hormones highlighting the role of CYP17A1 (grey boxes) in adrenal steroidogenesis. CYP17A1 performs a hydroxylation reaction on the Δ5 pregnenolone and the Δ4 progesterone (solid arrow). The respective 17α-hydroxyl forms can either be converted into glucocorticoids in the zona fasciculate or undergo second round to catalysis by CYP17A1. In humans, this second, 17,20-lyase reaction primarily produces DHEA in the zona reticularis (solid arrow), while the lyase reaction producing androstenedione is very inefficient (broken arrow). Broken box arrows summarize subsequent enzymatic steps involving different enzymes in the production of steroid hormones. Relevant carbon numbering is shown on the steroid skeleton.
That CYP17A1 lies at the heart of human steroidogenesis, as described above, has been elucidated through a long series of functional studies too extensive to review here. However, until recently there were no structures of CYP17A1, limiting our ability to understand the rich biochemistry of this enzyme, rationalize the many clinically-relevant mutations, or effectively target its inhibition—as is currently being exploited for the treatment of prostate cancer. Since 2012, however, a wealth of structural information has become available for CYP17A1 with different substrates and inhibitors, information which is beginning to help understand key functional aspects of this long-enigmatic enzyme.
2. Key spectral and biochemical characteristics of CYP17A1
Like all enzymes of the cytochrome P450 superfamily, the CYP17A1 active site contains a heme prosthetic group. These enzymes received their name from the wavelength of the absorption spectroscopy Soret band, which is at 450 nm when the central heme iron is reduced and carbon monoxide is bound to it directly. A useful laboratory tool, this state is not physiologically relevant.
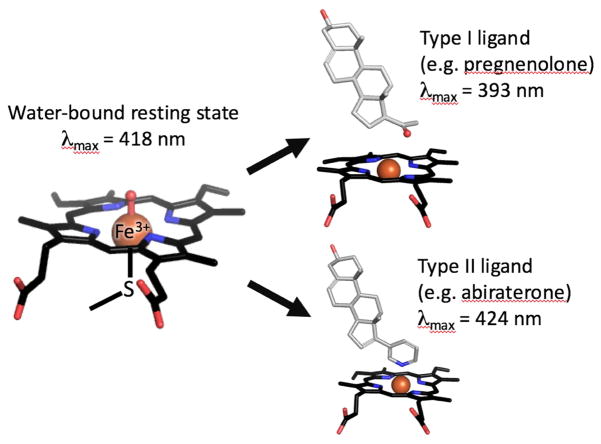
As isolated, the resting state of the CYP17A1 heme iron is typically ferric (Fe3+) and hexacoordinated (Figure 2). In addition to the conserved bonds with four nitrogens in the heme pyrrole rings and the proximal Cys thiolate, the sixth coordination heme-iron of CYP17A1 is bound to water, which is readily observed as an absorbance maximum at 417 nm. When substrates bind in the active site, this water is often displaced and the resulting 5-coordinate system is identified by a blue-shift to ~393 nm (type I ligand). In contrast, iron coordination to a nitrogenous ligand will red-shift the maximum to ~424 nm (type II ligands). These spectral properties have been used to interrogate CYP17A1 substrate and inhibitor binding modes and binding affinities. CYP17A1 incubation with pregnenolone, progesterone, 17α-hydroxypregnenolone, or 17α-hydroxyprogesterone each result in type I spectral shifts, typically because the substrate is binding adjacent to, but not directly to the heme iron (DeVore and Scott, 2012). In contrast, binding of the clinical inhibitor abiraterone results in a type II shift, indicating the pyridine nitrogen coordinates directly to the iron.
Figure 2.
Heme-iron in different coordination state is shown by stick model. Absorbance maxima at 417 nm is representative of water coordinated oxidized heme-iron (resting state), while type I ligand and type II ligand shift absorbance maxima towards blue spectrum (393 nm) and red spectrum (424 nm), respectively.
CYP17A1 catalysis initiates with the displacement of water from hexacoordinated heme-iron, increasing the reduction potential and facilitating CPR reduction of the heme-iron to the ferrous state. The subsequent events in CYP17A1 hydroxylation are typical of P450 reactions overall and are reviewed in detail by Porubek (Porubek, 2013). Briefly, they involve binding of molecular oxygen to the heme iron and a second reduction by CPR to generate three important sequential intermediates (1) a ferric peroxoanion, (2) a ferric hydroperoxo complex, and (3) a ferryl oxo form following cleavage of the oxygen-oxygen bond. In the hydroxylation reaction this latter ferryl oxo species abstracts a hydrogen from the steroid substrate C17 position to form a free radical, which then rebounds to yield the 17α-hydroxy product (Guengerich, 2007).
The CYP17A1-mediated 17,20-lyase reaction is a carbon-carbon bond cleavage that is not typical for P450 enzymes and various key catalytic intermediates have been suggested (Akhtar et al., 2011; Yoshimoto and Guengerich, 2014; Gregory et al., 2013). Recent studies from two research groups have proposed that the peroxyanion species is the important species (Akhtar et al., 2011; Khatri et al., 2014), with the distal oxygen involved in a nucleophilic reaction and homolytic cleavage of the O-O bond resulting in deacetylation and generation of a free carbon radical. Subsequent hydrogen abstraction by the Fe(III)-O complex is proposed to afford the double bond yielding the ketone product. However, there remains support in the field for alternate key catalytic intermediates, including the ferryl oxo involved in hydroxylation.
