Abstract
Unlike other amino acids, the branched chain amino acids (BCAAs) largely bypass first pass liver degradation due to a lack of hepatocyte expression of the mitochondrial branched chain aminotransferase (BCATm). This sets up interorgan shuttling of BCAAs and liver-skeletal muscle cooperation in BCAA catabolism. To explore whether complete liver catabolism of BCAAs may impact BCAA shuttling in peripheral tissues, the BCATm gene was stably introduced into mouse liver. Two transgenic mouse lines with low and high hepatocyte expression of the BCATm transgene (LivTg-LE and LivTg-HE) were created and used to measure liver and plasma amino acid concentrations and determine whether the first two BCAA enzymatic steps in liver, skeletal muscle, heart, and kidney were impacted. Expression of the hepatic BCATm transgene lowered the concentrations of hepatic BCAAs while enhancing the concentrations of some nonessential amino acids. Extrahepatic BCAA metabolic enzymes and plasma amino acids were largely unaffected and no growth rate or body composition differences were observed in the transgenic animals as compared to wild type (WT) mice. Feeding the transgenic animals a high fat diet did not reverse the effect of the BCATm transgene on the hepatic BCAA catabolism nor did the high fat diet cause elevation in plasma BCAAs. However, the high fat diet fed BCATm transgenic animals experienced attenuation in the mammalian target of rapamycin (mTOR) pathway in the liver and had impaired blood glucose tolerance. These results suggest that complete liver BCAA metabolism influences the regulation of glucose utilization during diet-induced obesity.
Keywords: CATm, BCAA metabolism, liver transgenic mouse, high fat diet, amino acids
1. Introduction
The important regulatory role of BCAAs (Leu, Ile, and Val) as nutrient signals promoting protein synthesis [1] and as donors of nitrogen to Ala and Gln synthesis in skeletal muscle and other tissues [2,3] have led to their use in treating medical conditions, and as nutritional supplements [4,5]. Abnormal BCAA concentrations, on the other hand, have been linked to metabolic, cardiovascular, and neurological disorders [6,7]. Because they are essential amino acids, BCAA homeostasis is regulated by BCAA catabolism and differs from that of other essential amino acids in several important aspects. First, BCAA catabolism involves two initial enzymatic steps, common to all three BCAAs; thus, the dietary intake of an individual BCAA influences the catabolism of all three [8]. The first enzymatic step is the reversible transamination catalyzed by the BCAT isozymes (mitochondrial BCATm and cytosolic BCATc) [9]. The overall reaction is a transfer of the α-amino group from a BCAA to α-ketoglutarate (α-KG) to form Glu and the respective branched chain α-keto-acid (BCKA) [10]. The second step is the oxidative decarboxylation of BCKAs to their respective branched chain acyl-CoA derivatives and the formation of NADH. This step is catalyzed by the branched chain α-keto acid dehydrogenase enzyme complex BCKDC [11], an intra-mitochondrial multienzyme complex, organized around a core scaffold of dihydrolipoamide acyltransferase (E2) subunits, to which multiple copies of the branched chain α-keto acid dehydrogenase (E1), and the dihydrolipoamide dehydrogenase (E3), are attached noncovalently [12]. BCKDC is regulated by several known mechanisms [13]; the primary mechanism is phosphorylation and inactivation of the α-subunit of E1 enzyme (E1α) at Ser293 by the branched chain α-keto acid dehydrogenase kinase (BDK) [14].
Another important feature of BCAA metabolism is that BCAAs largely bypass liver degradation and travel via the circulation to peripheral tissues where they are transaminated by BCATm. Accordingly, BCATm is expressed in nearly all rodent, non-human primate, and human tissues with the exception of liver tissues [11]. Thus, transamination of BCAAs is largely extrahepatic and BCAA catabolic enzymes exhibit a wide tissue distribution. One consequence of the limited hepatic transamination of BCAAs, and distribution of BCATm and BCKDC activities in body tissues, is the considerable interorgan shuttling of BCAAs and their metabolites. This interorgan shuttling evolved not merely to conserve BCAAs, but also because BCAAs play an important role in nutrient signaling and body nitrogen metabolism. A sharp rise of plasma Leu is observed in response to a meal that may promote Leu signaling via complex I of mTOR pathway (mTORC1) in peripheral tissues and stimulate protein synthesis [15]. Additionally, BCAAs contribute nitrogen to the Glu-Gln shuttle between neurons and astrocytes in the brain and Glu and Ala metabolism between skeletal muscle and liver [3,16].
Different animal models of obesity and human studies have revealed that the plasma concentrations of BCAAs are elevated during obesity [17–20]. Elevation in plasma BCAA concentrations as well as supplementation with BCAAs are described as beneficial in improving body weight control, adiposity, muscle glucose uptake, glucose tolerance, and resistance to diet induced obesity [18,21]. Tissue specific alterations in BCAA metabolism can contribute to elevations in plasma BCAAs during obesity. In liver and adipose tissues from Zucker rats, low expression levels of BCATm and BCKDC were associated with elevated plasma BCAAs [19]. Likewise, BCKDC was found to be significantly lower in subcutaneous adipocytes of obese humans [18]. However, incomplete, or impaired BCAA metabolism has been also associated with increased sensitivity to insulin resistance and diabetes [22].
The goal of this study was to create a mouse with the complete BCAA catabolic pathway present in the liver potentially restricting the interorgan shuttling of BCAAs. To achieve this goal, the BCATm gene (Bcat2) was stably introduced into mouse liver creating a unique transgenic mouse model capable of both hepatic transamination and oxidation of BCAAs. As a result, hepatic but not plasma concentrations of BCAAs (with the exception of Leu) were lowered along with an enhanced liver nonessential amino acid formation. This animal was then challenged with a high fat diet during which liver BCAA oxidative capacity was enhanced further; the higher capacity to metabolize BCAAs in the liver, however, prevented the elevation of plasma BCAAs in response to the high fat diet and resulted in a lower BCAA/AAA (aromatic amino acid) ratio along with higher blood glucose concentrations.
2. Methods
2.1. Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee at Virginia Tech and Wake Forest University School of Medicine. WT and transgenic mice, carrying the mouse Bcat2 gene encoding BCATm protein in their liver (liver transgenic LivTg mice), were used throughout the study, and were from the same background, C57BL/6 (see generation of transgenic mice below). WT and LivTg mice were housed in colony cages in a temperature controlled room with a 12-hour light/12-hour dark cycle and were given free access to water and offered a standard rodent chow (Harlan-Teklad 2018). Both male and female mice were used in most of the experiments with essentially the same results. Either mixed gender or male data are shown in the manuscript. Average animal age was 30 weeks and 5–12 animals per group were used during the experiments.
