Abstract
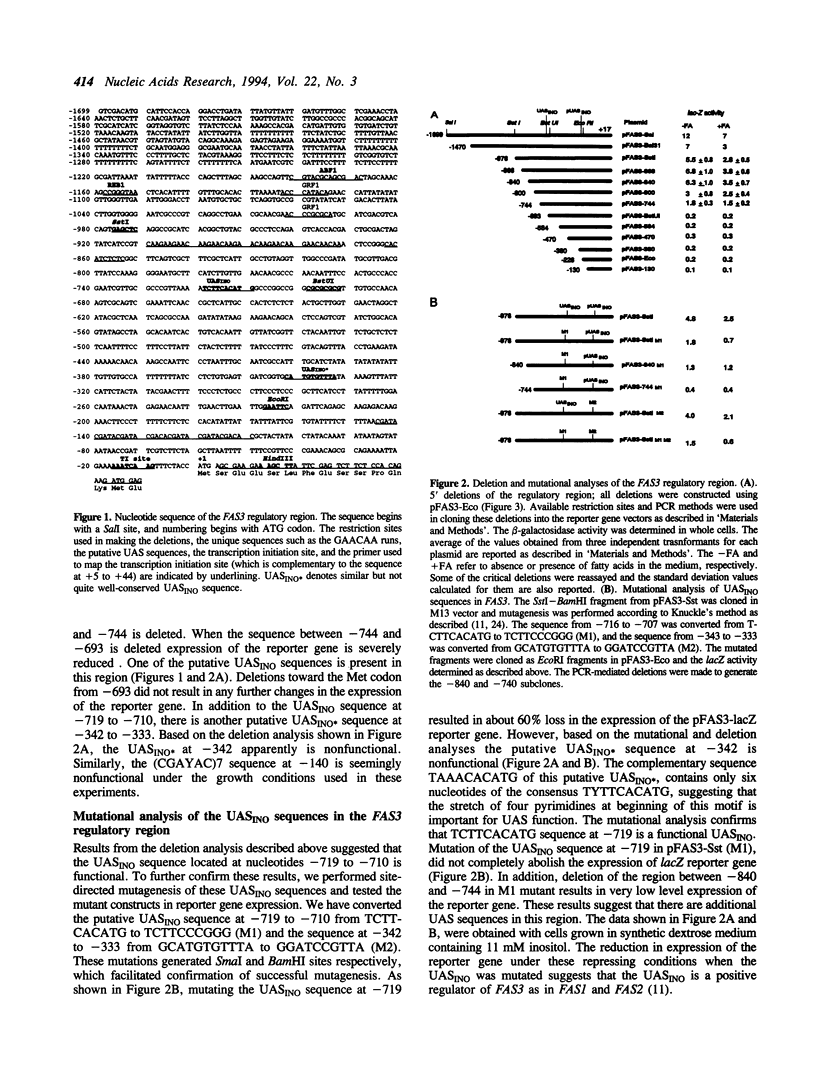
We have determined the sequence of the FAS3/ACC regulatory region and mapped the transcription initiation site. In this sequence, there are two putative UASINO sequences. Deletion and mutation analyses revealed that the UASINO sequence at nucleotides -719 to -710 is functional. The expression of FAS3-lacZ reporter genes and the measurement of mRNA levels in regulatory mutants of phospholipid biosynthesis clearly indicated that FAS3 is regulated by inositol and choline. Previous studies have shown that the genes coding for fatty acid synthase, FAS1 and FAS2, are regulated by inositol (Chirala, S.S. [1992] Proc. Natl. Acad. Sci. USA 89, 10232-10236). Thus all three genes involved in saturated fatty acid biosynthesis are coordinately regulated with phospholipid biosynthesis. Comparison of the UASINO sequences present in FAS1, FAS2, and FAS3 suggested that the functional sequence of this UAS element is YTTCACATG. However, even when the functional UASINO was mutated, substantial expression of the FAS3-lacZ reporter gene was observed. Deletion analysis, electrophoretic mobility shift assays, and expression using a heterologous reporter gene showed that the region between nucleotides -840 and -736 has two UAS elements. The same sequence seems to be responsible for fatty acid-mediated repression of FAS3. The presence of these additional UAS sequences explains why yeast does not require fatty acids even when repressing amounts of inositol and choline are present in the medium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Feel W., Chirala S. S., Wakil S. J. Cloning of the yeast FAS3 gene and primary structure of yeast acetyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4534–4538. doi: 10.1073/pnas.89.10.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailis A. M., Lopes J. M., Kohlwein S. D., Henry S. A. Cis and trans regulatory elements required for regulation of the CHO1 gene of Saccharomyces cerevisiae. Nucleic Acids Res. 1992 Mar 25;20(6):1411–1418. doi: 10.1093/nar/20.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Kimmerly W. J., Rine J., Kornberg R. D. Two DNA-binding factors recognize specific sequences at silencers, upstream activating sequences, autonomously replicating sequences, and telomeres in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jan;8(1):210–225. doi: 10.1128/mcb.8.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Lue N. F., Kornberg R. D. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988 Dec;8(12):5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman G. M., Henry S. A. Phospholipid biosynthesis in yeast. Annu Rev Biochem. 1989;58:635–669. doi: 10.1146/annurev.bi.58.070189.003223. [DOI] [PubMed] [Google Scholar]
- Chasman D. I., Lue N. F., Buchman A. R., LaPointe J. W., Lorch Y., Kornberg R. D. A yeast protein that influences the chromatin structure of UASG and functions as a powerful auxiliary gene activator. Genes Dev. 1990 Apr;4(4):503–514. doi: 10.1101/gad.4.4.503. [DOI] [PubMed] [Google Scholar]
- Chirala S. S. Coordinated regulation and inositol-mediated and fatty acid-mediated repression of fatty acid synthase genes in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10232–10236. doi: 10.1073/pnas.89.21.10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirala S. S., Kasturi R., Pazirandeh M., Stolow D. T., Huang W. Y., Wakil S. J. A novel cDNA extension procedure. Isolation of chicken fatty acid synthase cDNA clones. J Biol Chem. 1989 Mar 5;264(7):3750–3757. [PubMed] [Google Scholar]
- Chirala S. S., Kuziora M. A., Spector D. M., Wakil S. J. Complementation of mutations and nucleotide sequence of FAS1 gene encoding beta subunit of yeast fatty acid synthase. J Biol Chem. 1987 Mar 25;262(9):4231–4240. [PubMed] [Google Scholar]