3. Key structural characteristics of CYP17A1
Human cytochrome P450 enzymes are ~55 kDa proteins anchored in the endoplasmic reticulum via an amino-terminal transmembrane anchor sequence and a secondary membrane-embedded portion of the remaining catalytic domain. When isolated from the membrane these exposed hydrophobic surfaces make structural studies challenging. As a result, the first membrane P450 structure was not solved until 2000. Crystallization of CYP2C5 was enabled by using a construct with a truncated N-terminal membrane anchoring helix and mutated residues in the membrane-binding face of the catalytic domain (Williams et al., 2000). Since then simple deletion and/or modification of the transmembrane helix has been used to produce catalytically-active forms enabling X-ray structures for many membrane P450 enzymes. Notably, crystallization of the full-length steroidogenic CYP19A1 (aromatase) enzyme purified from human placenta yielded a nearly identical structure to the truncated recombinant protein (Cα root mean square deviation of 0.4 Å). These approaches and structures have yielded wealth of structural and functional information about human P450 enzymes, including the first CYP17A1 structures in 2012.
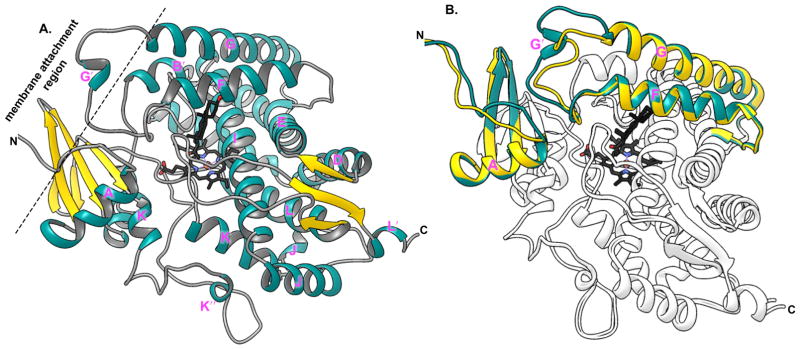
The overall CYP17A1 structure (DeVore et al., 2012; Petrunak et al., 2014) (Figure 3a) is consistent with the high conservation of general topography among P450 enzymes even given large variations in sequence conservation (Pochapsky et al., 2010). This means that the CYP17A1 protein is largely α-helical with twelve major helices (labeled A–L) and a few minor helices (indicated by prime symbols).
Figure 3.
Ribbon representation of crystal structure of CYP17A1 highlighting characteristic structural features. A. Structure of progesterone (black sticks) bound CYP17A1 A105L mutant (PDB id: 4nkx) showing typical P450 structural folds with iron coordinated heme in the active site. B. Overlay of the two molecules of CYP17A1 A105L from one crystal symmetric unit is shown. A/B (shown in dark cyan) and C/D (gold) highlight the variation in the backbone structure of helix F and G structure, which interacts with the ER membrane.
Different P450 enzymes typically demonstrate the greatest structural variation in regions involving substrate binding in the active site and substrate access to this otherwise buried active site (often including the B′ helix and the region between the F and G helices). Different molecules of CYP17A1 also show structural variations, even within a single crystal. Structural differences between the two CYP17A1 conformations occur at N-terminus, a loop between the β1 strands, and the F–G region (Petrunak et al., 2014) (Figure 3b). These regions all lie on the face of the protein thought to interact with the membrane and from which hydrophobic substrates are though to enter (Figure 3a).
Conformational changes in the F–G region have been proposed to permit ligand penetration and egress from the otherwise buried active site (Poulos and Johnson, 2015; De Lemos-Chiarandini et al., 1987). In the soluble bacterial P450 BM3 the presence or absence of substrate-modulated conformational changes were correlated with opening or closing of an access channel from the protein surface into the active site (Ravichandran et al., 1993; Li and Poulos, 1997). Transitions between open and close state was also suggested from the studies on rabbit CYP2B4 crystal structures. In that case the B′ to C region and the F–G region dissociated to form a broad cleft into the active site in the ligand free state (Scott et al., 2003) but were repositioned to close off access in a ligand-bound state (Scott et al., 2004). Thus, the conformational changes observed in this same F–G region of CYP17A1 may similarly be relevant to membrane binding and/or ligand access.
Initial crystal structures of CYP17A1 were determined with the steroidal inhibitors abiraterone and galeterone/TOK-001. Both form a covalent coordinate bond between their respective nitrogen heterocycles and the heme iron, consistent with the type II spectral shifts observed through absorption spectroscopy shifts. The steroidal core packs against the I helix that forms one wall of the active site and makes an angle of 60° from the heme plane. The other notable interaction observed was a hydrogen bond between the the C3 hydroxyl of the inhibitors and the side chain of Asn202 in helix F (DeVore et al., 2012). This work suggested that steroidal substrates might bind in a similar manner and prompted detailed comparisons of CYP17A1 structure and biochemistry.
