Abstract
The rodent postrhinal cortex (POR), homologous to primate areas TH/TF and the human ‘parahippocampal place area’, has been implicated in processing visual landmark and contextual information about the environment. Head direction (HD) cells are neurons that encode allocentric head direction, independent of the animal’s location or behavior, and are influenced by manipulations of visual landmarks. The present study determined whether the POR plays a role in processing environmental information within the HD circuit. Experiment 1 tested the role of the POR in processing visual landmark cues in the HD system during manipulation of a visual cue. HD cells from POR lesioned animals had similar firing properties, shifted their preferred firing direction following rotation of a salient visual cue, and in darkness had preferred firing directions that drifted at the same rate as controls. Experiment 2 tested the POR’s involvement in contextual fear conditioning, where the animal learns to associate a shock with both a tone and a context in which the shock was given. In agreement with previous studies, POR lesioned animals were able to learn the tone-shock pairing, but displayed less freezing relative to controls when reintroduced into the environment previously paired with a shock. Therefore, HD cells from POR lesioned animals, with demonstrated impairments in contextual fear conditioning, were able to use a visual landmark to control their preferred direction. Thus, despite its importance in processing visual landmark information in primates, the POR in rats does not appear to play a pivotal role in controlling visual landmark information in the HD system.
Keywords: spatial navigation, visual landmark, context, fear conditioning
Introduction
An animal’s ability to determine location and direction relative to its surroundings is critical to its survival. Recordings in rodents have identified several functional cell types throughout the limbic system that support an internal representation of the environment, including head direction (HD) cells, which fire as a function of an animal’s directional heading in the horizontal plane, independent of its location or ongoing activity (Taube et al., 1990a; Taube, 2007). During navigation, HD cells are able to maintain an accurate representation of directional heading through the monitoring of idiothetic (‘self-motion’) information by continuously integrating internal cues; however, in the absence of visual information, HD cell tuning is subject to drift over time (Mizumori and Williams, 1993; Goodridge and Taube, 1998). This drift can be corrected by landmark information in order to maintain an accurate representation of space. HD cells are influenced by salient visual cues that serve as landmarks (Taube et al., 1990b; Goodridge and Taube, 1995). Following a 90° rotation of a salient visual landmark in the absence of other polarizing cues, the preferred firing direction (PFD) of HD cells show a corresponding shift. Several brain structures appear to play a role in binding visual cues to the HD system, including the postsubiculum (PoS) and retrosplenial cortex (RSP), and lesions of these structures disrupt the influence of visual cues over the HD signal in several brain areas (Goodridge and Taube, 1997; Clark et al., 2010; Yoder et al., 2015). Visual information presumably enters the HD circuit through the direct projection from visual areas 17/18b to the PoS, but it might also enter through the parallel area 17/18b → RSP → PoS pathway (Vogt and Miller, 1983; reviewed in Yoder et al., 2011).
The parahippocampal cortex is another possible route for visual information to enter the HD circuit. Imaging studies in humans have demonstrated parahippocampal involvement when subjects passively viewed scenes such as landscapes and cities, but not when viewing objects or faces; this pattern of response gave rise to the name ‘parahippocampal place area’ (PPA; Epstein and Kanwisher, 1998). Damage to this region in humans has been associated with selective deficits in memory for scenes and while navigating through unfamiliar visual environments (Epstein et al., 2001; Kornblith et al., 2013). The postrhinal cortex (POR) is thought to be the rodent homolog of the primate parahippocampal areas TH/TF (Von Bonin and Bailey, 1947; Burwell et al., 1995) and has been implicated in processing contextual and visuospatial information (Bucci et al., 2002; Norman & Eacott, 2005). Neurons displaying aspects of location-specific firing, which included split or multiple subfields, have also been described here (Burwell and Hafeman, 2003; cf. Fyhn et al., 2004). The POR receives extensive input from brain structures involved in visuospatial processing, such as visual and retrosplenial cortices, lateral dorsal thalamus, parasubiculum and entorhinal cortex (Burwell and Amaral, 1998; Agster and Burwell, 2009; reviewed in Furtak et al., 2007). Importantly, many of these areas contain HD cells. Taken together, these observations suggest that the POR contributes to perceptual processing of spatial and contextual environmental information.
The present study investigated the involvement of the POR in processing visual and spatial information in the HD circuit. HD cells were recorded from the anterodorsal thalamus (ADN) in rats with POR lesions. Animals were tested in two experiments. Experiment 1 involved recording HD cells while a salient visual cue card was rotated within the environment, out of view of the animal. Additionally, HD cells were recorded in the same environment in the dark with the cue card removed to assess the role of the POR in monitoring self-movement cues. Experiment 2 tested the same animals in a contextual fear conditioning task where an aversive foot shock was paired with a tone and a context. We report that animals with POR lesions are able to use a visual landmark to maintain a directional representation, and are also able to maintain an accurate representation of direction in the absence of visual landmarks. However, these animals show a deficit in their ability to associate a shock with the context in which it was received, while maintaining an association between a tone and a shock, suggesting that the POR is recruited during higher level processing of the visual world, beyond that of a simple polarizing stimulus.
Materials and Methods
General Methods
Subjects
All experimental procedures were approved by Dartmouth College Institutional Animal Care and Use Committee. Female Long-Evans Hooded rats (Harlan Laboratories; Indianapolis, IN, age: 4–10 mo., weight: 247–374 g at time of surgery) were kept on a 12-hour light/dark cycle and were pair housed prior to surgery and housed individually following surgery. Rats had ad libitum access to food and water prior to screening, and then were food restricted to 85% of their pre-surgical weight during subsequent screening and recording sessions.
Electrodes
Each animal received implantation of a drivable chronic multichannel electrode array (see Kubie, 1984). This array consisted of a modified circular 11-pin Augat plug (ten outer pins plus one center pin) connected to a 26 gauge stainless steel cannula. Ten 25 μm nichrome wires (California Fine Wire, Grover Beach, CA) were threaded through the cannula and wrapped around each outer pin after insulation was stripped, one wire per pin. The cannula was soldered to the center pin for a ground reference. The male pins were coated with epoxy to ensure stability, and acrylic was used to attach three drive screws. Plastic cuffs were attached to the drive screws, which would later be connected to dental cement contacting the animal’s skull, which served as an anchor. Prior to surgery, the wires were cut and spread apart, electroplated to 150–250 kΩ resistance, sterilized and coated with polyethylene glycol to ensure rigidity during implantation.
Surgery
Rats were anaesthetized with 1–3 % isoflurane gas and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and the incisor bar was raised or lowered to ensure bregma and lambda were in the horizontal plane. Eight rats received bilateral injections of 100 mM N-methyl-D aspartic acid (NMDA, Sigma-Aldrich, St. Louis, MO) into the POR. NMDA was used to avoid damage to fibers of passage. For NMDA injections, a glass pipette was pulled and the tip broken back to a diameter of ~ 50 μm. Following injection, the pipette was left in place for a minimum of 5 min to promote diffusion of NMDA (or saline for sham controls). Additionally, one rat received 1 mA current for 10s per site using the same coordinates as the other POR lesion animals. Control animals consisted of 4 rats that received only an electrode array implant, 11 rats that had insect pins inserted just dorsal to the POR but no current injected, and 1 rat that received injections of 0.9% saline into the POR. Table 1 displays the coordinates and injection volumes.
Table 1.