2.2. Generation of transgenic mice overexpressing mouse BCATm in the liver
A full-length mouse Bcat2 cDNA (GenBank accession number NC_000073.6) was cloned into pLIV.11 vector that contains the constitutive human apoE gene promoter and its hepatic control region, to direct the transgene expression in liver [23]. After digestion with SpeI and Sall, a fragment containing the human ApoE promoter, the mouse Bcat2 cDNA, the poly(A) signal from the human ApoE gene, and the hepatic control region, was isolated and microinjected into the male pronucleous of fertilized eggs from C57BL/6 females in the Transgenic Mouse Core Facility of Wake Forest University Health Sciences [24]. Ear DNA PCR analysis was performed to identify founder mice that harbored the integrated BCATm transgene in the liver. As a result, two female founder mice, with different levels of BCATm expression, were determined to exhibit germline transmission. Two transgenic mouse lines, with low and higher expression levels of the BCATm transgene in their liver (LivTg-LE [low expressor], LivTg-HE [high expressor], respectively), were confirmed by Western blotting and used in this study. They were maintained as hemizygotes by breeding with WT C57BL/6 mice, while nontransgenic WT littermates were used as controls. To genotype, ear DNA was lysed with an alkaline lysis reagent (25 mM NaOH, 0.2 mM EDTA) at 95°C for 1 h and neutralized with an equal volume of 40 mM Tris-HCl, and used for PCR analysis with the following primers: BCATmLivTgFor 5’ –ATGGCTGCAGCCACACTAGGA −3’; BCATmLivTgRev 5’ –GTGACCATACCAACACCCA −3’. A PCR product of 560 bp was produced from BCATmLivTg DNA, but not from WT DNA. Vldlr (very-low-density-lipoprotein receptor) was used as a positive loading control with the following primers: VldlrFor 5’ – GCAGATGAGTTCACTGGCTCC −3’; VldlrRev 5’ –AGGGCAGTTGACCTCATCGCT −3’ and with a PCR product of ~400 bp.
2.3. High fat diet study
WT and LivTg-HE male mice were offered a high fat diet (60% of Kcal from fat, Research Diets D12492, see supplemental Table 1) at the age of 6 weeks. Total duration of the high fat diet study was 18 weeks and the actual mouse age at the end of the high fat diet feeding was 24 weeks. At 0, 3, 9, and 12 weeks of the high fat diet study, body composition of WT and LivTg-HE mice was evaluated by using Bruker Live Mice Analyzer (BRUKER LF-90 TD-NMR) (Bruker Optics, Inc) as described (17). At 0 and 14 weeks of the high fat diet feeding, WT and LivTg-HE mice were subjected to a glucose tolerance test (GTT). At the end of the high fat diet study (18 weeks), all mice were fasted for 12 h and gavaged with Leu (118 mM Leu diluted in 0.5% carboxymethylcellulose (wt:v) in distilled water). Leu was injected with a feeding needle through the mouse mouth (1.35 mg/g body weight) (23). At 30 min following Leu administration, mice were euthanized by asphyxiation with carbon dioxide and cervical dislocation then blood and organs were collected.
2.4. Western Blotting analysis
Frozen powdered liver, muscle, kidney, and heart tissues were ground and re-suspended in an extraction buffer (25 mM HEPES, pH 7.0, 0.4% CHAPS, 1 mM DTT) containing 1 mM EDTA, 1 mM EGTA, 5 mM benzamidine, 0.5 mM microstatin, 33 mM β-glycerophosphate, 0.5 mM thiamine pyrophosphate (TPP), 1 mM sodium orthovanadate, 7.2 mM potassium fluoride (KF) and 1:100 dilution of Calbiochem protease inhibitor cocktail set III (EMD Biosciences, La Jolla, CA). Each extract was sonicated twice, centrifuged at 13,200 rpm for 15 min at 4°C, and supernatants were collected. Total protein content, protein separation, and Western Blotting were performed as described [25]. E1α and E2 peptide antibodies [26] were used to detect BCKDC. In the case of the E1α subunit of BCKDC, an antibody that detects a phosphorylation at serine 293 (pS293) on the E1α subunit was used. A reversible phosphorylation of S293 on the E1α subunit by the BDK enzyme [14] causes inactivation of BCKDC and was utilized to measure the activity state of BCKDC. BCAT isozyme-specific antisera were raised in rabbits using purified recombinant human BCATm, and affinity purified as described [11,27]. For mTOR signaling detection, 4E-BP1 (eukaryotic translation initiation factor 4E binding protein 1), P-4E-BP1 (Thr37/46), S6K (70-kDa ribosomal protein S6 kinase) and P-S6K (Thr389) along with β-tubulin, and pan-actin antibodies were purchased from Cell Signaling Technologies (Danvers, MA). Image J software [28] was used to quantify each protein band and normalize to a loading control (pan actin or β-tubulin) before calculating the ratio between phosphorylated and total E1α, 4E-BP1, and/or S6K.
2.5. BCAT enzyme activity
Total BCAT activity in liver extracts was measured at 37°C in 25 mM potassium phosphate buffer, pH 7.8, using 1 mM α-keto[1-14C]isovalerate (KIV) and 12 mM Ile as described [9]. A unit of enzyme activity (U) was defined as 1 µmol of Val formed per minute at 37°C.
2.6. Glucose tolerance test (GTT)
GTT was performed in overnight fasted mice. Mice were injected intraperitoneally with 10% glucose at 2.0 mg/g body weight; and tail vein blood glucose was measured at 15, 30, 60, 90 and 120 min after injection.
2.7. Amino acid analysis
Amino acid concentrations in liver tissue and mouse plasma from WT, LivTg-LE, and LivTg-HE mice were measured by HPLC after O-phthaldialdehyde derivatization on a Supelcosil™ LC-18 column (15 cm × 4.6 mm, 3 µm) (Sigma, St. Louis, MO) as described previously [29]. All samples were deproteinized with 1.5 M perchloric acid (HClO4) and neutralized with 2 M potassium carbonate (K2CO3).
2.8. Statistical Analysis
One-way ANOVA was used to assess the difference between WT, LivTg-LE, and LivTg-HE mice on a standard rodent chow diet. Two tailed non-paired t-test was used to assess the difference between WT and LivTg-HE mice on a high fat diet. Values are means ± SEM and P ≤ 0.05 was considered significantly different.
3. Results
3.1. Expression of the BCATm transgene in the mouse liver
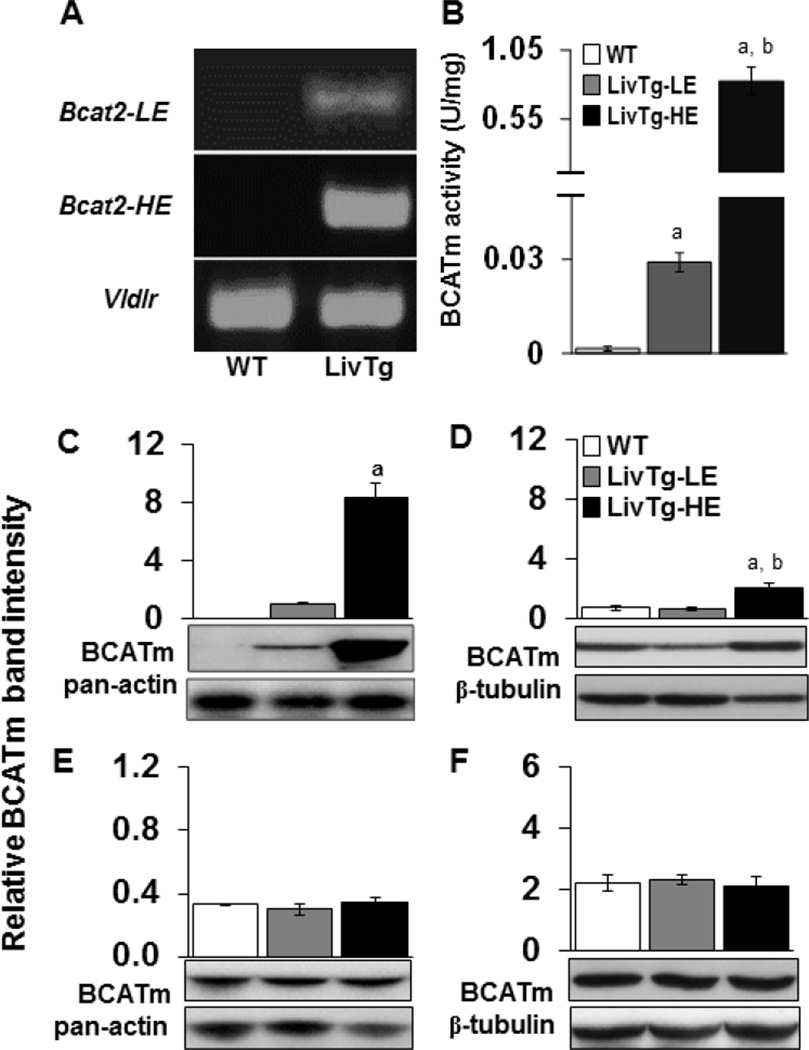
Two transgenic lines, expressing a BCATm transgene (Bcat2) in the liver, were established with a PCR product of 560 kb (Fig. 1A). WT mice did not produce the Bcat2 band consistent with a lack of BCATm expression in rodent liver [11]. Vldlr was used as a positive loading control, with a PCR product of about 400 kb (Fig. 1A). Introduction of the BCATm transgene into mouse liver resulted in an active BCATm protein as measured by the BCAT activity assay. As shown in Fig. 1B, liver BCAT activity in LivTg-LE and LivTg-HE was 0.029 and 0.83 U/mg protein, respectively, compared to <0.001 U/mg protein in the control mouse livers. WT animals did not have detectable BCATm protein in their liver, while LivTg-HE had around 8.8–fold higher liver BCATm protein expression as compared to LivTg-LE (Fig. 1C), suggesting the activity assay was more sensitive than Western blotting. Comparison of BCATm protein expression (transgenic and endogenous BCATm) in kidney, muscle, and heart revealed that BCATm protein expression was 3.2-fold higher in kidney of LivTg-HE but unchanged in muscle or heart tissues as compared to WT or LivTg-LE animals (Fig. 1D–F). This finding is consistent with low expression of ApoE in kidney [30] being responsible for increased BCATm expression in the kidney of LivTg-HE animals. In summary, the BCATm transgene was stably introduced in the liver of two transgenic lines, expressing low (LivTg-LE) and high (LivTg-HE) levels of hepatic BCATm.
