Abstract
Red blood cell transfusions in the setting of trauma is a double edged sword, as it is a necessary component for life-sustaining treatment in massive hemorrhagic shock, but also associated with increased risk for nosocomial infections and immune suppression. The mechanisms surrounding this immune suppression are unclear. Using supernatant from human packed red blood cell (RBC), we demonstrate that clearance of E. coli by macrophages is inhibited both in vitro and in vivo using a murine model of trauma and hemorrhagic shock. We further explore the mechanism of this inhibition by demonstrating that human stored RBCs contain soluble high mobility group box 1 protein (HMGB1) which increases throughout storage. HMGB1 derived from the supernatant of human stored RBCs was shown to inhibit bacterial clearance, as neutralizing antibodies to HMGB1 restored the ability of macrophages to clear bacteria. These findings demonstrate that extracellular HMGB1 within stored RBCs could be one factor leading to immune suppression following transfusion in the trauma setting.
Keywords: High mobility group box 1, Transfusion, Storage lesion, Innate Immunity, Massive Transfusion
Introduction
Transfusion of packed red blood cells (RBCs) has been linked to an increased risk of nosocomial infection and a state of relative immunosuppression (1). Although a necessary part of resuscitation for many patients, RBC transfusion is associated with an increased possibility of ventilator acquired pneumonia (2,3) and secondary infections (4). Some authors have hypothesized that these adverse events are increased in patients undergoing massive transfusion (5), which may be due to a two-hit phenomenon, with injury serving as the first hit and RBC transfusion providing the second hit (6). An area of significant interest and a source of significant controversy is the role of storage duration in outcomes following RBC transfusion. Many of the deleterious effects of transfusion are thought to be amplified in older RBCs and may be related to changes in RBC membranes or in soluble mediators released from RBCs or leukocytes. Prospective and retrospective analyses have suggested an increased risk of mortality (7), multiple system organ failure (MSOF) (5), need for ICU stay (7), and, importantly, an increased risk of nosocomial infection associated with older RBCs compared to younger red cell units (4,8). Although recent randomized, prospective data suggest no difference in outcomes between fresh versus longer stored units, these studies were not performed in the setting of massive transfusion and focused on leukoreduced blood (9, 10).
The constellation of biochemical and mechanical changes that occur in RBCs over the duration of storage has been termed the ‘storage lesion. We and others have argued that at least some of the potential deleterious clinical effects assigned to the storage lesion mirror those seen with excessive innate immune activation (11). Elements within stored RBC units have the capacity to act as damage associated molecular pattern (DAMP) proteins. DAMPs are endogenous mediators of inflammation, which contribute to activation of innate immune pathways. Excessive DAMP release as well as exogenous administration (12) has been linked to negative outcomes. Several DAMPs signal through the critical innate immune receptor toll-like receptor 4 (TLR4), a receptor also linked to host responses to pathogens (13). One such DAMP, and a key regulatory molecule in the host response to infection, is high mobility group box 1 (HMGB1) (12). HMGB1 is a non-histone DNA binding protein that is passively released from dead or dying cells or released by activated cells during inflammation and has an important role in the host inflammatory response (14). In experimental models of trauma, HMGB1 contributes to both pro-inflammatory responses (15) and immunosuppression (16). HMGB1 has been previously shown to be present in stored human RBC units (17); however, the effects of HMGB1 administration as part of transfused RBCs remain unexplored. Previous authors have shown that exogenous HMGB1 can attenuate host response to pathogens (18) through dysregulation of phagocytosis and bacterial clearance (18-20).
In this study we hypothesize that the HMGB1 that accumulates in stored RBC units, when administered exogenously in large quantities such as would be possible in the setting of massive transfusion, can impair microbial clearance. To test this hypothesis, we will examine the in vitro effects of exogenous HMGB1 (both recombinant and contained within stored RBC units) on the phagocytosis of E.coli by macrophages and inflammatory cytokine production. In order to test the in vivo relevance of these findings, we have devised a murine model of trauma and hemorrhage that involves resuscitation of mice with the acellular supernatant of stored human RBCs (containing HMGB1), followed by an infectious challenge to model the scenario of nosocomial infection.
Materials and Methods
Animals
C57BL/6 WT mice (8-12 weeks, male) were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were housed in accordance with University of Pittsburgh (Pittsburgh, PA, USA) and National Institutes of Health (NIH; Bethesda, MD, USA) animal care guidelines in specific pathogen-free conditions with 12-hr light-dark cycles and free access to standard feed and water. All animal experiments were approved conducted in accordance with the guidelines set forth by the Animal Research and Care Committee at the University of Pittsburgh.
Human RBC and macrophage experiments
Following approval of the Institutional Review Board (PRO08010232) at the University of Pittsburgh, whole blood was collected from healthy volunteers following informed consent. Samples were stored in a de-identified fashion in accordance with IRB regulations. Experimental details for individual assays using human cells are outlined below.
Packed RBC supernatant preparation
The fresher (5 day) and older (42 day) packed RBC units preserved in CPD/AS-5 were acquired from the Institute for Transfusion Medicine and the Central Blood Bank with IRB approval (PRO09100378). RBCs were maintained under FDA mandated storage conditions throughout the duration of storage. For analysis, ten (10) units were randomly selected from the indicated day of storage. Stored units underwent gentle centrifugation 2000 × G, 4C] to separate the supernatant from cellular component. The acellular supernatants of these packed RBC units were stored at -80 C for future use. All experiments utilized non-leukoreduced RBC units except for quantification of HMGB1, where, as indicated, leukoreduced PRBC supernatant was included for comparison.
Peritoneal Macrophage Isolation
Brewer thioglycollate (2 ml, 3%; Sigma Aldrich Corp., St. Louis, MO, USA) was injected intraperitoneally (i.p.) in C57BL/6 mice to stimulate macrophage recruitment. After 5 days, a peritoneal wash was performed with 0.2mM RPMI-EDTA solution to collect peritoneal macrophages (PM). Cells were subsequently washed twice with PBS, counted, and suspended in RPMI media. Cells were plated immediately for use.