- Guarente L., Yocum R. R., Gifford P. A GAL10-CYC1 hybrid yeast promoter identifies the GAL4 regulatory region as an upstream site. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7410–7414. doi: 10.1073/pnas.79.23.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Pinkham J., Wei R., Miller R., Guarente L. The HAP3 regulatory locus of Saccharomyces cerevisiae encodes divergent overlapping transcripts. Mol Cell Biol. 1988 Feb;8(2):655–663. doi: 10.1128/mcb.8.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasslacher M., Ivessa A. S., Paltauf F., Kohlwein S. D. Acetyl-CoA carboxylase from yeast is an essential enzyme and is regulated by factors that control phospholipid metabolism. J Biol Chem. 1993 May 25;268(15):10946–10952. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Parthasarathy S., Numa S. Evidence that acyl coenzyme A synthetase activity is required for repression of yeast acetyl coenzyme A carboxylase by exogenous fatty acids. Proc Natl Acad Sci U S A. 1976 Feb;73(2):386–390. doi: 10.1073/pnas.73.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaki T., Nikawa J., Hosaka K., Yamashita S. Functional analysis of the regulatory region of the yeast phosphatidylserine synthase gene, PSS. J Bacteriol. 1991 Dec;173(24):7992–7995. doi: 10.1128/jb.173.24.7992-7995.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziora M. A., Chalmers J. H., Jr, Douglas M. G., Hitzeman R. A., Mattick J. S., Wakil S. J. Molecular cloning of fatty acid synthetase genes from Saccharomyces cerevisiae. J Biol Chem. 1983 Oct 10;258(19):11648–11653. [PubMed] [Google Scholar]
- Lopes J. M., Henry S. A. Interaction of trans and cis regulatory elements in the INO1 promoter of Saccharomyces cerevisiae. Nucleic Acids Res. 1991 Jul 25;19(14):3987–3994. doi: 10.1093/nar/19.14.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A. H., Chirala S. S., Mody N. H., Huang W. Y., Wakil S. J. Primary structure of the multifunctional alpha subunit protein of yeast fatty acid synthase derived from FAS2 gene sequence. J Biol Chem. 1988 Sep 5;263(25):12315–12325. [PubMed] [Google Scholar]
- Nikoloff D. M., Henry S. A. Genetic analysis of yeast phospholipid biosynthesis. Annu Rev Genet. 1991;25:559–583. doi: 10.1146/annurev.ge.25.120191.003015. [DOI] [PubMed] [Google Scholar]
- Pazirandeh M., Chirala S. S., Wakil S. J. Site-directed mutagenesis studies on the recombinant thioesterase domain of chicken fatty acid synthase expressed in Escherichia coli. J Biol Chem. 1991 Nov 5;266(31):20946–20952. [PubMed] [Google Scholar]
- Rose T. M., Schultz E. R., Todaro G. J. Molecular cloning of the gene for the yeast homolog (ACB) of diazepam binding inhibitor/endozepine/acyl-CoA-binding protein. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11287–11291. doi: 10.1073/pnas.89.23.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Lebert C., Höltke J., Roberts L. M., Schweizer E. Molecular cloning of the yeast fatty acid synthetase genes, FAS1 and FAS2: illustrating the structure of the FAS1 cluster gene by transcript mapping and transformation studies. Mol Gen Genet. 1984;194(3):457–465. doi: 10.1007/BF00425558. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Roberts L. M., Höltke H. J., Takabayashi K., Höllerer E., Hoffmann B., Müller G., Köttig H., Schweizer E. The pentafunctional FAS1 gene of yeast: its nucleotide sequence and order of the catalytic domains. Mol Gen Genet. 1986 Jun;203(3):479–486. doi: 10.1007/BF00422073. [DOI] [PubMed] [Google Scholar]
- Schüller H. J., Hahn A., Tröster F., Schütz A., Schweizer E. Coordinate genetic control of yeast fatty acid synthase genes FAS1 and FAS2 by an upstream activation site common to genes involved in membrane lipid biosynthesis. EMBO J. 1992 Jan;11(1):107–114. doi: 10.1002/j.1460-2075.1992.tb05033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller H. J., Schorr R., Hoffmann B., Schweizer E. Regulatory gene INO4 of yeast phospholipid biosynthesis is positively autoregulated and functions as a transactivator of fatty acid synthase genes FAS1 and FAS2 from Saccharomyces cerevisiae. Nucleic Acids Res. 1992 Nov 25;20(22):5955–5961. doi: 10.1093/nar/20.22.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. R., Craig E. A. Transcriptional regulation of an hsp70 heat shock gene in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 May;7(5):1906–1916. doi: 10.1128/mcb.7.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier J. M. Regulatory DNA-binding proteins in yeast: an overview. Yeast. 1990 Jul-Aug;6(4):271–297. doi: 10.1002/yea.320060402. [DOI] [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]