Fig. 1.
Tissue expression of BCATm in mice with low and high expression of the BCATm transgene. (A) Genomic PCR analysis of Bcat2 gene, which encodes BCATm, from ear DNA of either LivTg-LE (Bcat2-LE) or LivTg-HE (Bcat2-HE) mice. The transgene allele is about 560 bp, Vldlr was used as a loading control, while WT did not produce Bcat2. (B) Liver BCATm activity (U/mg = 1 µmol Val formed/min/mg protein at 37oC) in WT (white bar), LivTg-LE (gray bar) and LivTg-HE (black bar) mice was measured as described in Methods. (C–F) Western blotting of total BCATm protein (transgenic plus endogenous BCATm) from liver (C), kidney (D), muscle (E), and heart (F) tissues of WT, LivTg-LE, and LivTg-HE mice consuming a standard rodent chow. Pan-actin or β-tubulin was used as a loading control. Data represent mean ± SEM, n=6–12, mixed gender (A, B) or male mice (C–F), average age 30 weeks, aP≤ 0.05 as compared to WT; bP≤ 0.05 as compared to LivTg-LE.
3.2. BCKDC activity state is increased in the liver of BCATm transgenic mice
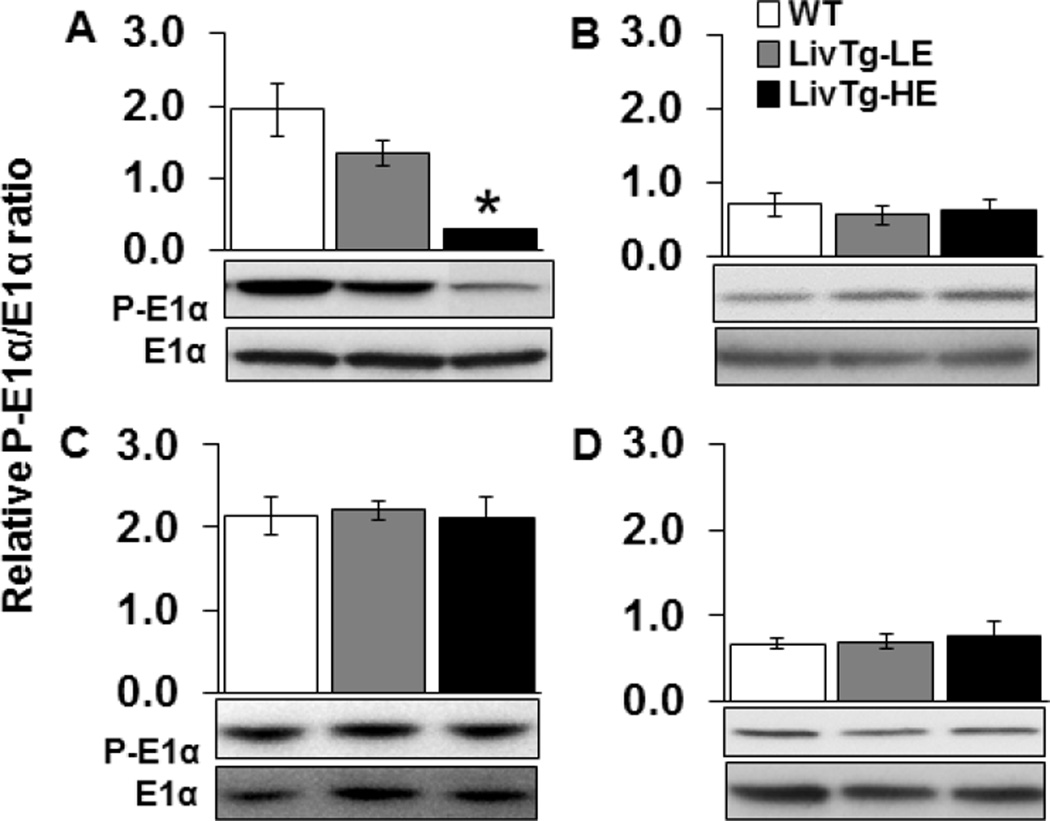
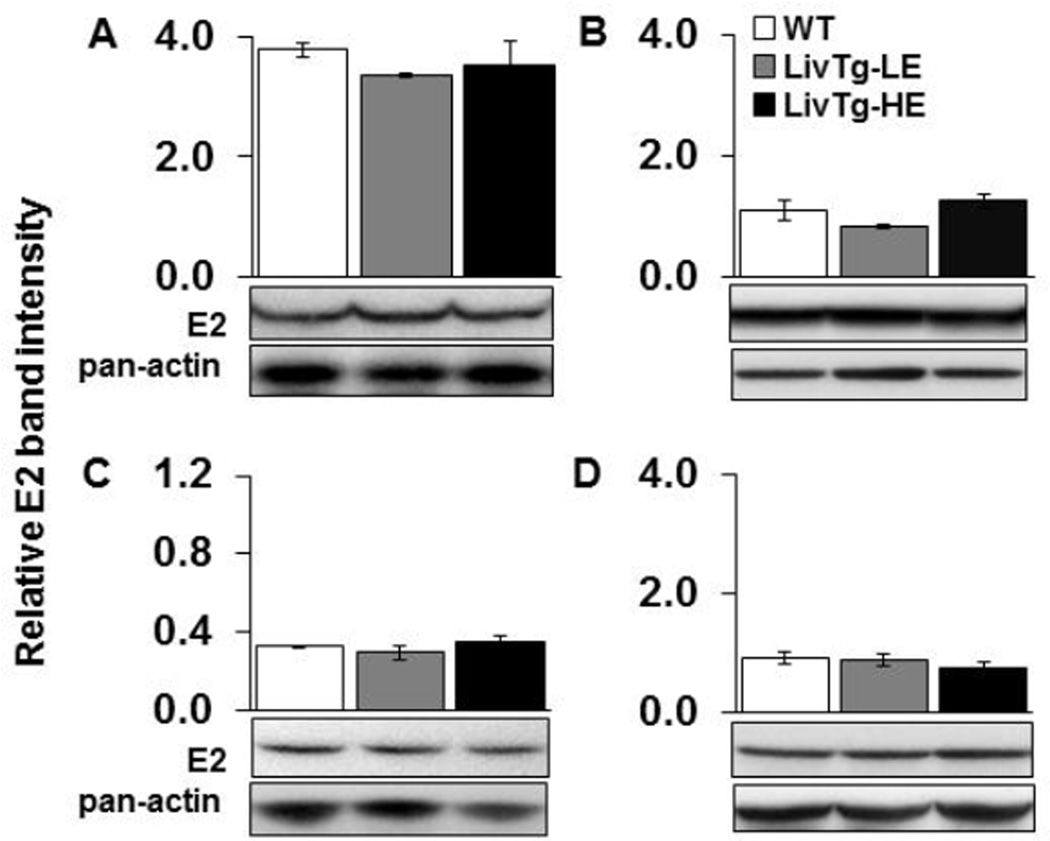
Activity of BCKDC is controlled by phosphorylation of the E1α subunit at Ser293 by BDK causing inactivation of the E1 enzyme and BCKDC [14,31]. To determine whether expression of BCATm in mouse liver results in compensatory alterations in BCKDC enzymes or activity state, phosphorylation of E1α, as well as levels of E1α protein and E2 enzyme protein were measured in liver, kidney, skeletal muscle, and heart tissues (Fig. 2 and 3). In the liver, the relative ratio between phosphorylated and total E1α was between 32% and 85% lower in LivTg-LE and LivTg-HE, respectively, as compared to WT (Fig. 2A). In contrast, the phosphorylation of E1α was unaffected in kidney, skeletal muscle, or heart tissues of the transgenic animals (Fig. 2B–D). E2 protein concentrations were unchanged in all transgenic mouse tissues examined (Fig. 3A–D). Therefore, introducing BCATm transgene in the liver increases BCKDC activity as seen by the decreased phosphorylation ratio between P-E1α and E1α. In the non-hepatic tissues, BCKDC activity and enzyme levels were unaffected so the small increase in the kidney BCATm was not sufficient to change activity state indicating that transgenic expression of BCATm in the mouse liver does not result in obvious alterations in BCAA catabolic enzymes outside the liver.
Fig. 2.
Expression of liver BCATm results in activation of hepatic but not extrahepatic BCKDC (E1α). E1α and P-E1α were determined by Western Blotting in liver (A), kidney (B), muscle (C), and heart (D) tissues of WT (white bar), LivTg-LE (gray bar), and LivTg-HE (black bar) mice consuming a standard rodent chow. Each graph shows the relative ratio between the phosphorylated and total form of E1α. Data represent mean ± SEM, n=6 male mice, average age 30 weeks. *P≤ 0.05 as compared to WT.
Fig. 3.
BCKDC (E2) is not altered in the transgenic animals when fed a standard rodent chow. E2 was determined by Western Blotting in liver (A), kidney (B), muscle (C), and heart (D) tissues of WT (white bar), LivTg-LE (gray bar), and LivTg-HE (black bar) mice consuming a standard rodent chow. Each graph shows the relative E2 band intensity normalized to pan-actin. Data represent mean ± SEM, n=6 male mice, average age = 30 weeks.
3.3. Liver BCATm transgene expression alters hepatic amino acid concentrations