E. coli Growth and Dilution
E. coli (ATCC 25922, American Type Culture Collection, Manassas, VA) were incubated in trypticase soy broth (TSB) at 37°C for 18 hours. Bacteria were collected by centrifugation and washed twice in PBS. Using spectrophotometry, bacterial stock were diluted to ∼1×108 colony forming units (CFU)/ml. This sample was then serially diluted and inoculated at varying doses as outlined below.
Macrophage Co-culture and Bacterial Clearance experiment
Peritoneal macrophages (PMs) were plated in 96-well plates at a concentration of 5×105 cells/well. PMs were co-cultured for 6 hours with either media alone or 20% D5 or D42 PRBC supernatant at 37°C and 5% CO2. After the 6hr co-culture, ∼1×103 E. coli were added for 2 hours again at 37°C and 5% CO2. After the 2hr incubation, E. coli CFUs were determined by serial dilution of the supernatant and plating on TSB agar plates and counting after 18 hour incubation. For evaluation of the effect of HMGB1, PMs were co-cultured in media with either recombinant HMGB1, generated as we have previously described (15) at concentrations of 1, 2, 5 μg/ml or BSA at similar concentrations as control at 37°C and 5% CO2 for 6 hours followed by E. coli inoculation and determination of bacterial clearance as described above. Finally, PMs were co-cultured in media with either an anti-HMGB1 neutralizing antibody (gift from Dr. Kevin Tracey, Feinstein Institute for Medical Research) at a concentration of 2.5ug/ml or non-immune rabbit IgG (Sigma-Aldrich, St. Louis, MO) control at the same concentration and the bacterial clearance model was carried out as described.
RAW 264.7 Experiments
RAW 264.7 macrophages were cultured and plated in 48-well plates at a concentration of 2×105 cells/well. Macrophages were co-cultured for 3 hours with either media alone or 20% D5 or D42 PRBC supernatant 37°C and 5% CO2. After 3hr co-culture, macrophages were stimulated with Ultrapure lipopolysaccharide (LPS) derived from E. coli (0111:B4, List Biological Laboratories, Inc, Vandell Way, CA) at a concentrations of 0.01ug/ml or media as previously described (19). After a total of 6 hours cells were harvested and RNA extracted for IL-6 and TNF-α expression analysis by RT-PCR.
Quantitative real-time PCR
RNA was isolated from RAW 264.7 macrophages using the RNeasy kit (Qiagen) and reverse transcribed to cDNA using Clontech cDNA synthesis kit (Clontech, Mountain View, CA). Quantitative real-time PCR was performed as previously described using Bio-Rad CFX System using primers verified for IL-6 and TNF-α (21). Expression levels are expressed as fold change relative to the housekeeping gene GAPDH.
Human Macrophage Isolation and Bacterial Clearance
Human monocyte-derived macrophages (HMDM) were prepared from blood of healthy adult volunteers. First, peripheral blood mononuclear cells (PBMC) were fractionated by Ficoll-Paque density gradient centrifugation (GE Healthcare, USA). CD14-positive monocytes were then selected by magnetically labeled beads, according to the manufacturer's instructions, using human CD14 MicroBeads, LS columns (Miltenyi Biotec, Auburn, CA). CD14+ monocytes were cultured and differentiated into macrophages over 9 days in RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin and 10 ng/ml M-CSF (R&D, Minneapolis, MN USA). Cells were grown at 37°C in 5% CO2. Medium was half changed at the 3rd, 5th and 7th day. After 9 days, differentiated macrophages were scraped, resuspended in antibiotic-free RPMI-1640 (10% FBS) and plated in a 96-well plate at a concentration of 1×105 cells/well. The co-culture and bacterial clearance experiments were then conducted as described earlier.
Phagocytosis Assay
The effect of older vs. fresher PRBC supernatant was assessed with the Vybrant Phagocytosis Assay Kit (Thermo Fischer, V-6694). Briefly, per manufacturer's recommendations, PMs were plated on a 96-well plate at a concentration of 1×106 cells/well. PMs were co-cultured with media, D5, or D42 PRBC supernatant as described above for 6 hours. Following co-culture incubation all media was removed and PMs were inoculated with fluorescein-labeled E. coli for 2 hours. Next, trypan blue was added to quench extracellular fluorescent E. coli and the plate was read. The effect of the D5 and D42 supernatant was calculated as a percentage compared to the control media reading (arbitrarily set at 100%).
Trauma and Hemorrhagic Shock (THS) Model
Mice were anesthesized with isofluorane anesthesia. The femoral artery was cannulated with tubing connected to a blood pressure monitor. Polytrauma consisted of bilateral pseduofracture and liver crush injury. As previously described, pseudofracture is performed by injection of 0.15cc of bone matrix, prepared from donor mice tibia and femur that is crushed and suspended in PBS, into the posterior muscles of the thigh (22). Liver crush was performed by a midline laparotomy, exposure of the right middle lobe of the liver, which was crushed 4 times with a 12.5cm curved-hemostat. Hemorrhage was performed by removal of 25% of circulating blood volume (based on 80ml blood/kg body weight) via a closed cardiac puncture with a 1cc syringe and 30-gauge needle. The laparotomy was closed and the mice were allowed to recover for 2 hours. Upon awakening they were given buprenorphine (1mg/kg) for analgesia. After the 2-hour recovery, mice were resuscitated with Ringer's lactate solution (LR), D5 PRBC supernatant, or D42 PRBC supernatant at a volume of three times the hemorrhage volume. Sham mice underwent groin exploration and femoral artery cannulation without polytrauma or hemorrhagic shock.