4. Structural basis of hydroxylation regiospecificity
The regiospecificity of CYP17A1 hydroxylation can vary among species. CYP17A1 enzymes from some higher primates and other mammalian species are able to hydroxylate the Δ4 progesterone at C16 in addition to the canonical hydroxylation at C17. In humans, 10–30% of progesterone gets converted into 16α-hydroxyprogesterone (Petrunak et al., 2014; Arlt et al., 2002; Swart et al., 1993). Notably, the Δ5 pregnenolone is not hydroxylated at C16 (Arlt et al., 2002; Swart et al., 1993; Hough et al., 2013). Although the physiological role of 16α-hydroxyprogesterone biosynthesis is not completely understood, elevated serum and urine levels of 16α-hydroxyprogesterone in humans corresponding to female reproductive status (Stiefel and Ruse, 1969) and disease states such as Cushing’s (Villee et al., 1962; Ward and Grant, 1963; Villee, 1964) and congenital adrenal hyperplasia (Janoski et al., 1969) may suggest an unknown role for 16α-hydroxyprogesterone. Circumstantial evidence for its role also comes from studies correlating abnormal male sexual development with higher serum 16α-hydroxyprogesterone levels (Storbeck et al., 2011). Regardless, the observed regioisomers suggest that the two hydroxylase substrates may bind to CYP17A1 a little differently.
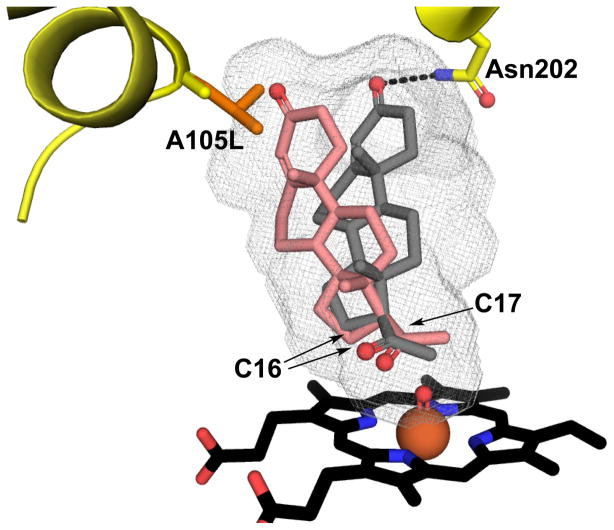
In 2010 Swart et. al. implicated Ala105 in determining the regioselectivity of Δ4 progesterone hydroxylation of steroid by CYP17A1 (Swart et al., 2010). Ala105, is conserved in human and chimpanzee CYP17A1, both of which can form 16α-hydroxyprogesterone. In other mammalian species the corresponding position is occupied by a leucine residues, and these species generate little or no 16α-hydroxyprogesterone product. In human CYP17A1 mutation of Ala105 to leucine significantly decreased production of 16α-hydroxyprogesterone with a simultaneous increase in 17α-hydroxyprogesterone formation (Swart et al., 2010). A homology model suggested that the bulky leucine side chain bordering the active site would create steric hindrance preventing the progesterone from positioning appropriately for the 16α-hydroxylase reaction to occur. (Swart et al., 2010). This prescient suggestion is consistent with recent CYP17A1 structures and additional functional information (Petrunak et al., 2014). First, comparison of CYP17A1 structures with the steroidal inhibitor abiraterone demonstrated that Leu105 did indeed fill up part of the distal active site cavity that was available with Ala105 (Figure 4). Second, a structure of CYP17A1 with progesterone revealed the steroidal substrate with the C3 ketone hydrogen bonding to Asn202 and C17 in a position most consistent with the major 17α-hydroxy metabolite. However, in the wild type enzyme with Ala105 space would also be available in the distal pocket for the C3 end of progesterone to shift toward Ala105, which would also position C16 at the other end of the steroid closer to the heme iron, promoting hydroxylation at this position (Petrunak et al., 2014) (Figure 4). Conversely, the presence of Leu105 would occlude this alternate distal space, likely restricting progesterone hydroxylation to the C17 position.
Figure 4.
Regioselective hydroxylation of progesterone at C16 and 17 by CYP17A1. In the most productive orientation C17 is directed towards iron for 17α–hydroxylation (grey sticks), while an alternate orientation is possible promoting C16 hydroxylation (salmon sticks). Longer side chain of leucine in the case of A105L mutant will fill some of the void in the active site that will create steric hindrance disfavoring 16α–hydroxylation.