Introducing BCATm transgene into mouse liver established hepatic BCATm activity and resulted in upregulation of BCKDC activity as measured by Western blotting. These changes in the first two catabolic enzymes in liver made increased first pass BCAA catabolism feasible, and if significant, could result in decreased plasma or tissue BCAA concentrations and increased net transfer of BCAA nitrogen to Glu (then Gln and Ala) in the liver. Therefore, liver and plasma amino acid concentrations were measured in LivTg-LE and LivTg-HE mice and compared with WT littermates. As shown in Table 1, liver BCAAs (Leu, Ile, and Val) were 2.1-, 2.2- and 3-fold higher in WT as compared to LivTg-HE mice. Statistically significant reductions in liver BCAA concentrations were also observed in the LivTg-LE mice (Table 1). The dispensable amino acids Glu and Asp were 2.8-fold and 84% higher in the liver of LivTg-HE animals. These results suggest increased liver transamination of BCAAs due to the expression of BCATm transgene especially in LivTg-HE animals. Note that increased Glu concentrations in the liver of LivTg-HE did not affect liver Gln and Ala concentrations, which is consistent with the interorgan shuttling of these amino acids. On the other hand, Ser, which is synthesized from Ala, was 50% higher in LivTg-HE animals. Tyr, Arg, and Cit were 44%, 85%, and 84% higher in the liver of LivTg-HE but unchanged in LivTg-LE as compared to WT (Table 1). Changes in Gly, Ser, and Tau may result from alterations in Met and one-carbon metabolism. Higher Cit may reflect increased urea cycle activity. Therefore, introducing BCATm in the liver lowered BCAA concentrations in liver, but only impacted hepatic metabolism of amino acids involved in the urea cycle (Arg, Asp, Cit, Glu) and sulfur amino acids (see Met and Tau) at very high levels of BCATm expression (LivTg-HE animals).
Table 1.
Liver amino acid concentrations (µmol/g wet tissue) of
| WT | LivTg-LE | LivTg-HE | |
|---|---|---|---|
| Leu | 0.36 ± 0.02 | 0.20 ± 0.05* | 0.17 ± 0.02* |
| Ile | 0.26 ± 0.01 | 0.13 ± 0.02* | 0.12 ± 0.01* |
| Val | 0.42 ± 0.03 | 0.20 ± 0.05* | 0.14 ± 0.02* |
| His | 0.59 ± 0.03 | 0.32 ± 0.29 | 0.42 ± 0.15 |
| Met | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.08 ± 0.01* |
| Phe | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.01 |
| Trp | 0.11 ± 0.00 | 0.10 ± 0.01 | 0.12 ± 0.01 |
| Thr | 0.28 ± 0.02 | 0.26 ± 0.04 | 0.33 ± 0.02 |
| Lys | 0.46 ± 0.02 | 0.44 ± 0.05 | 0.47 ± 0.06 |
| Glu | 1.27 ± 0.13 | 1.33 ± 0.50 | 3.53 ± 0.60* |
| Gln | 5.62 ± 0.27 | 5.97 ± 1.88 | 5.27 ± 1.08 |
| Asp | 0.39 ± 0.03 | 0.39 ± 0.13 | 0.72 ± 0.21* |
| Asn | 0.21 ± 0.01 | 0.20 ± 0.04 | 0.25 ± 0.02 |
| Ala | 3.44 ± 0.14 | 3.83 ± 0.32 | 3.40 ± 0.91 |
| Gly | 2.76 ± 0.06 | 2.50 ± 0.26* | 3.34 ± 0.55 |
| Ser | 0.24 ± 0.02 | 0.20 ± 0.06 | 0.36 ± 0.04* |
| Tyr | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.13 ± 0.01* |
| Arg | 0.33 ± 0.02 | 0.33 ± 0.03 | 0.61 ± 0.16* |
| Tau | 18.8 ± 0.18 | 17.5 ± 2.16 | 16.05 ± 0.39* |
| Orn | 0.85 ± 0.06 | 0.86 ± 0.08 | 0.72 ± 0.21 |
| Cit | 0.37 ± 0.02 | 0.37 ± 0.03 | 0.68 ± 0.18* |
WT, LivTg-LE, and LivTg-HE male mice were fed a standard rodent chow and after euthanasia liver was collected and used in HPLC assay (see Methods). Data represent mean ± SEM, n=5–9, age between 24–33 weeks,
P≤0.05 as compared to WT mice.