Second-Hit Model
Twenty-four hours after THS, mice were inoculated via intraperitoneal injection with 5×104 CFU E. coli (23). Twenty-four hours after E. coli inoculation, mice were sacrificed by cardiac puncture technique. Peritoneal washings were obtained, serially diluted, plated on TSB agar plates and cultured overnight at 37°C. The following day colony forming units (CFUs) were counted.
Quantification of HMGB1
HMGB1 concentration was determined from the supernatant of packed RBC via ELISA (Shino-test; Tokyo, Japan). Flow cytometry was performed prior to centrifugation from blood pooled from 3 random samples for FACS analysis of HMGB1 following permeabilization (identified using PE conjugated human anti-HMGB1, 2.5ug/ml (Biolegend, San Diego, CA) expression on leukocytes (identified using FITC anti-CD45, 1ug/ml (Biolegend, San Diego, CA) and RBCs (identified using FITC human anti-235b, 2.5ug/ml (Biolegend, San Diego, CA)). Flow cytometry was performed using a FACSCanto flow cytometer using DIVA software (BD Biosciences).
Statistics
All data are presented as mean ± SD for n ≥ 3 unless stated otherwise in the figure legends. Statistical significance was determined with the 2-tailed Student's t test or 1-way ANOVA with Tukey's post-hoc test using Graph Pad Prism software (GraphPad). A p value of less than 0.05 was considered significant.
Results
RBC supernatant inhibits bacterial clearance and impairs macrophage function
The ability of peritoneal macrophages to clear pathogens was assessed in vitro in the presence of fresher and older RBC supernatants. E. coli were cultured with thioglycollate-stimulated peritoneal phagocytes that were exposed to fresher or older RBC supernatants, while cell culture media treated with storage preservative alone was used as a control. As demonstrated in Figure 1, there was no significant change in microbial clearance from macrophages cultured with fresher RBC supernatant (CFU/ml, values are 107, 697.5±185.5 vs. 755.0±157.0, p=0.304). However, pre-treatment of macrophages with older RBC supernatant significantly impaired the clearance of E. coli, resulting in higher CFUs (indicating less clearance of bacteria) (CFU/ml ×107: 930.6±133.8, p=0.0017 vs. control). Importantly, there were no significant differences in the number of peritoneal macrophages post-treatment, nor were there any significant changes post-incubation with bacteria in any of the treatment groups (data not shown). In order to assess the effects of RBC supernatant on cytokine gene expression by macrophages, we pre-treated isolated macrophages with RBC supernatant as above and then exposed the cells to LPS to induce cytokine message production. As demonstrated in Figure 1 (B,C) using RT-PCR with values relative to GAPDH, supernatants from both fresher and older RBC markedly impaired the gene expression of IL-6 and TNF-α by macrophages, with the effects most pronounced with the older RBC supernatant. Of note, gene expression, as demonstrated by others, does not necessarily correspond to protein production or cytokine release (24). Multiple concentrations of LPS were tested in the system with consistent inhibition of cytokine gene expression by D42 supernatant, although inhibition by D5 supernatant could be overcome when LPS concentration reached 1ug/ml (data not shown).
Figure 1. Supernatant from older RBC units impairs macrophage phagocytosis and cytokine production.
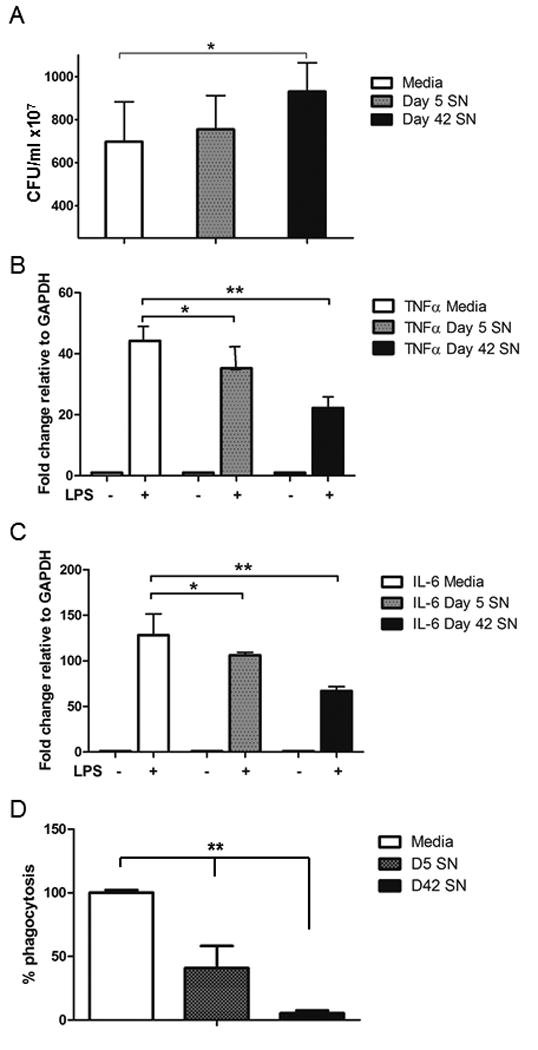
Murine peritoneal macrophages were pre-treated with either media alone, fresher (day 5) RBC supernatant, or older (day 42) RBC supernatant prior to exposure to an inoculum of E. coli. Treatment with older RBC supernatant resulted in a decreased clearance of E. coli as reflected by an increase in the colony forming units (CFU) (A). Pre-exposure to stored RBC supernatant reduced the cytokine gene expression of IL-6 (B) and TNFα (C) in macrophages in response to an LPS challenge as measured by RT-PCR fold change relative to the housekeeping gene, GAPDH. Clearance of bacteria was confirmed to occur through phagocytosis by quantifying the percent internalization of fluorescent labelled E.coli after treatment with D5 or D42 supernatant compared to control (media internalization arbitrarily set at 100% to display differences relative to media control). *p<0.002, **p<0.001. N=pooled data of 3 separate experiments with 3 replicates per group for all assays.