Injection sies
| A/P (Lambda) | M/L (Bregma - Lambda) | D/V (skull) | Volume |
|---|---|---|---|
| +0.4 | ±3.8 | −6.5/−6.2 | 0.2–0.4 μL |
| −0.1 | ±3.7 | −6.0/−5.7 | 0.2–0.4 μL |
| −0.6 | ±3.4 | −4.6/−4.3 | 0.2–0.4 μL |
| −1.0 | ±3.1 | −4.5/−4.0 | 0.2–0.4 μL |
A/P and M/L coordinates are relative to Lambda, and D/V is relative to the skull surface. All injections are administered at a 22° M/L angle.
After NMDA injections or sham lesion, the lesion holes were covered with bone wax (Ethicon, San Lorenzo, PR). Skull screws (Small Parts, Miramar, FL) were inserted into frontal, parietal and occipital bones for holding the electrode in place. A hole was drilled above the ADN and the electrode was lowered until it was just dorsal to the ADN (coordinates: −1.9 A/P, 1.3 M/L (from Bregma), −3.8 D/V (below dura)). The electrode array was attached to the skull with dental Grip Cement (Dentsply International, Milford, DE). Animals were allowed 1 week to recover from surgery prior to screening procedures.
Signal processing and recording procedures
After the animals had recovered from surgery, they were trained to forage for 20 mg sugar pellets (Bio-Serv, Frenchtown, NJ) in a gray wooden cylinder (76 cm diameter x 51 cm height) in a dedicated recording room. A black curtain surrounded the cylinder to minimize extra-maze visual cues, and white noise was played over a speaker. Eight uniformly spaced 25-watt DC lights illuminated the room. The floor of the cylinder was covered with gray paper, and a white cue card subtending 100° of arc was taped to the inside cylinder wall, which provided a polarizing visual cue that could be used as a landmark reference.
The screening procedure consisted of connecting the electrode to a headstage composed of a red and green light-emitting diode (LED) separated by 11 cm, which allowed for tracking the position and direction of the animal, as well as 10 field effect transistors (FETs, one for each electrode wire). The FETs amplified the neural signal in a source-follower configuration before the signal entered a cable tethered to an overhead commutator. The signal was then amplified (P5 series; Grass Technologies, West Warwick, RI), band-pass filtered (300 Hz to 30 kHz, 6 dB/octave), and then sent to a dual time/amplitude window discriminator (Model DDIS-1; Bak Electronics, Mount Airy, MD). The corresponding waveform was displayed on an oscilloscope (Model 2214; Tektronix, Beaverton, OR) and played through a speaker. The windows were adjusted to isolate waveforms from single neurons. An overhead video camera (SONY XC-711; Tokyo, Japan) captured the x,y coordinates of the red and green LEDs at 60 Hz, which was combined with the output pulse generated by the window discriminator and sent to a computer (Macintosh G4) running LabView software (version 5.0; National Instruments, Austin, TX).
Upon detection and isolation of a HD cell, an 8 min baseline recording session was conducted, which consisted of having the animal forage for sugar pellets in the circular arena with the room door closed and lights on. If the waveform isolation quality was acceptable (Rayleigh vector length > 0.3; signal-noise ratio > 4.0, see below), the animal underwent a landmark rotation session and subsequent dark session (experiment 1). Animals were also concurrently trained in a contextual fear conditioning task (experiment 2).
Experiment 1: Landmark Cue Rotation
To determine if the POR plays a role in binding visual and spatial information to the HD signal, animals underwent a landmark cue rotation test (Fig. 1A). Following an initial 8 min baseline recording session (Standard 1) the animal was removed from the cylinder and placed in a cardboard box with the lid closed. The floor paper was changed to eliminate olfactory cues, and the cue card was rotated 90° clockwise or counterclockwise. The animal received a 30 sec period of disorientation, where the animal was placed in an opaque cardboard box and carried around the room before being replaced in the cylinder (refer to Dudchenko et al., 1997), and another 8 min session was recorded (Rotation). Following this cue rotation session, a second standard session (Standard 2) was recorded with the cue card in the original location for 8 min.
Figure 1. Experimental Protocol.
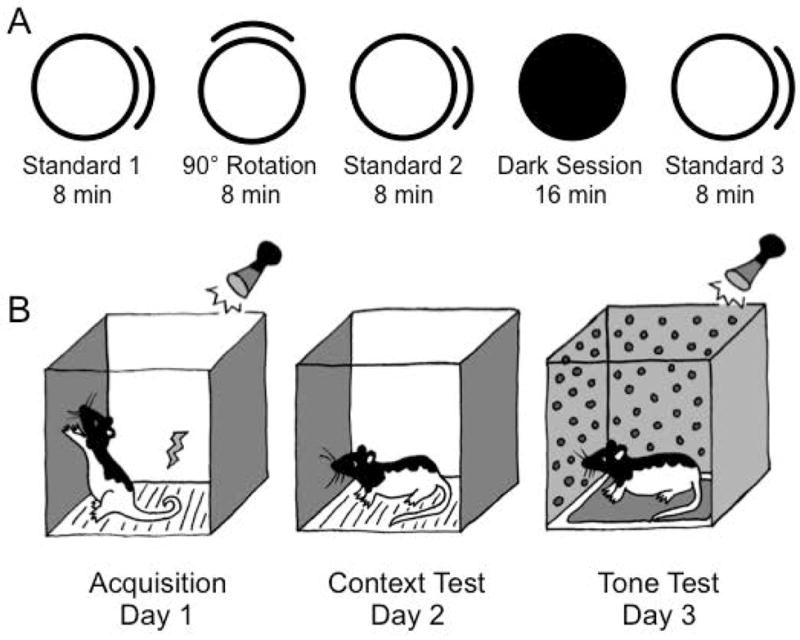
After successful isolation of a HD cell, a landmark cue rotation (A, experiment 1) was conducted in which a cue card was rotated out of view of the animal (90° cue rotation) and in the absence of other polarizing stimuli (dark session). Contextual fear conditioning (B, experiment 2) took place over 3 days. Acquisition occurred on day 1 and consisted of three tone-shock pairings. The context test occurred on day 2, where the rat was replaced in the shock context, but no shock or tone was delivered. The tone test occurred on day 3 and consisted of placing rats in a ‘novel’ context with 20 tone presentations. Freezing to either context (day 2) or tone (day 3) served as an index of conditioned fear.
To determine if the POR is involved in maintaining the stability of the HD signal over time, HD cells were then recorded in the absence of visual cues in a 16 min dark session. Following removal of the animal from the cylinder, the cue card was removed and the lights were turned off before returning the animal to the cylinder to forage for sugar pellets (Dark session). The distance traveled during this session was also compared between groups. Finally, in order to determine if the preferred firing direction was maintained across multiple sessions, a third standard session was recorded with the cue card in the original position (Standard 3). In total there were five sessions recorded for this experiment: Standard 1 → 90° Cue Rotation → Standard 2 → Dark → Standard 3. Before and between each session, the floor paper was replaced and the animal was disoriented (see above), and the experimenter randomly entered and exited the curtain.
Experiment 2: Contextual Fear Conditioning
Animals were concurrently trained in a contextual fear conditioning paradigm (Fig. 1b), which took place in a standard operant conditioning chamber (24 × 30.5 × 29 cm; MED Associates, Inc., St. Albans, VT). The conditioning chamber consisted of a clear Plexiglas top, front, and back walls, aluminum sidewalls, and a metal grid floor. The box was located inside a sound-attenuating chamber (62 × 56 × 56 cm) containing a speaker and a 6 watt house light, which provided background illumination. A camera was also located inside the chamber, which was connected to a DVD recorder for offline behavioral analysis. The apparatus was connected to a computer running MED-PC IV (Med Associates, Inc.; St. Albans, VT), which controlled the house light, speaker, and shock delivery.