Why is pregnenolone not hydroxylated at C16 as well as C17 like progesterone? The more a steroid reorients to place C16 for optimal hydroxylation, the more the bond between the C3 substituent and Asn202 would need to be weakened or disrupted. Of course this is one of two structural changes between progesterone and pregnenolone. The progesterone C3 ketone can only serve as a hydrogen bond acceptor and thus can only bond with the NH of the Asn202 side chain. The C3 hydroxyl of pregnenolone can serve as either hydrogen bond donor or acceptor, so can interact with Asn202 via either the side chain NH or the oxygen. In fact, binding studies support that pregnenolone binds more tightly to the wild type enzyme than progesterone. Thus, stronger, more versatile interactions between Asn202 and the C3 OH of pregnenolone may mean that this ligand does not as readily adopt the second orientation nearer to A105 and thus C16 is not positioned for hydroxylation.
In conclusion, the available structural and functional data support the basic assertion that the sterics of the B′ residue at position 105 and strength of the C3-N202 interactions dictate hydroxylase regioselectivity by modulating steroid positioning between two orientations along its long axis.
5. Pregnenolone vs. progesterone hydroxylation
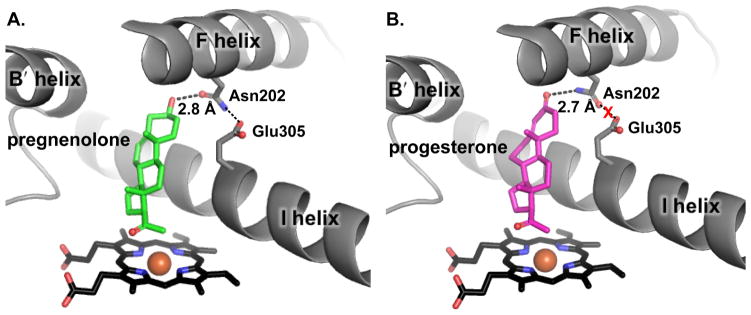
Earlier studies by Auchus et al. (1998) in a yeast system demonstrated that the Vmax for CYP17A1 17α-hydroxylation reaction is within two-fold for the Δ5 pregnenolone vs. Δ4 progesterone substrates (Auchus et al., 1998). Our group observed similar kcat values for 17α-hydroxylation of these Δ5 (0.39 min−1) and Δ4 (kcat ~ 1.0 min−1) steroids by CYP17A1 (Petrunak et al., 2014). However, the Km of pregnenolone was almost 11-fold lower than that for progesterone. Additionally, pregnenolone and its 17α-hydroxylated product bind CYP17A1 with higher affinities (Kd < 100 and 210 nM, respectively) than the progesterone and its 17α-hydroxylated product (Kd ~ 230 and 331 nM, respectively) (DeVore et al., 2012; Petrunak et al., 2014).
Structures of CYP17A1 with both hydroxylase substrates (Figure 5) provided mechanistic insight into these functional observations. Although the orientations of both of steroids were consistent with an orientation favoring hydrogen abstraction from C17, the hydrogen bonding network around Asn202 and pregnenolone (C3 hydroxyl) showed subtle differences in comparison to progesterone (C3 keto) (Petrunak et al., 2014). As stated previously, the C3 hydroxyl of pregnenolone can serve as either hydrogen bond donor or acceptor to the Asn202 side chain, while the C3 keto substituent can only serve as acceptor. When the C3 OH acts as donor to the N202 oxygen acceptor, then the N202 side chain NH is available to donate a hydrogen bond to the side chain oxygens of E305 in the I helix. In contrast, the C3 keto acceptor of progesterone must interact with NH donor of N202, leaving the N202 side chain oxygen acceptor oriented toward the E305 side chain acceptor and preventing formation of that hydrogen bond. The direction of these effects are consistent with the larger dissociation and Michaelis (Km) constants for CYP17A1/progesterone vs. CYP17A1/pregnenolone (Petrunak et al., 2014).
Figure 5.
Substrate selectivity for 17α–hydroxylation reaction by CYP17A1. Pregnenolone and progesterone both show similar orientation with respect to the heme plane but the C3 alcohol of pregnenolone makes a hydrogen bond with Asn202 of CYP17A1, which in turn forms a hydrogen bond with Glu305 indicating higher affinity of pregnenolone for CYP17A1. (B) Although C3 keto of progesterone forms a hydrogen bond with Asn202, but with this arrangement Asn202 cannot simultaneously hydrogen bond with Glu305.
6. 17,20-Lyase: 17α-hydroxypregnenolone vs. 17α-hydroxyprogesterone substrates
One of the most unusual and intriguing aspects of CYP17A1 function is the carbon-carbon bond cleavage between 17 and 20 carbon or 17,20-lyase activity. While very early work suggested two separate proteins carried out hydroxylation and lyase reactions, later work on bovine CYP17A1 demonstrated capacity for both enzymatic reactions (Zuber et al., 1986). The realization that CYP17A1 is a dual function enzyme prompted the suggestion of a “bi-lobed” active site with different ligand orientations for the two catalytic activities. Recent structures of human CYP17A1 do not support this topographical concept (DeVore et al., 2012) (Petrunak et al., 2014), but do provide some insight into the substrate selectivity of the lyase reaction.