In order to determine whether the impaired clearance of microbes was due specifically to phagocytosis, we utilized a commercially available phagocytosis assay using peritoneal macrophages incubated with fluorescent E. coli to determine the rate of internalization. Pre-incubation with RBC supernatant significantly inhibited phagocytosis of E. coli, with the most pronounced effect occurring in the macrophages exposed to day 42 supernatant (Figure 1d).
Solube factors from older RBC supernatant impair microbial clearance after THS
The transfusion of packed RBC units of older storage age has been shown to increase the infectious complications following trauma (25). Following the identification of impaired clearance of E. coli by macrophages treated with older (42 day old) RBC supernatant, we next sought to explore the in vivo relevance of these findings in a murine model of trauma and hemorrhage. The supernatant of fresher (5 day) or older (42 day) RBC were used as resuscitative fluid in order to evaluate role of soluble mediators in the packed RBC units in bacterial clearance following experimental trauma. The volume of supernatant (15cc/kg) was chosen to reflect a massive transfusion resuscitation to account for the significant blood loss in our model (16). Mice were injected with E. coli at 24 hours following THS. Mice resuscitated with supernatant from older RBC showed impaired clearance of E.coli compared to mice resuscitated with supernatant from fresher RBC or Lactated Ringer's [CFU/ml ×107 Older RBC Supernatant: 36.6±10.2 vs Fresher RBC Supernatant: 17.3±6.8 vs LR: 19.2±4.4, sham (out of box control): 3.7±2.2p<0.05] (Figure 2). There was no difference in survival of animals through the duration of the model.
Figure 2. Transfusion of older human RBC supernatant impairs in vivo clearance of E. coli following murine trauma and hemorrhagic shock as well as clearance by human macrophages.
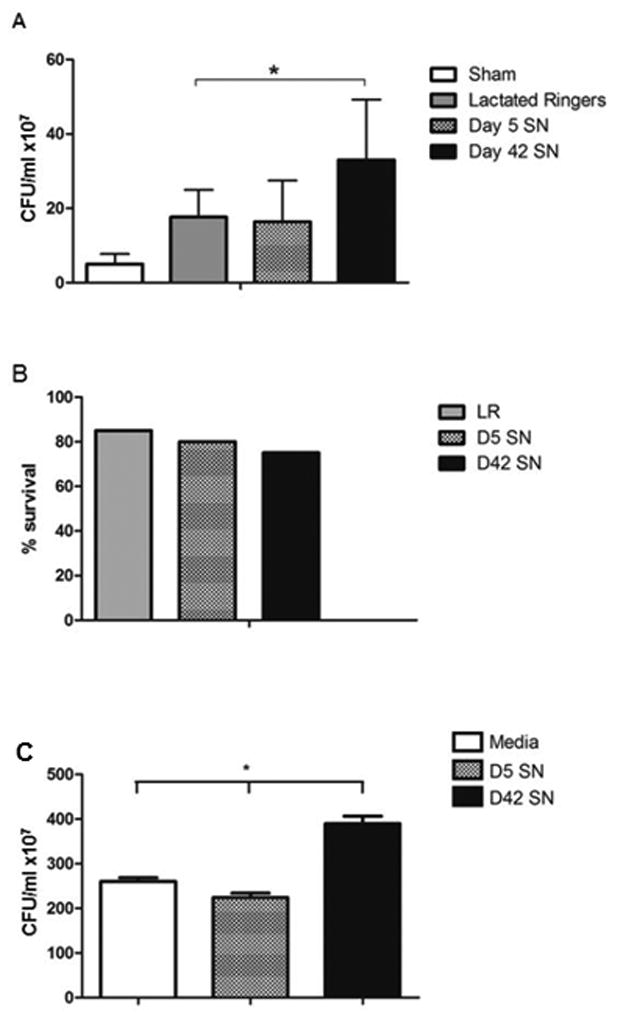
Mice were subjected to a polytrauma model including hemorrhagic shock and resuscitation with either LR or stored human RBC supernatants (SN). Following trauma, E.coli was placed into the peritoneal cavity to mimic an infectious insult, and the clearance of bacteria was assessed by harvesting peritoneal washings. All three experimental groups had a significant increase in residual (non-cleared) E. coli compared to sham. Resuscitation with older (day 42) RBC supernatant led to a marked increase in CFU (indicating a decrease in bacterial clearance) compared to LR (A). There was no difference in survival between groups in the model (B). (C) Human-monocyte derived macrophages were pre-treated with media, D5 SN, or D42 SN prior to exposure to E.coli and clearance was assessed as described. *p<0.05. N=15 mice (5 animals per group, results pooled from 3 separate experiments), **p<0.0001. N=26 per group (6 replicates from 4 separate experiments).
Older blood supernatant inhibits human macrophage clearance of E.coli
Human monocyte-derived macrophages (HMDM) were prepared from blood of healthy adult volunteers and treated with either media alone, day 5 supernatant, or day 42 supernatant. Following incubation with E. coli, microbial clearance was assessed. Similar to the findings using murine macrophages, bacterial clearance was markedly inhibited (resulting in higher measure CFU, or more residual bacteria) following exposure to day 42 supernatant (CFU/ml ×107, media 260.7±39 vs day 5 SN 224.3±54 vs day 42 SN 389.6±98, p<0.0001 for day 42 vs media by ANOVA) (Figure 2c).