Contextual fear conditioning occurred over 3 days. Acquisition (day 1) consisted of three 10 sec tone presentations (1.5 kHz, 78 dB), which co-terminated with the delivery of a 1.0 sec 1.0 mA constant current foot shock. Each tone-shock paring was separated by a 64 sec inter-trial interval (ITI), and presentation of the first tone was preceded by a 3 min period in which no tone or shock occurred. The context test occurred the next day (day 2), when the animals were returned to the shock context, but importantly no tone or shock was delivered. The tone test occurred on day 3, and consisted of placing the animals into a ‘novel’ context, which was the same box as days 1 and 2, but with polka-dot wall paper covering the sides, a manila folder covering the floor grid, and a smear of Vick’s Vapor Rub placed underneath the floor grid in order to provide a novel odor. The animal received 20 presentations of a 10 sec tone (1.5 kHz, 80 dB, 30 sec ITI) during this session. Freezing was assessed offline (see below).
In order to determine if freezing during fear conditioning was a result of the animal’s locomotion alone, as opposed to the conditioned stimuli, rats were placed in an open field chamber immediately following the final session of fear conditioning on day 3. This test consisted of an arena (43.2 × 43.2 × 30.5 cm) composed of Plexiglas walls and floor (Med Associates, Inc., St. Albans, VT). Sixteen photobeams were arranged 5.5 cm apart, and movement was assessed as a function of the number of photobeam interruptions that occurred in a 10 min session.
Data Analysis
HD cell tuning curves
During recording, video tracking and spike data were acquired at 60 Hz by a computer running LabView software (National Instruments; Austin, TX). The angle between the red and green LEDs was used to determine the directional heading of the animal for each video frame, which was then sorted into sixty 6° bins. The average firing rate for each HD was determined by dividing the total number of spikes in each 6° bin by the amount of time the animal spent facing that direction. To determine if a cell’s firing distribution was non-uniform and directional, it was subjected to a Rayleigh test; it was considered directional if the mean vector length r > 0.3, which corresponds to an alpha level of ~0.05 for the sample sizes used in this study (Batschelet, 1981; Clark et al., 2012; Yoder & Taube, 2009). Cells that met this criterion during the first standard session were considered HD cells.
HD cell characteristics
All results are reported as mean ± standard error of the mean (SEM) unless otherwise indicated. The proportion of HD cells recorded in POR lesion and control groups was calculated by dividing the total number of HD cells recorded in each group by the number of putative ADN neurons recorded, which was determined by counting the number of cells recorded between the first and last HD cell encountered across all electrode wires. The cell’s PFD, peak firing rate, directional firing range, background firing rate, directional information content and mean vector length were determined during the first standard session of the cue rotation experiment. The PFD was defined as the 6° bin that contained the maximal firing rate (referred to as the peak firing rate). The directional firing range was defined as the width of the base of a triangle fit to the HD x firing rate function, and the background firing rate was the average rate of firing outside this range. Finally, the directional information content (Skaggs et al., 1993) was determined to be the amount of information conveyed by each spike, calculated from the following formula:
where pi is the probability that the head is pointed in the ith directional bin, λi is the mean firing rate for bin i, and λ is the mean firing rate for all directional bins. A Student’s t-test was performed to assess any differences between groups for the above parameters.
HD cell shift analysis
To determine the amount the PFD shifted for each cell following a cue card rotation, a cross-correlation analysis was performed between sessions. The shift in the PFD was defined as number of degrees (in 6° bins) in which the HD x firing rate function needed to be shifted between sessions to obtain the highest Pearson correlation (see Goodridge and Taube, 1995). This comparison was performed for the following sessions: Standard 1 → 90° Cue Rotation, Standard 1 → Standard 2 and Standard 1 → Standard 3. The mean resultant vector for each comparison was then computed for each group. To determine if the distribution of shift values within each group were significantly clustered (i.e., non-uniform), shift values were subjected to a Rayleigh test. A sample was said to be significantly clustered if r > 0.3, which corresponds to an alpha level of ~0.05 for the sample sizes we tested (Batschelet, 1981). A one-sample V test was then used to compare each group against expected shift values of 0° and 90°. Finally, a Watson-Williams F-test was used to determine if shifts differed significantly between groups.
Dark session analysis
To determine how stable the HD signal was in the absence of visual landmarks, HD cells were recorded in the cylinder with the lights turned off and the cue card removed for 16 min. The heading of the animal (in 6° bins) was recorded according to when the HD cell’s firing rate reached 75% of its maximum rate, and a scatterplot of HD x time was created to determine the drift of the PFD over time. Drift within a session was determined by fitting a line to the HD x time function, and the slope of the line (°/min) was defined as the average drift across the session; a negative slope indicated drift in the clockwise direction, and a positive slope indicated drift in the counterclockwise direction. Additionally, the standard deviation of the cell’s PFD within the session was used as another measure of stability, since a drifty HD cell will have a larger variance than a stable cell. A Student’s t-test was performed to determine if there were any differences between groups in the dark. In addition, distance traveled during the dark session was computed by measuring the distance the rat’s head moved every second and a Student’s t-test was used to determine if there was a difference in locomotion between groups.
Fear conditioning analysis
Freezing was used as the index of conditioned fear (Blanchard and Blanchard, 1969; Fanselow, 1980) and was defined as the absence of all movement apart from breathing. Scoring of freezing behavior was performed offline using a stopwatch and computer with a DVD player. Freezing was assessed for each epoch by noting the presence or absence of freezing, and the frequency of freezing was converted to a percentage for each epoch. Freezing during acquisition (day 1) was assessed every 8 sec for four 64 sec consecutive epochs: baseline (first 64 sec period during pre-shock exploration) and each period following shock delivery. Freezing during the context test (day 2) was assessed every 8 sec for twenty 64 sec epochs. Finally, freezing to a tone in a ‘novel’ context (day 3) was assessed every 2 sec during each 10 sec tone presentation, for a total of 20 tone presentations. Day 2 was divided into five 320 sec blocks for analysis, and day 3 into five blocks of 4 tone presentations. ANOVA with repeated measures was used to compare freezing at different time points across groups.
Movement analysis
For the 10 min open field test, which immediately followed the Tone Test, the entire session was divided into ten 60 sec blocks, and the total distance traveled for each block was recorded for each animal. ANOVA with repeated measures was used to assess any group differences in locomotion for each block.