In contrast, with the exception of Leu which was significantly lower (37% less) in LivTg-HE animals, plasma amino acid concentrations were largely unchanged in the transgenic animals (Table 2). Decreases in Ile and Val did not reach significance. Plasma Tyr was significantly higher by 48% and 60% in LivTg-LE and LivTg-HE, respectively (Table 2), and it was also elevated in the liver of LivTg-HE animals (Table 1). Thus, the results suggest the mice were able to compensate for local changes in liver amino acid concentrations and/or metabolism while plasma amino acid concentrations were for the most part maintained within the range observed in WT animals.
Table 2.
Plasma amino acid concentrations (µM) of fed mice
| WT | LivTg-LE | LivTg-HE | |
|---|---|---|---|
| Leu | 141.6 ± 9 | 117.9 ± 12 | 89.8 ± 11* |
| Ile | 92.3 ± 5 | 88.1 ± 13 | 72.5 ± 10 |
| Val | 214.3 ± 18 | 233.5 ± 43 | 185.5 ± 26 |
| His | 85.1 ± 6 | 113.7 ± 11* | 96.5 ± 9 |
| Met | 84.2 ± 9 | 96.6 ± 11 | 89.2 ± 11 |
| Phe | 75.0 ± 5 | 95.0 ± 12 | 82.0 ± 7 |
| Trp | 89.8 ± 7 | 118.3 ± 19 | 102.4 ± 8 |
| Thr | 103.5 ± 10 | 121.4 ± 19 | 121.0 ± 16 |
| Lys | 241.7 ± 2 | 278.6 ± 30 | 288.5 ± 32 |
| Glu | 40.6 ± 2 | 50.0 ± 13 | 46.2 ± 6 |
| Gln | 1093.2 ± 77 | 1235.8 ± 88* | 977.3 ± 93 |
| Asp | 12.7 ± 1 | 16.7 ± 5 | 14.4 ± 3 |
| Asn | 57.3 ± 6 | 72.0 ± 11 | 60.4 ± 10 |
| Ala | 257.3 ± 23 | 321.3 ± 22 | 279.5 ± 36 |
| Gly | 280.5 ± 15 | 310.4 ± 30 | 197.2 ± 14 |
| Ser | 142.0 ± 10 | 155.0 ± 15 | 138.3 ± 15 |
| Tyr | 50.5 ± 4 | 74.8 ± 13* | 81.0 ± 11* |
| Arg | 133.2 ± 12 | 141.5 ± 16 | 150.7 ± 17 |
| Tau | 576.7 ± 26 | 579.0 ± 73 | 534.5 ± 33 |
| Orn | 51.4 ± 9 | 46.3 ± 13 | 52.2 ± 14 |
| Cit | 61.5 ± 4 | 72.0 ± 8 | 63.0 ± 1 |
WT, LivTg-LE, and LivTg-HE male mice were fed a standard rodent chow before drawing blood for analysis (see Methods). Data represent mean ± SEM, n=5–9, age between 24–33 weeks,
P≤0.05 as compared to WT mice.
3.4. Liver BCATm transgenic mice do not show altered growth rate, organ weight, or body composition
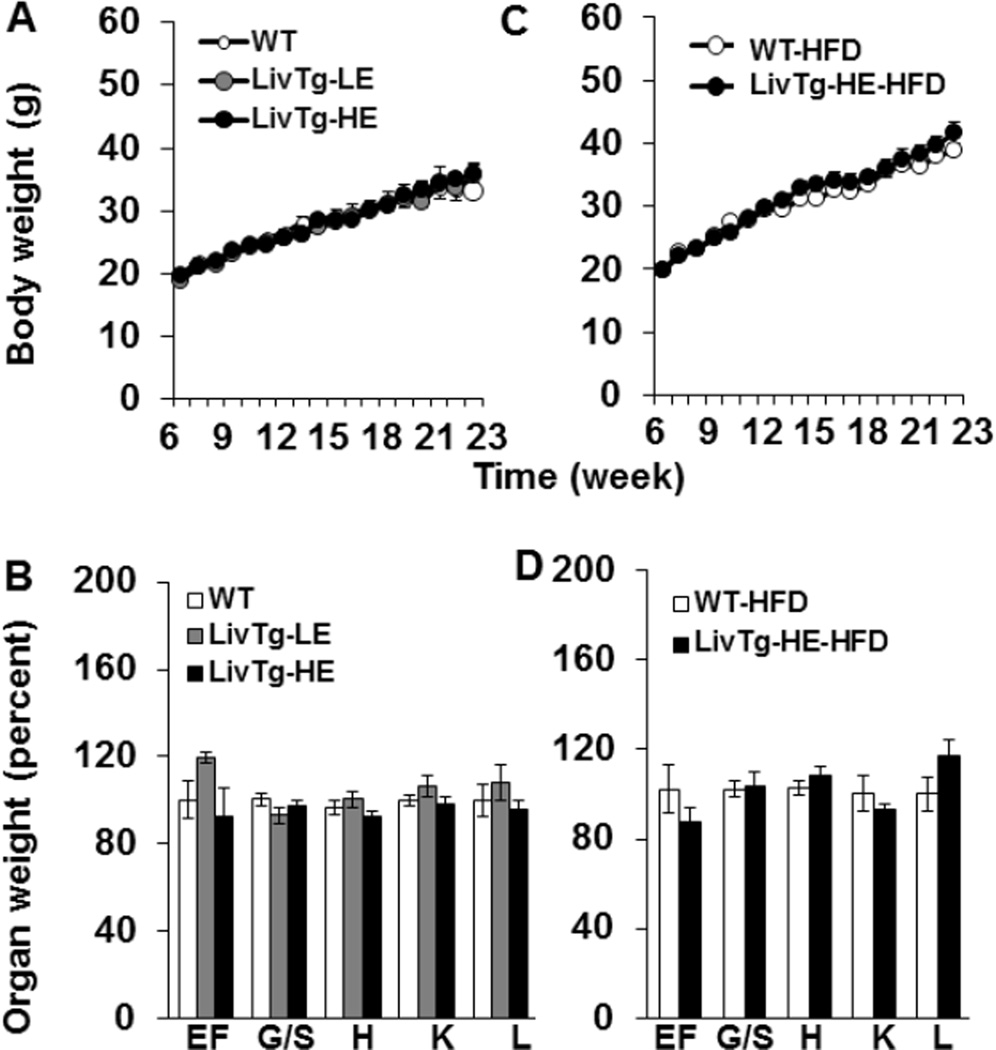
When the transgenic animals were fed a standard rodent chow diet, there were no noticeable differences in their growth rate or organ weights as compared to WT animals (Fig. 4A,B). Because global BCATm−/− mice are resistant to diet-induced obesity [20] and Ile and Leu are ketogenic amino acids, the impact of a high fat diet on LivTg-HE animals was also tested. LivTg-HE animals were chosen because they showed more pronounced changes in liver BCAA metabolism and Leu plasma concentrations (Fig. 1– 2, and Table 2). WT and LivTg-HE animals were fed a high fat diet (60% Kcal) for 18 weeks starting at 6 weeks of age. Although the high fat diet feeding resulted in increased body weight over time, no differences were observed in either body weight or organ weights between WT and LivTg-HE mice (Fig. 4C, D). Likewise, body composition, expressed as a percent of body weight, showed that all animals exhibited an increased fat mass and body fluid by around 5-fold and 26%, respectively, and a decreased lean mass (around 25%), after 12 weeks on the high fat diet with no differences between WT and LivTg-HE mice (Table 3). Thus, expression of BCATm in mouse liver does not alter growth rate, organ weights, or body composition of LivTg-HE mice on either standard rodent chow or high fat diet.