HMGB1 is present in RBC supernatants
HMGB1 has been shown to be a key mediator in the pathogenesis of organ injury and a contributor to immunosuppression following trauma and hemorrhagic shock. To determine if extracellular levels of HMGB1 increased with longer RBC storage, HMGB1 levels were measured in supernatants of stored RBC over time. Whole blood was collected from healthy volunteers without leukoreduction and the mean HMGB1 level was determined to be 0.9519 ng/ml as a baseline for circulating levels of HMGB1 prior to blood storage. We found that there was a significant increase in supernatant HMGB1 levels over time (Fresher: 69.147 ng/ml ± 2.2 vs. Older: 238.32 ng/ml ± 13.5, p<0.0001) (Figure 3). In order to assess whether the source of HMGB1 in the RBC supernatants was derived from the leukocyte component of stored RBCs, we performed the same studies in leukoreduced (LR) RBCs and found lower levels of HMGB1. Despite the reduction in concentration, an increase in HMGB1 levels was still observed with storage (Fresher LR: 13.636 ng/ml ± 0.43 vs. Older LR: 87.180 ng/ml ± 2.02, p<0.001). Because HMGB1 release was seen even in leukoreduced RBC we assessed whether HMGB1 could found associated with the actual RBC. FACS analysis for CD-235a allowed for sorting of RBCs, which were stained for HMGB1 and analyzed. Shown in Figure 3 are representative tracings from FACS analysis of permeabilized leukocytes (B) and RBCs from randomly selected, non-leukoreduced day 42 units (C). Leukocytes were chosen as a positive control and express robust levels of HMGB1, which is not associated with RBCs. RBCs of shorter storage duration were also tested and also failed to express HMGB1 (data not shown).
Figure 3. HMGB1 accumulates in stored RBC units.
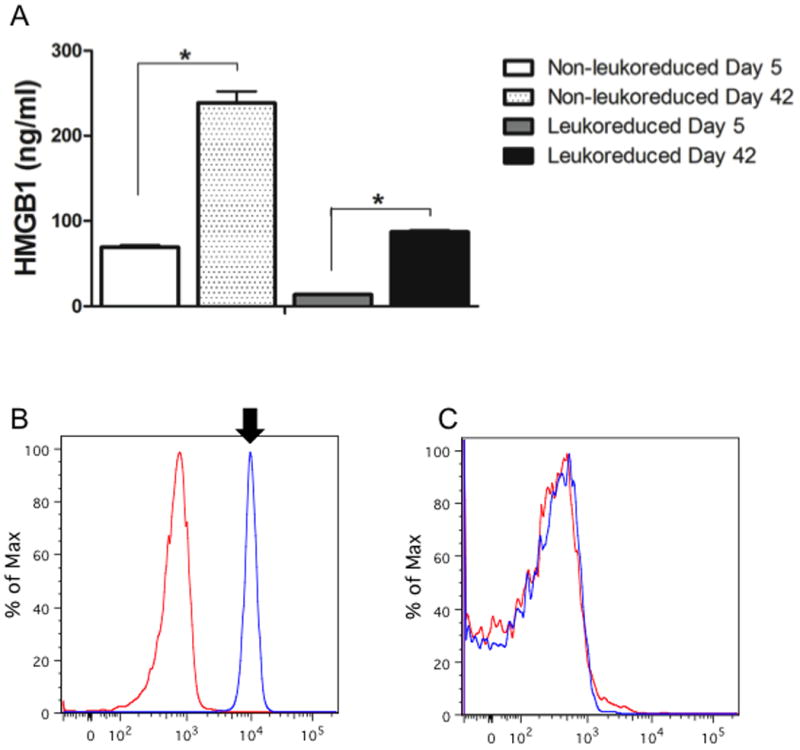
Concentration of HMGB1 was measured by ELISA in the supernatant of leukoreduced and non-leukoreduced RBC supernatant preparations from fresher (storage day 5) and older (storage day 42). Large quantities of HMGB1 were detected within the older RBC supernatant as compared to fresher supernatant, although leukoreduction resulted in a reduction of HMGB1 by approximately 2.5 fold (A). In order to determine the cell type which served as a source of HMGB1 in stored RBC units, flow cytometry was performed utilizing antibody sort for leukocytes (anti-CD45) and RBCs (anti-CD235a). Representative images of FACS sorted leukocytes demonstrated a robust expression of HMGB1 (B, arrow) compared to isotype, while no HMGB1 was detected on permeabilized RBCs (C). *p<0.0001. N=10 pooled units of RBCs per group, results run in triplicate for HMGB1 ELISA.
HMGB1 derived from RBC supernatants inhibits bacterial clearance
It has been previously demonstrated that HMGB1 inhibits phagocytosis in pulmonary leukocytes (19). Given our findings of impaired bacterial clearance after trauma, transfusion, and E. coli infection, and given the observation that HMGB1 accumulates in stored RBC units, we sought to assess whether HMGB1 could inhibit bacterial clearance in peritoneal macrophages. Peritoneal phagocytes were cultured with E. coli after incubating with either recombinant HMGB1 or bovine serum albumin (BSA) as a control protein. As shown in Figure 4a, HMGB1 strongly inhibited bacterial clearance, as measured by an increase in CFUs in macrophages treated with HMGB1. (CFU ×107 BSA: 300.7 ± 15.9 vs. HMGB1: 443.0 ± 14.8, p<0.05). Having confirmed that exogenous administration of HMGB1 can reduce clearance of bacteria, we next sought to remove HMGB1 from the RBC supernatant to see whether we could reverse the effect on bacterial clearance seen with exposure of macrophages to older RBC supernatants. Treatment of older supernatant with a HMGB1 neutralizing antibody resulted in a significant and marked increase in bacterial clearance by peritoneal macrophages (as measured by decreased remaining bacteria/CFU) as compared to IgG control (CFU ×107 IgG: 983.7 ± 52.1 vs. anti-HMGB1: 810.2 ± 46.0, p<0.05). These data support the hypothesis that HMGB1 present in stored RBC units inhibits phagocytosis by peritoneal macrophages.
Figure 4. HMGB1 in stored RBC units regulates phagocytosis of E.coli by isolated peritoneal macrophages.