Histological analysis
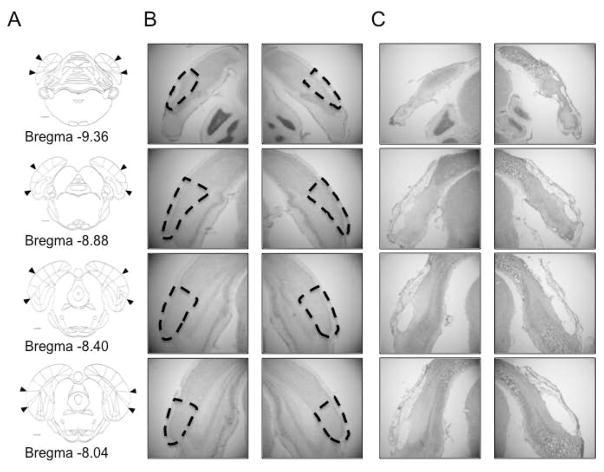
Upon complete advancement of the electrodes through ADN, animals were deeply anesthetized and anodal current was passed through several wires in order to mark the location of their termination. Rats were then perfused intracardially with 0.9% saline followed by a 10% formalin solution. Following fixation, the brain was removed from the skull and postfixed with 10% formalin containing a small amount of potassium ferrocyanide (~2%), which produced a Prussian blue reaction where the ferrous metal had been deposited from the anodal current. Following 24 h of postfixation, brains were then cryoprotected in a 20% sucrose solution for 48 h, followed by slicing into 30 μm sections with a cryostat. After allowing the tissue to dry for 24 h, sections were stained with thionin, and lesion size and electrode placement were evaluated with a light microscope. The location of the postrhinal cortex was determined according to Burwell (2001). Briefly, the POR is located at the caudal pole of the rat brain. Rostrally, the POR shares a border with the caudal subiculum. Ventrally, the POR shares a border with the lateral entorhinal cortex or parasubiculum, depending on rostrocaudal location, and dorsally with visual and association cortex (see Figure 2A for coronal sections illustrating the location of the POR).
Figure 2. POR lesions.
A) The rostrocaudal extent of the postrhinal cortex shown in coronal sections on a line drawing. B–C) photomicrographs taken from a rat with no lesion (B) and from one animal with an extensive POR lesion (C). Damage ranged from 75–98% bilaterally across the entire rostrocaudal extent of the POR in 9 animals (mean damage = 88 ± 2%). The brain depicted in C had 85% damage to the POR. Arrowheads (A) and dashed lines (B) demarcate the borders of the POR.
Analysis of lesion size was conducted using a microscope (Zeiss Axioskop; Jena, Germany) connected to a computer running Stereo Investigator 9 (MicroBrightField, Inc.; Williston, VT). Four representative A/P slices per hemisphere were chosen, based on Paxinos and Watson (2008), to estimate the rostrocaudal extent of the lesion: −8.04 mm, − 8.40 mm, −8.88 mm and −9.36 mm relative to Bregma. To determine lesion size for each slice, a contour was made that outlined the POR, and another contour was made containing the lesion. Lesion size per slice was determined by dividing the number of 50 μm pixels contained within each lesion contour by the total number of 50 μm pixels in the POR contour. The total lesion size per hemisphere was determined by taking the total number of 50 μm pixels contained in the four lesion contours divided by the total number of 50 μm pixels within the four POR contours. This procedure was repeated for the lateral and medial entorhinal cortices, retrosplenial cortex, post- and parasubiculum, and visual and association cortex just dorsal to the POR in order to assess the amount of surrounding tissue damage. All electrode tracks from both lesion and control animals where HD cells were identified were verified to have passed through the ADN.
Results
Postrhinal Lesions
A total of 25 animals were used in two experiments (16 in experiment 1, 25 in experiment 2; 7 lesioned and 9 control animals were used in both experiments). Nine animals had lesions of the postrhinal cortex, with damage ranging from 75–98% (mean: 88 ± 2%; Table 2), 12 animals had sham lesions and 4 animals had no lesion. Additionally, NMDA lesions frequently cause damage to tissue overlying the target region, and this damage occurred in the majority of our POR lesioned animals. Damage to the overlying visual cortex ranged from 23–58% (mean: 38 ± 3%) and included areas TeA, V2L, V1M, V1B and V2MM (Paxinos and Watson, 2008).
Table 2.
Postrhinal Lesions
| Animal ID | −9.36 mm (%) | −8.88 mm (%) | −8.40 mm (%) | −8.04 mm (%) | Average Lesion Size (%) |
|---|---|---|---|---|---|
| JP152 | 100 | 100 | 95 | 80 | 94 |
| JP158 | 65 | 65 | 95 | 75 | 75 |
| JP166 | 95 | 100 | 95 | 100 | 98 |
| JP167 | 70 | 90 | 100 | 100 | 90 |
| JP170 | 80 | 100 | 95 | 80 | 89 |
| JP171 | 70 | 100 | 90 | 75 | 84 |
| JP172 | 75 | 95 | 98 | 95 | 91 |
| JP173 | 50 | 100 | 95 | 95 | 85 |
| JP174 | 100 | 80 | 75 | 100 | 89 |
| Mean ± SEM | 78 ± 6 | 92 ± 4 | 93 ± 2 | 89 ± 6 | 88 ± 2 |
POR damage for each A/P location in each animal. Slices were chosen in each hemisphere based on the section which corresponded most to the template (Fig. 2; Paxinos and Watson, 2008).
HD cells were identified in 7 animals that had POR lesions exceeding 84% (experiment 1; mean damage = 89 ± 2%). Bilateral damage extended throughout the entire rostral-caudal extent of the POR, typically with more damage rostrally. In addition, HD cells were recorded in 9 of the 16 control animals (6 animals with sham lesions and 3 animals with implants only). A representative example of an animal with extensive lesions of the POR is shown in Figure 2.
HD cell characteristics
A total of 233 units were recorded in 16 animals, which included 113 units in 7 POR lesioned animals and 120 units in controls. Seventy-seven (out of 113) units were considered to be neurons recorded from the ADN in POR lesioned animals (i.e., units recorded between the first and last HD cell, inclusive), and 47 of these putative ADN neurons (61.0%) were classified as HD cells according to our criteria (Rayleigh vector length > 0.3). Of the 120 units recorded in control animals (6 shams, 3 implant-only), 45 of them were considered putative ADN neurons, and 30 of these ADN neurons (66.7%) were classified as HD cells. The proportion of HD cells did not differ between shams and implant-only controls (X2(1) = .049, p = .856; sham: 65.5%, n = 6 animals; implant: 68.8%, n = 3 animals) and we combined these two groups to investigate the frequency at which we encountered HD cells in our recordings. The POR lesioned animals did not differ from this combined control group in terms of the proportion of HD cells recorded (X2(1) = .386, p = .534; POR lesioned: 61.0%, n = 7 animals; control: 66.7%, n = 9 animals). Moreover, the proportion of HD cells in putative ADN neurons is consistent with previous studies (Taube, 1995).
HD cell characteristics did not differ significantly between the 6 rats with sham lesions and the 3 rats with only implants in terms of background firing rate (t(16) = 0.249, p = .806), peak firing rate (t(16) = 0.598, p = 0.559), directional firing range (t(16) = 0.299, p = .769), directional information content (t(16) = 2.009, p = .062) and Rayleigh vector length (t(16) = 0.197, p = .847). Thus, these animals were pooled together to form one group (‘control,’ n = 9 animals). Of the 76 ADN HD units recorded, there were no differences between groups in terms of background firing rate (t(41) = 0.043, p = .966), peak firing rate (t(41) = 0.442, p = 0.661), directional firing range (t(41) = 0.553, p = .584), directional information content (t(41) = 1.560, p = .126), or mean Rayleigh vector length (t(41) = 0.824, p = .415). Table 3 summarizes the mean values for each of these parameters. Because the above parameters are influenced by the presence of multiple cells recorded simultaneously, sessions in which two or more cells were recorded simultaneously were excluded from this analysis (POR lesioned: n = 25 sessions; Control: n = 18 sessions).
Table 3.