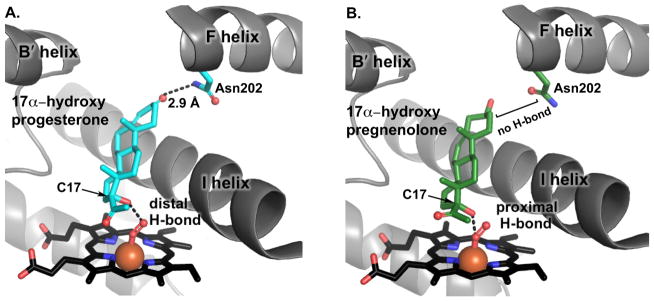
Species-specific selectivity has been observed in the utilization of different substrates for the 17,20-lyase reaction. For example, while the rodent and porcine enzymes can utilize both 17α-hydroxyprogesterone and 17α-hydroxypregnenolone as lyase substrates producing the corresponding androstendione and DHEA, human CYP17A1 utilizes 17α-hydroxypregnenolone as a lyase substrate much more efficiently than 17α-hydroxyprogesterone (Fluck et al., 2003). This is true despite both substrates having similar binding affinities (Petrunak et al., 2014). The underlying basis of this selectivity was addressed in recent studies by Gregory et. al. (2013) and Petrunak et. al. (2014) using resonance Raman spectroscopy and X-ray crystallography, respectively (Gregory et al., 2013; Petrunak et al., 2014). The resonance Raman studies suggested that the C17 hydroxyl of pregnenolone likely hydrogen bonds with the proximal oxygen of Fe-O-O, while the data is consistent with the C17 hydroxyl of progesterone hydrogen bonding to the distal oxygen of Fe-O-O (Gregory et al., 2013).
Recent structures of the CYP17A1 with lyase substrates are mostly consistent with this concept (Petrunak et al., 2014) (Figure 6). Although the A105L mutant was used in these studies, the steroidal inhibitor abiraterone binds identically to both wild type and A105L enzymes. In these structures, both CYP17A1 conformations had the poor lyase substrate 17α-hydroxyprogesterone bound in the active site very similar to the progesterone hydroxylase substrate, with similar distances, angles, and bonding between the C3 keto and Asn202 side chain. However, in the structure of CYP17A1 with 17α-hydroxypregnenolone multiple ligand positions were observed. In one CYP17A1 conformation 17α-hydroxypregnenolone was orientated and positioned as observed for all of the other substrate structures, but the second CYP17A1 conformation had 17α-hydroxypregnenolone located closer to the heme and farther from Asn202. If the heme is modeled with O2 bound, the results are consistent with the resonance Raman data. When located further from the heme the C17 hydroxyl appears most likely to interact with the distal oxygen of the Fe-O-O catalytic intermediate, thereby promoting O-O bond cleavage leading to the canonical ferryl oxo “compound I”. In contrast, when 17α-hydroxypregnenolone is located closer to the heme, the C17 hydroxyl appears most likely to interact with the proximal oxygen of Fe-O-O, thereby promoting the C-C bond cleavage of the lyase reaction (Petrunak et al., 2014). Of course both resonance Raman and crystallographic experiments were conducted in the absence of b5, which solution NMR studies have demonstrated further alter the conformation of CYP17A1 structural units that are likely to control the height and positioning of the substrate (vide infra). Thus, the current proposal is that small changes in the positioning of 17α-hydroxy substrates above the heme iron may control substrate selectivity for the lyase reaction.
Figure 6.
Substrate selectivity for 17,20 lyase reaction by CYP17A1. (A) H-bond between Asn202 and C3 keto of 17α–OH progesterone places the substrate father away from the active site but closer to distal oxygen of peroxy intermediate, an interaction that is not conducive to the lyase reaction (B) In contrast, 17α–OH pregnenolone positions itself closer to proximal oxygen of peroxy intermediate facilitating nucelophilic attack of the distal oxygen on C20 to perform lyase reaction.
7. Structural basis of b5 interaction with CYP17A1
Studies have shown that the kinetics of the human CYP17A1 17,20-lyase reaction are greatly increased in the presence of a membrane bound heme protein, cyt b5 (Miller, 2009; Akhtar et al., 2005). Although, the CYP17A1 is expressed in both the zona faciculata and zona reticularis, the lyase reaction crucial for androgen production proceed efficiently only in the latter compartment, which has higher expression of b5 (Auchus and Rainey, 2004). Another line of evidence suggests that the production of androgenic hormones during adrenarch is driven by the increase in b5 concentrations that occur during this developmental phase (Nguyen et al., 2009). So how does b5 selectively facilitate the lyase reaction?
Although, b5 is thought to be capable of electron delivery to some P450 enzymes and some CYP17A1 experiments also support this idea (Zhang et al., 2008; Duggal et al., 2016), most of the literature on CYP17A1 suggests that b5 instead modulates CYP17A1 activity allosterically (Khatri et al., 2014) (Auchus et al., 1998). Several lines of evidence define the CYP17A1/b5 interface. The anionic b5 residues E48 and E49 were initially identified by mutagenesis (Naffin-Olivos and Auchus, 2006) and further confirmed by a two dimensional NMR binding study (Estrada et al., 2014) to be involved in CYP17A1 binding. As for the CYP17A1 half of the interface, early studies highlighted mutations of residues R347 and R358 in patients with selective 17,20-lyase deficiency and normal 17α-hydroxylation activity (Geller et al., 1997). Subsequent, mutagenesis and NMR studies confirmed that the cationic residues R347, R358, and R449 on the CYP17A1 proximal surface form part of the interface with b5 (Estrada et al., 2014).