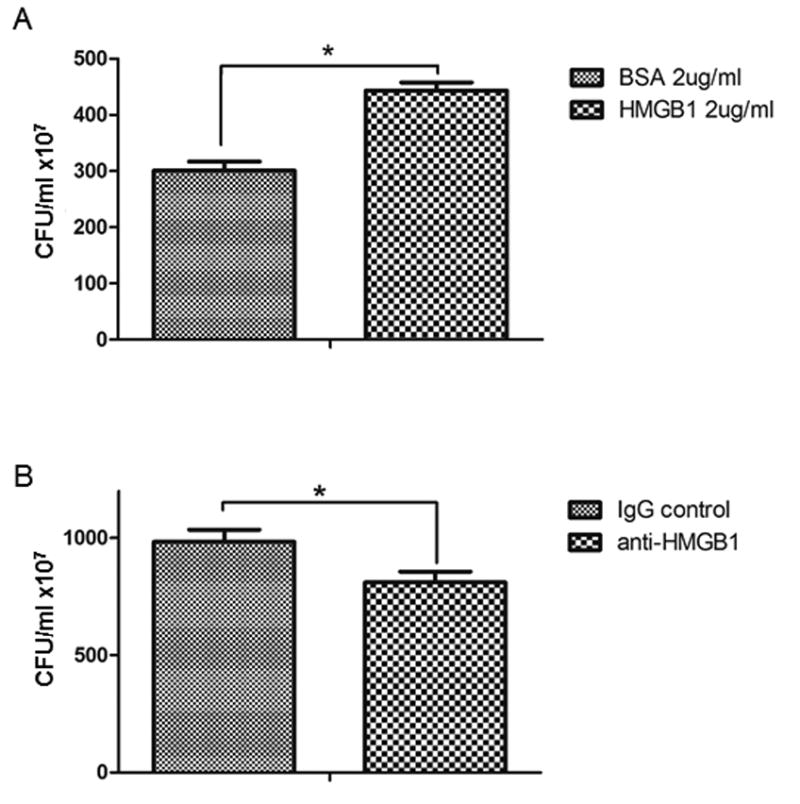
In order to test the specific effects of HMGB1 on macrophage phagocytosis, peritoneal macrophages were treated with recombinant HMGB1 or albumin (BSA) as a control prior to exposure to E.coli. HMGB1 markedly reduced the clearance of bacteria as demonstrated by the increase in CFU (A). In order to specifically implicate HMGB1 as a causative agent in the older (storage day 42) RBC supernatant, the supernatant was treated with either a control antibody (isotype IgG) or a neutralizing antibody directed at HMGB1 (anti-HMGB1). Neutralization of HMGB1 resulted in a significant increase in E.coli clearance as demonstrated by a decrease in CFU relative to control antibody (B). *p<0.05. N=27 (pooled results of 3 separate experiments, 9 samples per group).
Discussion
The transfusion of stored RBC units has been linked to an increase in nosocomial infection in patients following injury (1-4), although the mechanism by which this occurs remains largely unexplored. We and others have previously observed that numerous components of stored RBCs act as innate immune modulators (11,26), and it is well characterized that innate immune signaling on macrophages regulates their function in the setting of trauma (26,27). Here we identify the DAMP, HMGB1 as a potential inhibitor of macrophage phagocytosis following accumulation in stored RBC units. HMGB1 has previously been identified as an inhibitor of phagocytosis of apoptotic neutrophils through the binding of phosphatidyl serine (20) and is well recognized as an important innate immune signal in the setting of trauma (28,29) and sepsis (30). HMGB1 has also been shown to induce pyroptosis in macrophages (31). High concentrations of HMGB1 have been detected early in the inflammatory response to trauma and hemorrhage (12), and multiple animal models have characterized the deleterious effects of exogenous HMGB1 administration (12,28,32). The functional consequence of HMGB1 accumulation in stored RBCs was previously unknown but now appears to contribute to reduced inflammatory signaling and impaired microbial clearance.
In a series of in vitro experiments, we found that pretreatment of isolated murine macrophages with human RBC supernatant impaired E. coli clearance. Following addition of a fixed volume of bacteria, more CFUs of E. coli were obtained from macrophages pretreated with the supernatant of older (42 day old) but not fresher (5 day old) RBC units or control. Recovery of higher CFU counts suggests impaired phagocytosis and killing of bacteria (23). Keeping with the observation that RBC supernatants may impair macrophage function, we identify a reduction in the ability of peritoneal macrophages to express cytokines. Both IL-6 and TNFα gene expression in response to LPS challenge were reduced in both RBC supernatant treatment groups, although the reduction was most pronounced in the older (day 42) supernatant group. We sought to validate these findings in vivo by devising a two-hit model – trauma/hemorrhage followed by intraperitoneal inoculation with E.coli. The two-hit approach of infection following hemorrhage has been utilized previously as a model of in vivo macrophage function in bacterial clearance (24). Resuscitation was performed using LR, fresher RBC supernatant, or older RBC supernatant. HMGB1 was shown to accumulate in stored RBC units, with the most pronounced effect in older RBC units (stored for 42 days). We show here that the source of the HMGB1 is predominantly leukocyte driven. RBCs were shown not to express HMGB1. The mechanism of HMGB1 release could be by leukocyte cell death, either necrosis or pyroptosis or active release following activation. Clearance of bacteria was substantially reduced in mice receiving treatment with older RBC supernatant. The finding of impaired microbial clearance was attributed to HMGB1, as a neutralizing antibody to HMGB1 was sufficient to reverse the increase in CFU, while exogenous administration of HMGB1 to media treated macrophages reproduced the findings seen with stored RBC supernatants. As an initial suggestion of potential relevance beyond the murine studies, we also have shown that day 42 supernatant inhibited bacterial clearance by human macrophages.