Head direction cell properties
| PORx (n = 25) | Control (n = 18) | p | |
|---|---|---|---|
| Background firing rate (Hz) | 1.76 ± 0.37 | 1.78 ± 0.25 | 0.966 |
| Peak firing rate (Hz) | 32.7 ± 2.6 | 31.0 ± 2.9 | 0.661 |
| Directional firing range (degrees) | 114.7 ± 4.9 | 110.4 ± 5.9 | 0.584 |
| Directional information content | 1.22 ± 0.12 | 0.96 ± 0.10 | 0.126 |
| Rayleigh vector length | 0.71 ± 0.03 | 0.67 ± 0.03 | 0.416 |
HD cells did not differ across groups in terms of background firing rate, peak firing rate, directional firing range, directional information content or mean Rayleigh vector length. PORx: n = 25 sessions; control: n = 18 sessions.
Landmark Cue Rotation
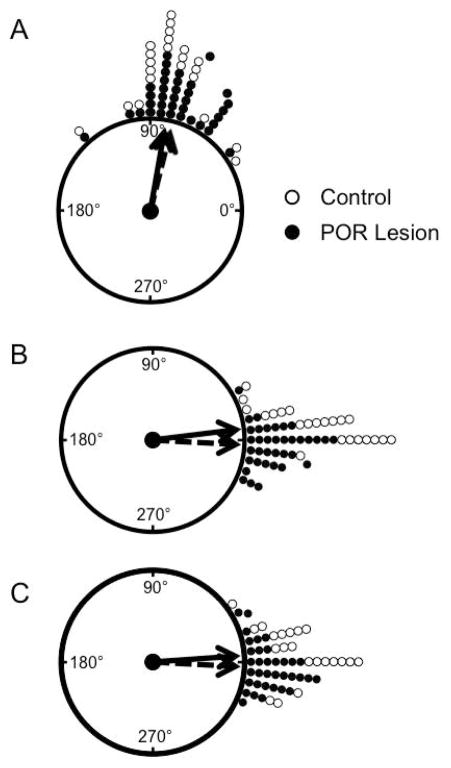
To determine if the POR contributes to landmark control of the HD circuit, HD cells were recorded following rotation of a salient visual landmark. Forty-seven HD cells were recorded in 36 cue rotation sessions in 7 POR lesioned animals, and 30 HD cells were recorded in 23 rotation sessions in 9 sham and unlesioned control animals. PFD shift values were averaged for sessions in which more than one cell was recorded simultaneously, given that HD cells tend to shift in register with each other (Taube et al., 1990b). On average the PFDs of HD cells in POR lesioned rats shifted 76.2 ± 2.8° (n = 36 sessions, 7 animals) while cells in control rats shifted 80.9 ± 4.2° (n = 22 sessions, 9 animals). A Rayleigh test revealed that cells in both POR lesioned and control animals responded with a similar PFD shift following a cue rotation (POR lesioned, r(36) = .958, z = 33.0, p < .001; control, r(22) = .940, z = 19.5, p < .001). Additionally, a V test indicated that in both groups this shift was not uniform, but had a directionality that was significantly clustered around an expected shift of 90° (POR lesioned: V = 33.5, p < .001; control: V = 20.4, p < .001). Finally, control and POR lesioned animals did not differ significantly from each other in terms of PFD shift following the cue rotation (F(1,56) = .884, p = .352). Taken together, these results suggest that the POR is not necessary to bind salient visual landmarks to the HD system during rotation of a salient visual cue, as lesions did not disrupt visual landmark control over the HD signal (Fig. 3A).
Figure 3.
Landmark Cue Rotation. Following a 90° cue rotation (A), HD cells tended to shift in register with the cue card in both POR lesioned and control animals. B–C) When the cue was returned to its original position, HD cell PFDs similarly shifted back to their original orientation in both groups following the rotation (B) and dark (C) sessions. POR lesion: filled circles, solid line; Control: open circles, dashed line. Arrows represent the mean resultant vector.
HD cell stability
To determine if the POR is involved in the stability of the HD signal over time, HD cells were recorded as the cue card was returned to its original position after the cue rotation session was complete. In this second ‘standard’ session, the PFD of cells in both control and POR lesioned groups shifted back to their original PFD (mean shift between standard sessions 1 and 2: POR lesioned: −2.93 ± 1.78°; control: 7.06 ± 1.79°). A V test indicated that these shifts were both directionality clustered around 0° (POR lesioned: V = 35.33; p < .001; control; V = 21.60; p < .001). However, there was a significant difference between groups in the mean shift between Standards 1 and 2 (F(1,56) = 13.5, p < .001). Further inspection revealed that HD cells in control rats tended to under-rotate following a return of the cue card to its original position, whereas HD cells in POR lesioned animals tended to over-rotate. Nonetheless, these data indicate that animals in both groups were able to use visual landmarks to update their PFDs relative to the cue card (Fig. 3B), indicating that the POR is not required for the stability of the HD signal over time.
Dark sessions
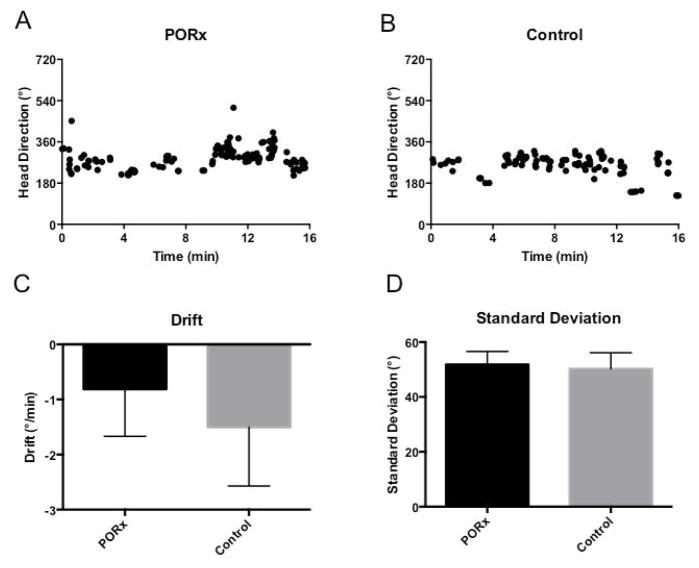
To determine if the POR is involved in updating the HD system in the absence of visual information, HD cells were recorded in the dark for 16 min with the cue card removed. Figure 4 illustrates representative HD cells recorded in the dark in both a POR lesioned (a) and a control (b) animal. HD cell PFDs in both groups tended to drift in darkness throughout the session (POR lesioned: −0.81 ± 0.85°/min, n = 27 sessions; control: −1.50 ± 1.07°/min, n = 15 sessions; Fig. 4C). One session in the lesion group was not included in this analysis because the drift value was greater than 1.5 times the inter-quartile range from the first quartile. A t-test revealed that there was no difference in the drift rates between groups (t(40) = .495, p = .623). The standard deviation (SD) of HD cell PFDs was used as a second measure of drift during darkness, as cells that are less stable over time tend to have a higher variance in a session than those that are more stable. As before, the standard deviation of the PFDs in the dark session did not differ between groups (t(41) = −.201, p = .842; POR lesioned: 51.8 ± 4.8°, n = 28 sessions; control: 50.2 ± 5.9°, n = 15 sessions; Fig. 4D). These results suggest that HD cells in both POR lesioned and control animals are able to integrate angular self-movement information in the absence of visual information.
Figure 4.
Dark Session. Representative examples from POR lesioned (A) and control (B) animals showing PFD drift over the course of a 16 min. dark session. There were no group differences in terms of drift within session (C) or standard deviation (D). PORx: POR lesion.