Fig. 4.
Expression of hepatic BCATm does not alter the growth rate or organ weights of the transgenic mice. (A,B) Growth curve (A) and organ weights (B) of WT (white circle/bar), LivTg-LE (gray circle/ bar), and LivTg-HE (black circle/ bar) consuming a standard rodent chow diet. (C,D) Growth curve (C) and organ weights (D) of WT (white circle/bar), and LivTg-HE (black circle/ bar) consuming a high fat diet (HFD). Data represent mean ± SEM, n=8–16 (A,B) and n=7–8 (C,D). Weekly weights were measured starting at 6 weeks, however the organ weights were measured at week 29 for mice on standard rodent chow diet and at week 24 for mice on HFD. Organ weights of LivTg-HE mice were expressed as a percent of WT organ weights. Organ weight abbreviations: epididymal fat (EF), gastrocnemius/soleus muscle (G/S), heart (H), kidney (K), and liver (L).
Table 3.
BCATm activity and body composition of WT and LivTg-HE mice before (0 weeks) and after 12 weeks of high fat diet (HFD)
| 0 weeks | 12 weeks | |||
|---|---|---|---|---|
| WT | LivTg-HE | WT-HFD | LivTg-HE-HFD | |
| BCATm activity (U/mg) | - | - | <0.0 ± 0.0 | 0.7a ± 0.1 |
| Body fat (% of BW) | 5.1 ± 0.5 | 5.5 ± 0.3 | 25.0b ± 1.4 | 26.0c ± 1.2 |
| Lean mass (% of BW) | 77.5 ± 0.6 | 77.9 ± 0.6 | 57.9b ± 1.2 | 56.1c ± 1.1 |
| Fluid (% of BW) | 6.9 ± 0.1 | 6.9 ± 0.1 | 8.7b ± 0.2 | 8.6c ± 0.1 |
Body fat, lean mass, and fluid were measured after 0, 3, 9 and 12 weeks of high fat diet feeding. The data from 0 and 12 weeks are shown. Data represent mean ± SEM, n=6–12 male mice,
P≤0.05 as compared to WT-HFD mice,
P≤0.05 as compared to WT or LivTg-HE mice at 0 weeks of high fat diet, respectively. BW, body weight.
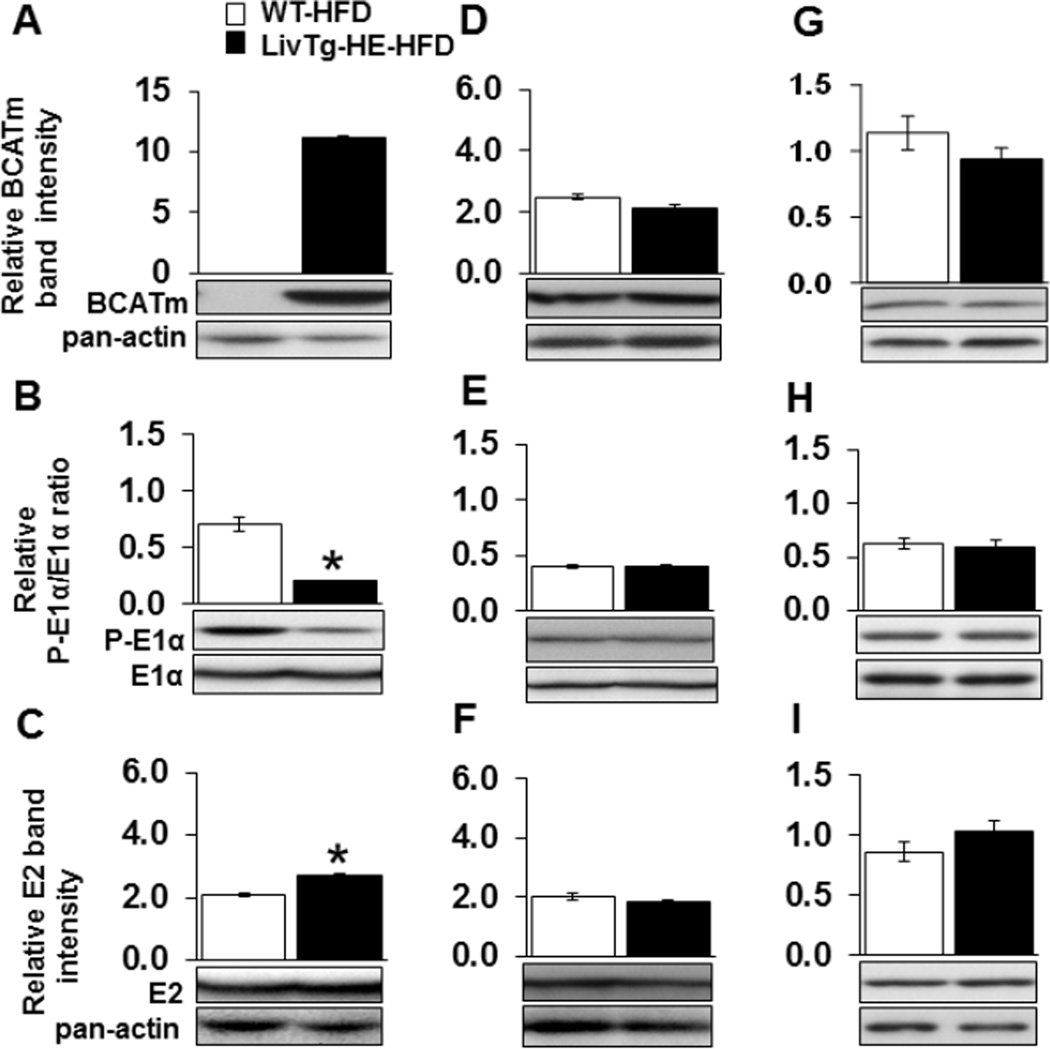
3.5. Hepatic BCATm and BCKDC activity state remain higher in LivTg-HE fed a high fat diet
High fat diet feeding did not cause alterations in the activity and expression of BCATm in the liver of LivTg-HE, which continued to express high concentrations of liver BCATm protein during the high fat diet feeding (Fig. 5A and Table 3). Additionally, the phosphorylation of liver E1α was 3.5-fold higher (Fig. 5B), and the expression of liver E2 enzyme was 30% less in WT as compared to LivTg-HE at the end of the high fat diet study (Fig. 5C). On the other hand, BCATm and BCKDC remained unaltered in skeletal muscle and heart of LivTg-HE compared to WT mice fed a high fat diet (Fig. 5D–I). Thus, in high fat diet fed LivTg-HE mice, liver BCKDC capacity and activity state were higher than in WT mice.
Fig. 5.