Previous authors have validated the use of stored human RBC supernatant in murine studies (16). A two-hit model of murine trauma and intraperitoneal infection allowed for the assessment of bacterial clearance following induction of inflammation, which has previously been shown to have important implications on macrophage function (23). A paucity of literature exists to explore the physiological effects of RBC transfusion on immune function in the setting of massive hemorrhage. The volume of RBC supernatant was chosen to best model a murine ‘massive transfusion.’ The acellular supernatant fraction was chosen as this was the fraction most likely to contain soluble HMGB1, as confirmed by the absence of HMGB1 on RBCs as shown by FACS analysis. Although a smaller volume of HMGB1 was still detected in leukoreduced RBC units, this could still be from residual leukocytes, as leukoreduction techniques have been shown to leave some residual leukocyte component (<5,000,000 WBCs/unit) and/or result in leukocyte injury/death and the potential for HMGB1 release (33). Furthermore, transfusion of the acellular human supernatant minimizes issues of xenogeneic immune activation, allowing for the ability to test the effects of human HMGB1, albeit necessarily in a murine model of trauma. In addition, we pooled 10 units of RBCs to create the supernatant used in these assays in order to minimize issues with inter-donor variability. The identification of potential immunomodulatory substances in stored RBC units may be of importance if the observational data suggesting an increase in nosocomial infection associated with older RBC transfusion persists in randomized prospective analyses of this patient population.
This study has a number of limitations. We sought to use human RBCs in order to assess the potential for inflammatory components within the stored units; however, the effects of human RBC supernatant within a murine system cannot be directly extrapolated to patient care. The finding of similar effect on human macrophages somewhat supports the validity of the observed effect of aged supernatant, however mechanistic studies in human cell lines would be required to understand potential clinical effects in further detail. Additionally, prior authors have shown that gene expression, which we show to be altered by exposure to RBC supernatant, does not necessarily correlate with cytokine release in macrophages (24). Although we used an acellular fraction, issues of immune stimulation cannot be excluded, although one would expect these to be the same for both durations of storage tested. In phagocytosis assays (Figure 1D), the effect of day 5 supernatant was more pronounced than on any of the other clearance assays performed (such as Figure 1A). Potential explanations for this difference include differences in the bacterial strain (the phagocytosis assay required a fluorescent labeled E.coli) as well as different endpoints (CFU versus fluorescent intensity) for the assays. We do not provide a mechanism for the HMGB1 effect in the present analysis. Multiple previous studies have evaluated the role of HMGB1 signaling through known innate immune receptors including TLR4 (12,13,15,28,34,35), TLR2, and the receptor for advanced glycation end products (RAGE) (13). We have recently shown that platelets express HMGB1 (35), which is implicated in thrombus formation and cell signaling, but this effect does not appear to be shared by RBCs according to our findings here. The specific effects of HMGB1 on macrophage phagocytosis are thought to occur through interference of the αvβ3 integrin of phagocytes (20). However, high levels of HMGB1 can also induce pyroptosis in macrophages through a RAGE-dependent mechanism (31). In these assays, we have chosen to focus on clearance of a gram-negative bacterium, which is a common nosocomial pathogen (36). However, our findings may be specific to E. coli and potentially may not be extrapolated to other forms of nosocomial infection; furthermore, it is not explicitly clear if impaired phagocytosis can be causally linked to the development of infection in this setting. In addition, the concentrations of recombinant HMGB1 used in the in vitro assays are substantially higher than measured concentrations in vivo. Although similar to concentrations used in other published studies (35), the need to use higher doses of recombinant protein to elicit an effect likely represents key differences in structure and function between recombinant and endogenous proteins. Nevertheless, the biological potential for stored RBCs to modulate macrophage function through HMGB1 adds to the body of biochemical evidence that defines the storage lesion.
In summary, we described the release of HMGB1 from RBC units throughout the duration of storage in a process largely dependent upon the leukocyte fraction. The accumulated HMGB1 was shown to be biologically active and impaired macrophage function and resulted in impaired microbial clearance in a two-hit model of trauma/hemorrhage and E.coli infection. These findings add to the growing body of literature regarding the effect of RBC storage, with particular emphasis on the role of the leukocyte. Release of inflammatory mediators such as HMGB1 may play a role in innate immune activation following RBC transfusion (11) and could have implications in the development of infectious and inflammatory complications. Strategies to remove HMGB1 from stored RBC could reduce the impact of massive transfusion on immune function. A dedicated study of the clinical outcomes of stored RBC transfusion in the setting of trauma and massive hemorrhage is warranted given the clinical controversy surrounding the effects of RBC storage.
Acknowledgments
The authors would like to acknowledge the contribution of Dr. Rami Namas to the measurement of HMGB1 levels in healthy volunteers as well as Mrs. Lauryn Kohut for her expertise in animal model work.
Source of funding: National Institutes of Health (P50GM053789 to T.R.B)
Footnotes
Address for reprints: same
There are no relevant conflicts of interest in the preparation and creation of this manuscript.