Following the dark session, HD cells were recorded in a final standard session (Standard 3) in which the cue card was returned to its original position. Once again, the PFDs of cells in both control and POR lesioned groups shifted back to their original PFDs (PFD difference between standard sessions 1 and 3: POR lesioned: −2.21 ± 2.02°; control: 4.84 ± 2.62°). A V test indicated that the PFDs in both POR lesioned and control animals reliably shifted back to their original values, and that the shifts did not differ from 0° (POR lesioned: V = 34.2 p < .001; control: V = 20.5, p < .001), suggesting that HD cells from both groups were able to maintain similar HD cell representations across sessions (Fig. 3C).
Contextual Fear Conditioning
Previous studies have shown that the POR plays a role in contextual fear conditioning, where a rat learns to associate an aversive stimulus (i.e., foot shock) with the context in which it was received (Bucci et al., 2000). To determine if the POR is involved in processing context, rats were concurrently run in a three-day contextual fear conditioning paradigm (see Materials and Methods above). Three animals in the control group were removed from analysis due to aberrant behavior. Two rats were statistical outliers during either acquisition or the tone test (i.e., they were greater than 1.5x outside the inter-quartile range). The third animal was removed due to hyperactivity during the context test (day 2); indeed, it was recorded as ‘immobile’ for only 1 time point during the entire session (1 out of 160 time points). In total, 22 animals were included in the analysis for this experiment (POR lesioned: n = 9; control: n = 13).
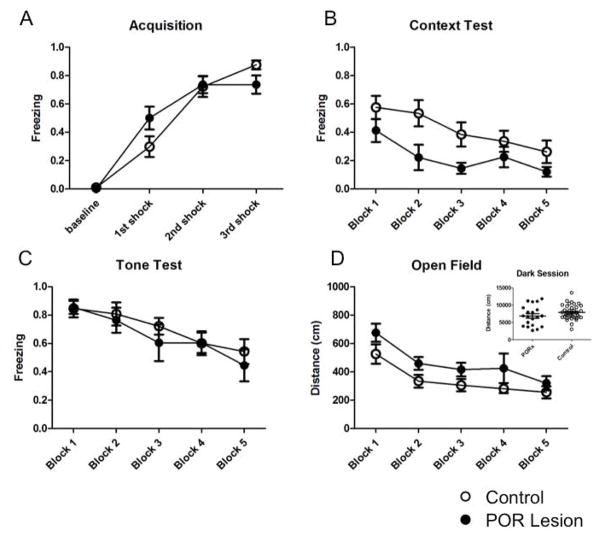
Freezing during training (day 1; Fig. 5A) was assessed before and immediately following delivery of each shock by sampling every 8 sec for four 64 sec epochs (baseline, 1st, 2nd and 3rd shock). Animals learned to associate the tone with the shock, as freezing in response to the tone increased significantly in both groups (F(3,60) = 123.6, p < 0.0001), with no significant differences between lesion and control animals (F(1,20) = 0.085, p = 0.774). There also was a significant block x group interaction effect (F(3, 60) = 4.57, p < 0.01); however, given that animals in both groups displayed elevated freezing levels by the end of the session, it is clear that both POR lesioned and control animals learned the tone-shock association.
Figure 5.
Contextual fear conditioning and open field test. Contextual fear conditioning consisted of 3 days of training and testing: A) acquisition, B) context test and C) tone test. D) Immediately following the last session of fear conditioning, animals were placed in an open field and total distance traveled was used as an index of differences in locomotion. Filled circles: POR lesion; open circles: control.
Freezing to context (day 2; Fig. 5B) was assessed by dividing the entire session (1280 sec) into five 256 sec blocks. Freezing was assessed every 8 sec throughout the session. Freezing levels were initially elevated for both groups; however, as expected, they decreased throughout the session, with a significant main effect of time (F(4,80) = 8.78, p < 0.0001). This decrease indicated that animals gradually extinguished their initial context-shock association in the absence of the tone or shock, and is consistent with previous studies (see Bouton, 2004). Importantly, there was a significant group difference, where POR lesioned animals froze less than controls (F(1,20) = 4.52, p < 0.05); this group difference was most evident for blocks 2 and 3 (see Fig. 5B). There was no significant time x group interaction (F(4,80) = 1.07, p = 0.377).
Freezing to the tone (day 3; Fig. 5C) was assessed by scoring freezing behavior every 2 sec during each 10 sec tone presentation for 20 presentations. The entire session was divided into five blocks of 4 tones each block. Freezing to the tone was elevated initially, but decreased significantly across blocks (F(4,80) = 11.94, p < 0.0001). There were, however, no group differences during this session (F(1,20) = 0.273, p = 0.607), indicating that POR lesioned animals were unimpaired in recalling the tone-shock association. There was no significant block x group interaction effect (F(4, 80) = 0.483, p = 0.749).
Open Field Test
To determine if any differences in freezing were due to motor impairments because of the lesion, a subset of animals (POR lesioned: n = 7; control: n = 6) were immediately tested in an open field test for 10 min following the last session of fear conditioning (Fig. 5D). The session was divided into five 120 sec blocks for analysis, and the total distance traveled during each block was used as an index of movement. Overall, there was a significant effect of time, where animals moved less over time (F(4,44) = 10.64, p < 0.0001). Interestingly, there was a significant effect of group, where lesion animals traveled more than controls (F(1,44) = 5.06, p = 0.046). However, overall distance traveled during the dark session (Experiment 1; Fig 5D inset) was the same between groups (t(54) = 1.476, p = 0.1457), suggesting that there was a difference in environmental exploration in the presence of visual cues, but no difference when visual cues were not available. There was no significant block x group interaction (F(4,44) = 0.22, p = 0.925), indicating that animals in both groups decreased their exploration as time elapsed. Taken together, the above results suggest that POR lesioned animals spent more time moving around and exploring their environment relative to controls when visual cues are available, but exploration is similar when moving around in the dark.
Discussion
The present set of experiments sought to understand the contribution of the postrhinal cortex in processing visual and spatial information during navigation, particularly with regards to the HD network. Information about visual landmarks is believed to enter the HD network directly through the area 17/18 → PoS pathway or indirectly through the area 17/18 → retrosplenial cortex → PoS pathway (Vogt and Miller, 1983; Yoder et al., 2011). Lesions to either the PoS or retrosplenial cortices disrupt the ability of visual landmarks to influence HD cell firing in multiple brain areas (Goodridge and Taube, 1997; Clark et al., 2010; Yoder et al., 2015). However, because processing landmark information requires the identification of visual objects that are suitable for landmarks, and because the ventral visual stream is thought to be involved in this type of processing (Ungerleider and Mishkin, 1982), it is possible that ventral visual stream areas might contribute critical information to the HD cell network about visual landmarks. Areas such as the PPA in humans are activated when subjects view images involving scenes or objects that can serve as landmarks (Epstein and Kanwisher, 1998). Consequently, we tested whether the POR, the homolog of the PPA in rodents, was involved in processing visual landmark information for the HD cell network. We found that the POR is not necessary for binding visual cues to a representation of direction in the HD circuit, as lesions do not affect the ability of HD cell PFDs to shift with the rotation of a visual landmark. The POR, however, is involved in contextual processing, as POR lesions disrupt an animal’s ability to associate a shock with the context in which it was experienced. These findings are consistent with other studies, which have demonstrated a role for the POR in encoding context (Bucci et al., 2000, 2002; cf. Burwell et al., 2004), location (Burwell and Hafeman, 2003), and conjoint item-location associations (Norman and Eacott, 2005; Furtak et al., 2012).