Expression of BCATm raises BCKDC activity in the mouse liver under high fat diet feeding. BCATm (A,D,G), E1α and P-E1α (B,E,H), and E2 (C,F,I) were determined by Western Blotting in liver (A,B,C), muscle (D,E,F), and heart (G,H,I) tissues of WT (white bar) and LivTg-HE (black bar) consuming a high fat diet (HFD). For E1α, each graph shows the relative ratio between the phosphorylated and total E1α protein. For BCATm and E2, each graph shows the relative band intensity normalized to pan-actin. Data represent mean ± SEM, n=6 male mice, age 24 weeks. *P≤ 0.05 as compared to WT.
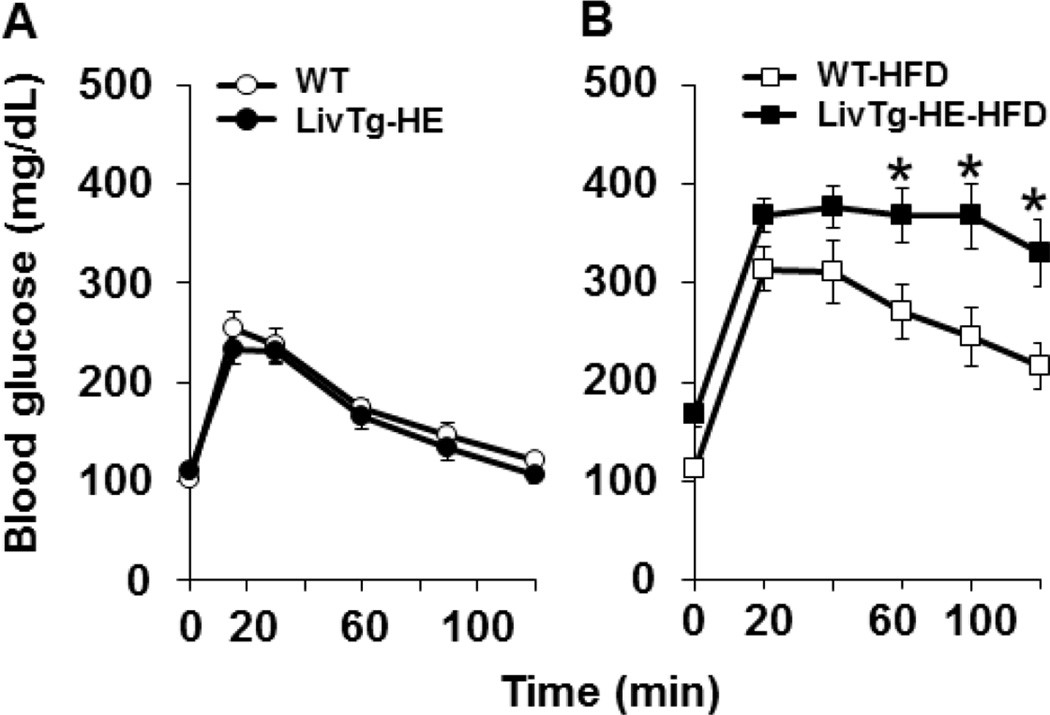
3.6. Blood glucose is affected by the liver BCATm transgene in mice fed a high fat diet
Since global loss of the mouse BCATm protein resulted in improved glucose tolerance during high fat diet feeding [20], the influence of introduction of BCATm on GTT was tested in the LivTg-HE and WT mice at the start and after 14 weeks on the high fat diet. Prior to introducing the high fat diet, no differences were observed in fasting blood glucose between LivTg-HE and WT animals (Fig. 6A). In contrast, fasting blood glucose was about 20% higher in LivTg-HE than in WT animals fed high the fat diet for 14 weeks (Fig. 6B). Following the glucose challenge, blood glucose concentrations remained significantly higher in LivTg-HE after 120 min (Fig. 6B) suggesting impaired glucose tolerance which resulted from the high fat diet being exacerbating in the LivTg-HE animals compared to WT.
Fig. 6.
High fat diet feeding results in higher blood glucose and impaired glucose tolerance in LivTg-HE mice compared to WT mice fed the same diet. (A, B) GTT. Blood glucose of WT and LivTg-HE mice was measured at 0 weeks (A) or after 14 weeks of a high fat diet feeding (HFD) (B). Data represent mean ± SEM, n=6–12, male mice, age 20 weeks, *P≤ 0.05 as compared to WT-HFD.
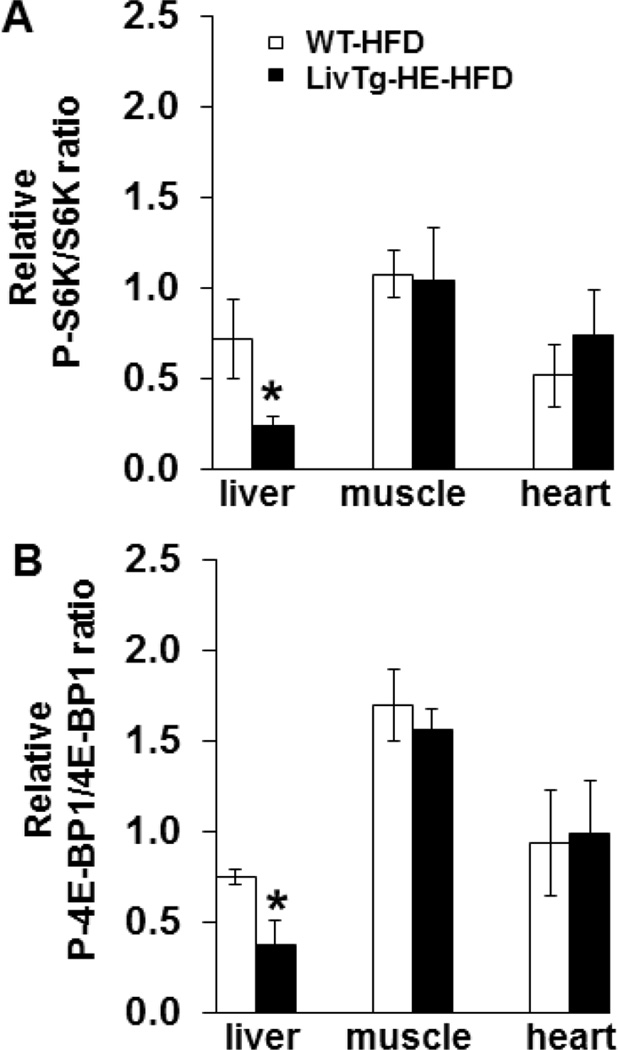
3.7. Hepatic but not peripheral mTORC1 signaling is attenuated in LivTg-HE mice fed a high fat diet
mTOR signaling pathway has been implicated in the regulation of glucose metabolism [25,32], while Leu is a known activator of mTORC1 [1]. To determine whether the increased blood glucose in LivTg-HE is associated with changes in mTORC1, the phosphorylation state and total protein concentrations of mTORC1 downstream targets (4E-BP1 and S6K) were measured in liver, skeletal muscle, and heart of high fat diet fed WT and LivTg-HE mice by Western blotting. As shown in Fig. 7A and B, the ratio of P-S6K and S6K and P-4E-BP1 and 4E-BP1 were significantly lower by 67% and 51%, respectively, in liver of LivTg-HE as compared to WT mice fed a high fat diet. However, no changes in mTORC1 signaling were observed in skeletal muscle or heart tissues in high fat diet fed LivTg-HE and WT mice (Fig. 7A,B). Likewise, no significant changes in mTORC1 signaling in liver or peripheral tissues were observed when transgenic animals were fed a standard rodent chow alone (data not shown). In conclusion, LivTg-HE mice fed a high fat diet had lower liver 4E-BP1 and S6K phosphorylation as compared to WT animals suggesting attenuation in liver mTORC1 signaling in response to increased hepatic BCAA (Leu) catabolism in these animals (see Fig. 5A–C), whereas, extrahepatic tissues were not affected.