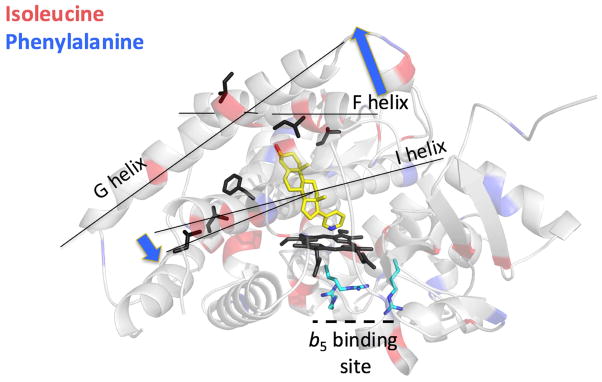
Even though b5 binds on the proximal face of CYP17A1, on the opposite side of the heme from the ligand, NMR results demonstrate that b5 causes changes in the CYP17A1 backbone in distant regions specifically those implicated in ligand access (Figure 7). For example, b5 binding results in conformational shifts for residues in one end of the central I helix and in the F and G helices, both regions known to reorient to promote ligand entry. Overall, this suggests that b5 binding on one side of CYP17A1 might promote retention of 17α-hydroxypregnenolone in the CYP17A1 active site to undergo the subsequent lyase reaction.
Figure 7.
Solution NMR studies tracking CYP17A1 isoleucine and phenylalanine backbone conformations upon binding of b5. Although b5 binds to arginine residues on the proximal face (cyan sticks), conformational changes are noted in the CYP17A1 backbone of residues (black stick side chains) on the opposite side of the heme (black sticks) and ligand (abiraterone, yellow sticks). These residues occur in one end of the central I helix and the F–G helices. Blue arrows indicate the types of motions these regions are observed to undergo in other P450 enzymes where structures of both open and closed ligand channels are available.
Notably, b5 binds in the same general area where CPR must bind to support either hydroxylase or lyase reactions. Consistent with partially overlapping binding sites for CPR and b5, addition of CPR to the preexisting CYP17A1/b5 complex results in the release of b5, as would be required for catalysis (Estrada et al., 2016; Estrada et al., 2014). Although CPR appears to bind with higher affinity to CYP17A1, NMR studies with its FMN-containing domain suggest that its binding does not cause these same structural changes observed in CYP17A1 when b5 binds CYP17A1 (Estrada et al., 2016). Overall, the disparate functional and structural information support an allosteric role for b5, potentially by modulating ligand access.
The importance of understanding the lyase reaction is reflected from the fact that selective lyase inhibitors are now being sought to improve prostate cancer treatment. The first-in-class CYP17A1 inhibitor abiraterone binds directly to the heme iron and fairly nonselectively inhibits both the 17α-hydroxylase and 17,20-lyase activity of CYP17A1. Inhibition of the former activity results in reduction in glucocorticoids and ultimately mineralocorticoid excess. Consequently, the prodrug version, abiraterone acetate, is currently prescribed with synthetic corticosteroid drug, prednisone, to overcome these side effects. Therefore, efforts are being made to develop novel molecules that would specifically block 17,20-lyase reaction without inhibiting the hydroxylase reaction.
8. Conclusion
This review highlights the structural changes in CYP17A1 associated with the substrate binding and accessory protein binding where they have some insights to correlate with the fascinating functional characteristics of this human steroidogenic enzyme. In contrast to early suggestions in the literature, it is now clear that hydroxylase and lyase substrates bind with similar orientations above the heme-iron. Furthermore, the structural data suggests that subtle details of sterics and hydrogen bonding between the C3 substituents and Asn202 likely underlie differential substrate metabolism for the 17α-hydroxylated substrates and the regioselectivity of hydroxylation. Moreover, NMR studies support allosteric control of the lyase reaction by b5 and suggest a potential mechanism via modulation of ligand access. In aggregate the recent structural insights are not only important in dissecting the dual functionality of CYP17A1, as well as have value in drug development of selective lyase inhibitors that may improve the treatment of prostate cancer.
Highlights.
CYP17A1 structures with steroidal substrates and inhibitors are reviewed.
Structures suggest subtle control of substrate positioning above the heme iron.
Cytochrome b5 allosterically alters the conformation of the CYP17A1 active site roof.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 GM076343 (to EES). The University of Kansas Protein Structure Laboratory was supported by National Institutes of Health Grants RR01778 and GM103420. X-ray data collection at the Stanford Synchrotron Radiation Lightsource was supported by National Institutes of Health Grants RR00129 and GM103393.
Footnotes
Conflict of interest statement
The authors declare that they have no competing interests.