References
- 1.Shorr AF, Jackson WL. Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care. 2005;11(5):468–72. doi: 10.1097/01.ccx.0000176689.18433.f4. [DOI] [PubMed] [Google Scholar]
- 2.Earley AS, Gracias VH, Haut E, Sicoutris CP, Wiebe DJ, Reilly PM, Schwab CW. Anemia management program reduces transfusion volumes, incidence of ventilator-associated pneumonia, and cost in trauma patients. J Trauma. 2006;61(1):1–5. doi: 10.1097/01.ta.0000225925.53583.27. [DOI] [PubMed] [Google Scholar]
- 3.Bochicchio GV, Napolitano L, Joshi M, Bochicchio K, Shih D, Meyer W, Scalea TM. Blood product transfusion and ventilator-associated pneumonia in trauma patients. Surg Infect (Larchmt) 2008;9(4):415–22. doi: 10.1089/sur.2006.069. [DOI] [PubMed] [Google Scholar]
- 4.Juffermans NP, Prins DJ, Vlaar AP, Nieuwland R, Binnekade JM. Transfusion-related risk of secondary bacterial infections in sepsis patients: a retrospective cohort study. Shock. 2011;35(4):355–9. doi: 10.1097/SHK.0b013e3182086094. [DOI] [PubMed] [Google Scholar]
- 5.Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010;137(1):209–20. doi: 10.1378/chest.09-0252. [DOI] [PubMed] [Google Scholar]
- 6.Hod EA, Zhang N, Sikol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT study: Anemia and blood transfustion in the critically ill –current clinical practice in the United States. Crit Care Med. 2004;32(1):39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 8.Vandromme MJ, McGwin G, Jr, Marques MB, Rue LW, 3, Weinberg JA. Transfusion and pneumonia in the trauma intensive care unit: an examination of the temporal relationship. J Trauma. 2009;67(1):97–101. doi: 10.1097/TA.0b013e3181a5a8f9. [DOI] [PubMed] [Google Scholar]
- 9.Lacroix J, Hebert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, Clayton L, McIntyre L, Callum J, Turgeon AF, Blajchman MA, Walsh TS, Stanworth SJ, Campbell H, Capellier G, Tiberghian P, Bardiax L, van de Watering L, van der Meer NJ, Sabri E, Vo D, ABLE Investigators; Canadian Critical Care Trials Group N Engl J Med. 2015;372(15):1410–18. [Google Scholar]
- 10.Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, Granger S, Bennett-Guerrero E, Blajchman MA, Scavo V, Carson JL, Levy JH, Whitman G, D'Andrea P, Pulkrabek S, Ortel TL, Bornikova L, Raife T, Puca KE, Kaufman RM, Nuttall GA, Young PP, Youssef S, Engelman R, Greilich PE, Miles R, Josephson CD, Bracey A, Cooke R, McCullough J, Hunsaker R, Uhl L, McFarland JG, Park Y, Cushing MM, Klodell CT, Karanam R, Roberts PR, Dyke C, Hod EA, Stowell CP. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 372(15):1419–29. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal MD, Raval JS, Triulzi DJ, Simmons RL. Innate immune activation after transfusion of stored red blood cells. Transfus Med Rev. 2013;27(2):113–8. doi: 10.1016/j.tmrv.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aneja RK, Tsung A, Sjodin H, Gefter JV, Delude RL, Billiar TR, Fink MP. Preconditioning with high mobility group box 1 (HMGB1) induces lipopolysaccharide (LPS) tolerance. J Leukoc Biol. 2008;84(5):1326–34. doi: 10.1189/jlb.0108030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson U, Tracey KJ. HMGB1 in sepsis. Scand J Infect Dis. 2003;35(9):577–84. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- 15.Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1538–44. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 16.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peltz ED, Moore E, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32(1):17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, Javdan M, Chavan SS, Miller EJ, Tracey KJ, Mantell LL. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas Aeruginosa pneumonia in cystic fibrosis. Mol Med. 2012;18(1):477–85. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel VS, Sitapara, Gore A, Phan B, Sharma L, Sampat V, Li JH, Yang H, Chavan SS, Wang H, Tracey KJ, Mantel LL. High Mobility Group Box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory ling injury in mice. Am J Respir Cell Mol Biol. 2013;48(3):280–7. doi: 10.1165/rcmb.2012-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friggerl A, Yang Y, Banerjee S, Park YJ, Liu G, Abraham E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am J Physiol Cell Physiol. 2010;299(6):C1267–76. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11(4):305–12. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 22.Darwiche SS, Kobbe P, Pfeifer R, Kohut L, Pape H, Billiar T. Pseudofracture: An Acute Peripheral Trauma Model. J Vis Exp. 2011;(50):e2074. doi: 10.3791/2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maung AA, Fujim S, MacConmara MP, Tajima G, McKenna AM, Delisle AJ, Stallwood C, Onderdonck AB, Mannick JA, Lederer JA. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J Immunol. 2008;180(4):2450–8. doi: 10.4049/jimmunol.180.4.2450. [DOI] [PubMed] [Google Scholar]
- 24.Zellweger R, Zhu XH, Wichmann MW, Ayala A, DeMaso CM, Chaudry IH. Prolactin administration following hemorrhagic shock improves macrophage cytokine release capacity and decreases mortality from subsequent sepsis. The Journal of Immunology. 1996;157:5748–5754. [PubMed] [Google Scholar]
- 25.Belizaire RM, Makley AT, Campion EM, Sonnier DI, Goodman MD, Dorlac WC, Friend LA, Lentsch AB, Pritts TA. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S128–33. doi: 10.1097/TA.0b013e3182606301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hod EA. Spitalnik: Stored red blood cell transfusions: Iron, inflammation, immunity, and infection. Transfus Clin Biol. 2012;19(3):84–9. doi: 10.1016/j.tracli.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564:83–8. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178(10):6573–80. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 29.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14(7-8):476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51(2):119–26. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Jiang Y, Wang J, Shi X, Liu Q, Liu Z, Li Y, Scott MJ, Xiao G, Li S, Fan L, Billiar TR, Wilson MA, Fan J. Macrophage endocytosis of high-mobility group box 1 triggers pyrooptosis. Cell Death Differ. 2014;(8):1229–39. doi: 10.1038/cdd.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, Bauer PM. High Mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med. 2013;18(1):1509–18. doi: 10.2119/molmed.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma RR, Marwaha N. Leukoreduced blood components: Advantages and strategies for its implementation in developing countries. Asian J Transfus Sci. 2010;4(1):3–8. doi: 10.4103/0973-6247.59384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J exp med. 2007;204(12):2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogel S, Bodenstein R, Chen Q, Feil S, Feil R, Rheinlaender J, Schaffer TE, Bohn E, Frick JS, Borst O, Munzer P, Walker B, Markel J, Csanyi G, Pagano PJ, Loughran P, Jessup ME, Watkins SC, Bullock GC, Sperry JL, Zuckerbraun BS, Billiar TR, Lotze MT, Gawaz M, Neal MD. J Clin Invest. 2015 Dec;125(12):4638–54. doi: 10.1172/JCI81660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]