Experiment 1
The proportion of HD cells recorded in the ADN did not differ significantly between POR lesioned and control animals and was consistent with previous studies (Taube, 1995). Basic HD cell characteristics were also similar between groups. We therefore conclude that POR lesions have minimal, if any, effect on the generation or stability of HD cell firing within a session. Next, the results of the landmark rotation test indicated that the POR is not necessary for binding salient visual landmarks to the HD system within the environment. Following a 90° rotation of the white cue card, HD cells in POR lesioned and control animals shifted a corresponding amount. The same was true when the cue card was returned to the original position following the rotation session, where the PFD of most cells returned to their original Standard 1 PFD.
While HD cells are sensitive to visual cue manipulations, HD cell firing still occurs in the absence of visual input. However, as would be expected in the absence of a reference cue, HD cell PFDs are less stable over time in the dark, suggesting that errors tend to accumulate within the HD network when the animal must rely on path integration to maintain its orientation (Mizumori and Williams, 1993; Goodridge and Taube, 1998). Results from the dark test are consistent with these studies and indicate that HD cells from both the lesion and control groups show a tendency to drift in the absence of visual input. Furthermore, the amount the PFD drifted did not differ between groups. These results suggest that the POR is not necessary for updating the HD signal with self-movement information during path integration in the absence of visual input.
Experiment 2
To assess the role of the POR in processing contextual visual object information independent of spatial processing, animals were trained to associate an electric shock with both a tone and a context, and conditioned freezing to both the tone and the context was measured as an index of fear (Fanselow, 1980). Signaled shocks were used because it allowed assessment of freezing due to a tone, a result of processing environmental information outside of the POR circuit thought to process context (Phillips and LeDoux, 1994; Bucci et al., 2000). There were no differences in freezing levels during acquisition (day 1), and animals in both groups showed comparable elevated freezing levels after the last tone-shock pairing. Freezing to context (day 2) was measured to assess the context-shock association. Overall freezing to the shock context decreased across the session in both groups; however, there was a significant reduction in freezing to context in animals with POR lesions compared to controls. Lastly, freezing during the tone (day 3) was measured to assess the tone-shock association. Animals in both groups showed an elevated level of freezing during tone presentation, with no differences between groups, indicating that the POR is not necessary for processing the tone-shock association, and that any deficit seen in context freezing was not due to differences in encoding or recall of the shock itself. Our results are in agreement with previous work suggesting that the POR plays a role in encoding environmental information in contextual fear conditioning (Bucci et al., 2000).
To determine if differences in freezing were due to overall differences in locomotion (i.e., hyperactivity) as a result of the lesion, rats were placed in an open field chamber following the final session of fear conditioning. There was an overall difference in total locomotion across the entire session, where POR lesioned animals traveled further than control animals. Because of this result we cannot exclude the possibility that POR lesioned animals froze less in the context test because of hyperactivity. However, there are several points to consider. First, this difference in locomotion only occurred in the presence of visual cues, as there was no difference between groups in the distance they traveled during the dark session (Experiment 1, Fig. 5D inset). One possible explanation for this pattern of results could be that the POR is recruited during the initial encoding of new configurations of visual stimuli, as would be the case when introducing animals to the open field for the first time. If the POR is damaged and rats are impaired at encoding a new environment, then locomotion might have remained elevated relative to controls as animals explored the novel open field environment. In contrast, the POR may not have been recruited when the animals explored the dark environment because the apparatus was familiar to them. Second, the similar amount of exploration in the dark suggests that any difference in freezing during the context test is unlikely to be explained solely on the basis of hyperactivity in the lesion group. Finally, despite the increased tendency for POR lesion animals to be more active in the open field, lesioned animals froze as much as controls in the tone test (Fig. 5C), indicating that any hyperactivity seen in the POR lesion group did not prevent them from freezing. Thus, the impaired freezing observed for the POR lesion group in the context test is more likely due to impaired ability in processing contextual information than to increased hyperactivity. Taken together, our findings indicate that lesions were effective in impairing freezing on the context test, but did not affect the ability of HD cells to remain in alignment with a landmark.
The postrhinal cortex and spatial navigation
The finding that the POR is not necessary for binding visual landmarks to the HD signal is somewhat surprising, given its connectivity to both visual areas 17/18 and to other areas of the brain involved in navigation. The POR is homologous to areas TH/TF in primates (Burwell et al., 1995), which is thought to occupy a position in the ventral or ‘what’ stream of visual processing. Conversely, other HD cell containing structures such as the PoS and retrosplenial cortex are believed to occupy a position in the dorsal or ‘where’ stream of visual processing (Ungerleider and Mishkin, 1982), and in particular in the branch of this stream that targets the medial temporal lobe component (Kravitz et al., 2011). Lesions of the PoS, and to a lesser extent the retrosplenial cortex, disrupt landmark control of HD cell responses (Goodridge and Taube, 1997; Clark et al., 2010; see Yoder et al., 2011 for review). Navigation, unlike simply reacting to stimuli automatically, is an online process that goes beyond mere perception and depends on recognizing objects as landmarks in order to maintain orientation and calculate a trajectory to a goal location. It therefore follows that damage to the ventral processing stream, which is involved in mnemonic object recognition, would affect an animal’s ability to use visual cues to determine direction. The absence of an effect due to the lesion raises the possibility that the lesion was incomplete or off-target. However, the fact that there was a deficit in processing context (which has been shown previously; see Bucci et al., 2000, 2002) independent of direction indicates that this structure plays a role in visual processing for context, but that it was not recruited when a single visual cue was informative about the spatial aspects of the environment in terms of landmark information. Taken together, it appears that environmental information enters the HD circuit through a variety of pathways, with each pathway making a unique contribution in reflecting real world events. The contribution of the POR appears to be contextual in nature, but by no means is it the only visual input, as the HD circuit can be influenced by visual objects independent of the POR.
Acknowledgments
We thank Jennifer M. Marcroft for technical assistance with surgical procedures and data collection, Bryn Adams for her artwork for the contextual fear conditioning experiment, and to David J. Bucci and Siobahn Robinson for their assistance with contextual fear conditioning and lesion quantification. This study was supported by NIH grant NS053907.