Disclosure statement
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL. Steroidogenic enzymes. Endocr Dev. 2008;13:1–18. doi: 10.1159/000134751. [DOI] [PubMed] [Google Scholar]
- Porubek D. CYP17A1: a biochemistry, chemistry, and clinical review. Curr Top Med Chem. 2013;13:1364–1384. doi: 10.2174/1568026611313120002. [DOI] [PubMed] [Google Scholar]
- Estrada DF, Laurence JS, Scott EE. Cytochrome P450 17A1 Interactions with the FMN Domain of Its Reductase as Characterized by NMR. J Biol Chem. 2016;291:3990–4003. doi: 10.1074/jbc.M115.677294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada DF, Skinner AL, Laurence JS, Scott EE. Human cytochrome P450 17A1 conformational selection: modulation by ligand and cytochrome b5. J Biol Chem. 2014;289:14310–14320. doi: 10.1074/jbc.M114.560144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. J Clin Endocrinol Metab. 2014;99:E518–527. doi: 10.1210/jc.2013-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami M, Homma K, Hasegawa T, Ogata T. Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development. Dev Dyn. 2013;242:320–329. doi: 10.1002/dvdy.23892. [DOI] [PubMed] [Google Scholar]
- DeVore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482:116–119. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. Mechanisms of cytochrome P450 substrate oxidation: MiniReview. J Biochem Mol Toxicol. 2007;21:163–168. doi: 10.1002/jbt.20174. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Wright JN, Lee-Robichaud P. A review of mechanistic studies on aromatase (CYP19) and 17alpha-hydroxylase-17,20-lyase (CYP17) J Steroid Biochem Mol Biol. 2011;125:2–12. doi: 10.1016/j.jsbmb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Yoshimoto FK, Guengerich FP. Mechanism of the third oxidative step in the conversion of androgens to estrogens by cytochrome P450 19A1 steroid aromatase. J Am Chem Soc. 2014;136:15016–15025. doi: 10.1021/ja508185d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MC, Denisov IG, Grinkova YV, Khatri Y, Sligar SG. Kinetic solvent isotope effect in human P450 CYP17A1-mediated androgen formation: evidence for a reactive peroxoanion intermediate. J Am Chem Soc. 2013;135:16245–16247. doi: 10.1021/ja4086403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri Y, Gregory MC, Grinkova YV, Denisov IG, Sligar SG. Active site proton delivery and the lyase activity of human CYP17A1. Biochem Biophys Res Commun. 2014;443:179–184. doi: 10.1016/j.bbrc.2013.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Cosme J, Sridhar V, Johnson EF, McRee DE. Mammalian microsomal cytochrome P450 monooxygenase: structural adaptations for membrane binding and functional diversity. Mol Cell. 2000;5:121–131. doi: 10.1016/s1097-2765(00)80408-6. [DOI] [PubMed] [Google Scholar]
- Petrunak EM, DeVore NM, Porubsky PR, Scott EE. Structures of human steroidogenic cytochrome P450 17A1 with substrates. J Biol Chem. 2014;289:32952–32964. doi: 10.1074/jbc.M114.610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochapsky TC, Kazanis S, Dang M. Conformational plasticity and structure/function relationships in cytochromes P450. Antioxid Redox Signal. 2010;13:1273–1296. doi: 10.1089/ars.2010.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos TL, Johnson EF. Structures of Cytochrome P450 Enzymes. In: Ortiz de Montellano P, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. Springer; New York: 2015. pp. 3–32. [Google Scholar]
- De Lemos-Chiarandini C, Frey AB, Sabatini DD, Kreibich G. Determination of the membrane topology of the phenobarbital-inducible rat liver cytochrome P-450 isoenzyme PB-4 using site-specific antibodies. J Cell Biol. 1987;104:209–219. doi: 10.1083/jcb.104.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KG, Boddupalli SS, Hasermann CA, Peterson JA, Deisenhofer J. Crystal structure of hemoprotein domain of P450BM-3, a prototype for microsomal P450’s. Science. 1993;261:731–736. doi: 10.1126/science.8342039. [DOI] [PubMed] [Google Scholar]
- Li H, Poulos TL. The structure of the cytochrome p450BM-3 haem domain complexed with the fatty acid substrate, palmitoleic acid. Nat Struct Biol. 1997;4:140–146. doi: 10.1038/nsb0297-140. [DOI] [PubMed] [Google Scholar]
- Scott EE, He YA, Wester MR, White MA, Chin CC, Halpert JR, Johnson EF, Stout CD. An open conformation of mammalian cytochrome P450 2B4 at 1.6-A resolution. Proc Natl Acad Sci U S A. 2003;100:13196–13201. doi: 10.1073/pnas.2133986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EE, White MA, He YA, Johnson EF, Stout CD, Halpert JR. Structure of mammalian cytochrome P450 2B4 complexed with 4-(4-chlorophenyl)imidazole at 1.9-A resolution: insight into the range of P450 conformations and the coordination of redox partner binding. J Biol Chem. 2004;279:27294–27301. doi: 10.1074/jbc.M403349200. [DOI] [PubMed] [Google Scholar]
- Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. Molecular evolution of adrenarche: Structural and functional analysis of p450c17 from four primate species. Endocrinology. 2002;143:4665–4672. doi: 10.1210/en.2002-220456. [DOI] [PubMed] [Google Scholar]
- Swart P, Swart AC, Waterman MR, Estabrook RW, Mason JI. Progesterone 16 alpha-hydroxylase activity is catalyzed by human cytochrome P450 17 alpha-hydroxylase. J Clin Endocrinol Metab. 1993;77:98–102. doi: 10.1210/jcem.77.1.8325965. [DOI] [PubMed] [Google Scholar]
- Hough D, Cloete SW, Storbeck K, Swart AC, Swart P. Cortisol production in sheep is influenced by the functional expression of two cytochrome P450 17alpha-hydroxylase/17,20-lyase (CYP17) isoforms. J Anim Sci. 2013;91:1193–1206. doi: 10.2527/jas.2012-5800. [DOI] [PubMed] [Google Scholar]
- Stiefel M, Ruse JL. Urinary excretion of 16-alpha-hydroxyprogesterone during pregnancy and the menstrual cycle. J Clin Endocrinol Metab. 1969;29:919–925. doi: 10.1210/jcem-29-7-919. [DOI] [PubMed] [Google Scholar]
- Villee DB, Dimoline A, Engel LL, Villee CA, Raker J. Formation of 16α-hydroxyprogesterone in hyperplastic adrenal tissue. J Clin Endocrinol Metab. 1962;22:726–734. doi: 10.1210/jcem-22-7-726. [DOI] [PubMed] [Google Scholar]
- Ward PJ, Grant JK. The metabolism of [4-14C]-progesterone by adrenocortical tissue from patients with cushing’s syndrome. J Endocrinol. 1963;26:139–147. [Google Scholar]
- Villee DB. Effects of Substrates and Cofactors on Steroid Synthesis in Hyperplastic Adrenals. J Clin Endocrinol Metab. 1964;24:442–455. doi: 10.1210/jcem-24-5-442. [DOI] [PubMed] [Google Scholar]
- Janoski AH, Roginsky MS, Christy NP, Kelly WG. On the metabolism of 16-alpha-hydroxy-C21-steroids. 3 Evidence for high rates of production of 16-alpha-hydroxyprogesterone and 16-alpha-hydroxypregnenolone in the salt-losing form of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1969;29:1301–1309. doi: 10.1210/jcem-29-10-1301. [DOI] [PubMed] [Google Scholar]
- Storbeck KH, Swart P, Africander D, Conradie R, Louw R, Swart AC. 16alpha-hydroxyprogesterone: origin, biosynthesis and receptor interaction. Mol Cell Endocrinol. 2011;336:92–101. doi: 10.1016/j.mce.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Swart AC, Storbeck KH, Swart P. A single amino acid residue, Ala 105, confers 16alpha-hydroxylase activity to human cytochrome P450 17alpha-hydroxylase/17,20 lyase. J Steroid Biochem Mol Biol. 2010;119:112–120. doi: 10.1016/j.jsbmb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Simpson ER, Waterman MR. Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science. 1986;234:1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- Fluck CE, Miller WL, Auchus RJ. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J Clin Endocrinol Metab. 2003;88:3762–3766. doi: 10.1210/jc.2003-030143. [DOI] [PubMed] [Google Scholar]
- Miller WL. Androgen synthesis in adrenarche. Rev Endocr Metab Disord. 2009;10:3–17. doi: 10.1007/s11154-008-9102-4. [DOI] [PubMed] [Google Scholar]
- Akhtar MK, Kelly SL, Kaderbhai MA. Cytochrome b(5) modulation of 17{alpha} hydroxylase and 17–20 lyase (CYP17) activities in steroidogenesis. J Endocrinol. 2005;187:267–274. doi: 10.1677/joe.1.06375. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Rainey WE. Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 2004;60:288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Corbin CJ, Pattison JC, Bird IM, Conley AJ. The developmental increase in adrenocortical 17,20-lyase activity (biochemical adrenarche) is driven primarily by increasing cytochrome b5 in neonatal rhesus macaques. Endocrinology. 2009;150:1748–1756. doi: 10.1210/en.2008-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hamdane D, Im SC, Waskell L. Cytochrome b5 inhibits electron transfer from NADPH-cytochrome P450 reductase to ferric cytochrome P450 2B4. J Biol Chem. 2008;283:5217–5225. doi: 10.1074/jbc.M709094200. [DOI] [PubMed] [Google Scholar]
- Duggal R, Liu Y, Gregory MC, Denisov IG, Kincaid JR, Sligar SG. Evidence that cytochrome b5 acts as a redox donor in CYP17A1 mediated androgen synthesis. Biochem Biophys Res Commun. 2016;477:202–208. doi: 10.1016/j.bbrc.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naffin-Olivos JL, Auchus RJ. Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry. 2006;45:755–762. doi: 10.1021/bi051623y. [DOI] [PubMed] [Google Scholar]
- Geller DH, Auchus RJ, Mendonca BB, Miller WL. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet. 1997;17:201–205. doi: 10.1038/ng1097-201. [DOI] [PubMed] [Google Scholar]