References
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19:1159–1186. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Taube JS. Neural correlates for angular head velocity in the rat dorsal tegmental nucleus. J Neurosci. 2001;21:5740–5751. doi: 10.1523/JNEUROSCI.21-15-05740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS. Lesions of the tegmentomammillary circuit in the head direction system disrupt the head direction signal in the anterior thalamus. J Neurosci. 2007;27:7564–7577. doi: 10.1523/JNEUROSCI.0268-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistic in Biology. New York: Academic Press; 1981. [Google Scholar]
- Blair HT, Cho J, Sharp PE. The anterior thalamic head-direction signal is abolished by bilateral but not unilateral lesions of the lateral mammillary nucleus. J Neurosci. 1999;19:6673–6683. doi: 10.1523/JNEUROSCI.19-15-06673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Sharp PE. Role of the lateral mammillary nucleus in the rat head direction circuit: a combined single-unit recording and lesion study. Neuron. 1998;21:1387–1397. doi: 10.1016/s0896-6273(00)80657-1. [DOI] [PubMed] [Google Scholar]
- Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser MB. Grid cells in the pre- and parasubiculum. Nat Neurosci. 2010;13:987–994. doi: 10.1038/nn.2602. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav Neurosci. 2000;114:882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behav Neurosci. 2002;116:479–488. [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J Comp Neurol. 2001;437:17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ. Perirhinal and postrhinal contributions to remote memory for context. J Neurosci. 2004;24:11023–11028. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Hafeman DM. Positional firing properties of postrhinal cortex neurons. Neuroscience. 2003;119:577–588. doi: 10.1016/s0306-4522(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: A review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Caballero-Bleda M, Witter MP. Projections from the presubiculum and the parasubiculum to morphologically characterized entorhinal-hippocampal projection neurons in the rat. Exp Brain Res. 1994;101:93–108. doi: 10.1007/BF00243220. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Bassett JP, Wang SS, Taube JS. Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. J Neurosci. 2010;30:5289–5302. doi: 10.1523/JNEUROSCI.3380-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Brown JE, Taube JS. Head direction cell activity in the anterodorsal thalamus requires intact supragenual nuclei. J Neurophysiol. 2012;108:2767–2784. doi: 10.1152/jn.00295.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW, Eichenbaum H, Rosenberg P, Eckmann KW. Afferent connections of the perirhinal cortex in the rat. J Comp Neurol. 1983;220:168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Goodridge JP, Taube JS. The effects of disorientation on visual landmark control of head direction cell orientation. Exp Brain Res. 1997;115:375–380. doi: 10.1007/pl00005707. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Deyoe EA, Press DZ, Rosen AC, Kanwisher N. Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cogn Neuropsychol. 2001;18:481–508. doi: 10.1080/02643290125929. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Etienne AS. The control of short-distance homing in the golden hamster. In: Ellen P, Thinus-Blanc C, editors. Cognitive Processes in Spatial Orientation in Animal and Man. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 223–251. [Google Scholar]
- Fanselow MS. Conditional and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical Analysis of Circular Data. Cambridge University Press; 1995. Revised edition. [Google Scholar]
- Furtak SC, Ahmed OJ, Burwell RD. Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron. 2012;76:976–988. doi: 10.1016/j.neuron.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17:709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating the semicircular canals of the squirrel monkey, Parts 1, 2, 3. J Neurophysiol. 1971;34:635–684. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS. Cue control and head direction cells. Behav Neurosci. 1998;112:749–761. doi: 10.1037//0735-7044.112.4.749. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Preferential use of landmark navigational system by head direction cells. Behav Neurosci. 1995;109:49–61. doi: 10.1037//0735-7044.109.1.49. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J Neurosci. 1997;17:9315–9330. doi: 10.1523/JNEUROSCI.17-23-09315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, van Dijk CA. Efferent connection of the dorsal tegmental region in the rat, studied by means of anterograde transport of the lectin phaseolus vulgari-leucoagglutinin (PHA-L) Brain Res. 1984;304:367–371. doi: 10.1016/0006-8993(84)90341-x. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zyo K. Retrograde double-labeling study of the mammillothalamic and mammillotegmental projections in the rat. J Comp Neurol. 1989;284:1–11. doi: 10.1002/cne.902840102. [DOI] [PubMed] [Google Scholar]
- Kornblith DJ, Cheng X, Ohayon S, Tsao DY. A network for scene processing in the macaque temporal lobe. Neuron. 2013;79:766–781. doi: 10.1016/j.neuron.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, Piette CE, Page H, Walters D, Marozzi E, Nardini M, Stringer S, Jeffery KJ. Weighted cue integration in the rodent head direction system. Phil Trans R Soc B. 2013;369:1–10. doi: 10.1098/rstb.2012.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubie JL. A driveable bundle of microwires for collecting single-unit data from freely-moving rats. Physiol Behav. 1984;32:115–118. doi: 10.1016/0031-9384(84)90080-5. [DOI] [PubMed] [Google Scholar]
- Liu P, Bilkey DK. The effects of NMDA lesions centered on the postrhinal cortex on spatial memory tasks in the rat. Behav Neurosci. 2002;116:860–873. doi: 10.1037//0735-7044.116.5.860. [DOI] [PubMed] [Google Scholar]
- Liu R, Chang L, Wickern G. The dorsal tegmental nucleus: an axoplasmic transport study. Brain Res. 1984;310:123–132. doi: 10.1016/0006-8993(84)90015-5. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;64:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Baker R. Anatomical connections of the nucleus prepositus in the cat. J Comp Neurol. 1985;237:377–407. doi: 10.1002/cne.902370308. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, et al., editors. Analysis of Visual Behavior. MIT Press; 1982. pp. 549–586. [Google Scholar]
- Mittelstaedt ML, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenshaften. 1980;67:566–567. [Google Scholar]
- Mizumori SJY, Williams JD. Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. J Neurosci. 1993;13:4015–4028. doi: 10.1523/JNEUROSCI.13-09-04015.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Compact. 6. San Diego: Academic Press; 2008. [Google Scholar]
- Phillips RG, LeDoux JD. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL. The firing of hippocampal place cells in the dark depends on the rat’s recent experience. J Neurosci. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Price JL, Hashikawa T. Cytoarchitectonic and chemoarchitectonic subdivisions of the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol. 2007;500:973–1006. doi: 10.1002/cne.21141. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Green C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J Neurosci. 1994;14:2339–2378. doi: 10.1523/JNEUROSCI.14-04-02339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PE, Tinkelman A, Cho J. Angular velocity and head direction signals recorded from the dorsal tegmental nucleus of Gudden in the rat: implications for path integration in the head direction cell circuit. Behav Neurosci. 2001;115:571–588. [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. In: Hanson SJ, Cowan JD, Giles CL, editors. Advances in Neural Information Processing Systems. Vol. 5. San Mateo, CA: Morgan Kaufmann; 1993. pp. 1030–1037. [Google Scholar]
- Stackman RW, Taube JS. Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J Neurosci. 1998;18:9020–9037. doi: 10.1523/JNEUROSCI.18-21-09020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: Organization of cortical inputs and interconnections with amygdala and striatum. Neurosciences. 1996;8:3–12. [Google Scholar]
- Suzuki WA, Amaral DG. The perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994a;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994b;14:1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: Origins and sensory-motor integration. Ann Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurophysiol. 1995;74:1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- Taube JS, Kesslak JP, Cotman CW. Lesions of the rat postsubiculum impair performance on spatial tasks. Behav Neural Biol. 1992;57:131–143. doi: 10.1016/0163-1047(92)90629-i. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990a;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990b;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The neocortex of Macaca mulatta. Urbana, IL: University of Illinois Press; 1947. [Google Scholar]
- van Groen T, Wyss JM. The postsubicular cortex in the rat: characterization of the fourth region of the subicular cortex and its connections. Brain Res. 1990;529:165–177. doi: 10.1016/0006-8993(90)90824-u. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor and postsubicular cortices. J Comp Neurol. 1983;216:192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Watson GS, Williams EJ. On the construction of significance tests on the circle and the sphere. Biometrika. 1956;43:344–352. [Google Scholar]
- Yeterian EH, Pandya DN. Corticothalamic connections of paralimbic regions in the rhesus monkey. J Comp Neurol. 1988;269:130–146. doi: 10.1002/cne.902690111. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Taube JS. Origins of landmark encoding in the brain. Trends Neurosci. 2011;34:567–571. doi: 10.1016/j.tins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Peck JR, Taube JS. Visual landmark information gains control of the head direction signal at the lateral mammillary nuclei. J Neurosci. 2015;35:1354–1367. doi: 10.1523/JNEUROSCI.1418-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. Head direction cell activity in mice: robust directional signal depends on intact otolith organs. J Neurosci. 2009;29:1061–1076. doi: 10.1523/JNEUROSCI.1679-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]