Fig. 7.
Phosphorylation of S6K and 4E-BP1 is lower in the liver of LivTg-HE mice fed a high fat diet (HFD) compared to WT mice fed the same diet. P-S6K, S6K, P-4E-BP1, and 4E-BP1 were determined by Western Blotting in liver, muscle, and heart of Leu-gavaged WT-HFD and LivTg-HE-HFD mice. Animals were sacrificed 30 min after Leu gavage. Each graph shows the relative ratio between the phosphorylated and total form of S6K (A) and 4E-BP1(B). Data represent mean ± SEM, n=5–6 male mice, age 24 weeks. *P≤ 0.05 as compared to WT-HFD.
3.8. Mice expressing hepatic BCATm transgene do not respond to a high fat diet with increased plasma BCAA concentrations
Plasma BCAAs are elevated in the obese state [33], and it has been suggested that BCAA metabolism may be impaired in obesity [22]. After 18 weeks on a high fat diet, all mice were gavaged with Leu to determine whether there were differences in the plasma clearance of Leu between the transgenic and WT animals following high fat diet feeding. Having in mind the higher capacity of the transgenic liver to catabolize Leu delivered from intestinal absorption, the rise in plasma Leu after gavage was blunted in LivTg-HE compared to WT animals (50% lower, Table 4) as well as the plasma concentrations of Ile and Val stayed lower (although not statistically significant) than in WT mice. These results suggest that the increased liver BCAA metabolism in LivTg-HE due to BCATm expression was not impacted by the high fat diet feeding. The aromatic amino acids, Phe, Trp, Tyr, were significantly higher by 42%, 26%, and 81% in LivTg-HE as compared to WT mice which led to a decreased plasma BCAA/AAA ratio from 4.53 in WT to 1.74 in LivTg-HE mice (Table 4). Although not statistically significant, plasma Gln was higher in the LivTg-HE mice whereas Ala was not affected (Table 4). The results demonstrate that transgenic expression of BCATm in the liver of LivTg-HE mice blunted the elevation in the plasma concentrations of Leu (BCAAs) and led to lower plasma BCAA/AAA ratio.
Table 4.
Plasma amino acid concentrations (µM) in fasted and Leu gavaged mice on high fat diet (HFD)
| WT -HFD | LivTg-HE-HFD | |
|---|---|---|
| Leu | 897.6 ± 230 | 449.8 ± 302* |
| Ile | 68.4 ± 12 | 55.8 ± 6 |
| Val | 167.1 ± 20 | 157.5 ± 15 |
| His | 25.1 ± 1 | 27.8 ± 3.3 |
| Met | 88.9 ± 11 | 100.7 ± 10 |
| Phe | 57.5 ± 3 | 81.6 ± 6* |
| Trp | 95.4 ± 8. | 125.2 ± 5* |
| Thr | 159.1 ± 14 | 200.9 ± 15 |
| Lys | 326.0 ± 37 | 381.1 ± 14 |
| Glu | 108.0 ± 14 | 126.1 ± 22 |
| Gln | 850.1 ± 68 | 1008.5 ± 79 |
| Asp | 48.0 ± 8 | 43.1 ± 10 |
| Asn | 40.3 ± 4 | 50.2 ± 4 |
| Ala | 1198.9 ± 102 | 1155.8 ± 87 |
| Gly | 393.5 ± 39 | 434.6 ± 21 |
| Ser | 161.8 ± 32 | 187.2 ± 17 |
| Tyr | 96.9 ± 12 | 175.2 ± 19* |
| Cit | 53.8 ± 4 | 70.9 ± 7 |
WT-HFD and LivTg HE-HFD male mice were fed HFD for 18 weeks, fasted for 12 h, then gavaged with Leu for 30 min before drawing blood for analysis (see Methods). Data represent mean ± SEM n=6–12 male mice, age 24 weeks,
P≤0.05 as compared to WT-HFD mice.
4. Discussion
The current study describes a unique mouse model where introduction of BCATm in the liver allows for complete catabolism of BCAAs in this organ potentially reducing or altering the direction of the interorgan shuttling of BCAA metabolites. Likewise, a stable expression of the BCATm transgene in mouse liver offers insight into how liver BCAA metabolism can affect extrahepatic metabolism and plasma circulating BCAAs under diet-induced obesity conditions. This is particularly relevant to metabolic health as changes in plasma concentrations of BCAAs occur in various physiological and pathological conditions [22,34,35].
Two transgenic lines that were established, LivTg-LE and LivTg-HE, expressed active liver BCATm enzyme and liver BCAA concentrations were decreased according to levels of BCATm expression. In contrast, plasma BCAAs remained largely unchanged with Leu being the only BCAA that decreased significantly in the plasma of LivTg-HE line. BCATm expression in the mouse liver also affected the activity of BCKDC as seen by the decreased phosphorylation of E1α subunit, which would support increased rates of BCAA oxidation. BCKDC is active in liver and is thought to be the primary site of BCAA oxidation in rodents [36,37]. On the other hand, BCATm is not normally expressed in rodent liver or primate hepatocytes. Both rodent and human studies [38,39] have suggested that BCAAs escape first pass metabolism in liver and are primarily taken up by skeletal muscle [36,40]. Because the transamination product of Leu, KIC, inhibits BDK [41], generation of KIC by BCATm in the liver mitochondria would be expected to increase the amount of active BCKDC. BCATm and E1α subunit form a metabolon, which facilitates BCAA oxidation [42]. The lower phosphorylation state of E1α subunit, which increases the amount of active enzyme, and the form of E1α subunit that can bind BCATm, likely enhanced BCAA oxidation thus increasing the capacity for first pass BCAA metabolism. However, it is noteworthy that the plasma and tissue amino acids were maintained close to normal levels except at very high levels of BCATm expression seen in the LivTg-HE animals. Tissues that are involved in BCAA metabolism and interorgan shuttling of BCAA metabolites such as skeletal muscle, kidney, and heart did not show apparent changes in BCAA catabolic enzymes, although BCATm protein expression was increased in the kidney of LivTg-HE, most likely due to the presence of low level kidney expression of the ApoE gene [30]. Although, at least quantitatively, skeletal muscle represents the most important site for BCAA transamination, the kidney may also contribute to whole body BCAA oxidation as it has high transaminase and oxidative capacities [36]. Measurable compensatory changes in BCAA catabolic enzyme expression in tissues other than the liver did not occur, and marked changes in plasma BCAAs were only significant for Leu. In the lactating rat, changes in the expression of BCAA metabolic enzymes in the mammary gland were tissue specific [43]. This gland became the major site of leucine removal and had increased BCATm and BCKDC activities [44], which is consistent with the hypothesis that BCAA metabolism is important for milk production where BCAA metabolism supports protein synthesis and provides nitrogen for Glu and Gln. Other conditions, such as starvation, however, are associated with a decreased activity of BCKDC especially in the skeletal muscle and are a part of the adaptive response of the whole body against protein wasting [45,46]. In the skeletal muscle, the BCAA concentrations can increase in response to starvation that may result from a conversion of unused BCKAs back to BCAAs [45]. Considering the differences of individual tissues in BCAA metabolism, the impact of starvation or other perturbations (trauma, chronic illnesses) on how BCAAs and BCKAs are utilized, may be tissue dependent [45,47–49]. Therefore, it would be of particular future importance to understand how the overexpression of the hepatic BCATm protein may impact the ability of the whole body to utilize or preserve BCAAs in response to stressors such as starvation and chronic illnesses.”
Hepatic concentrations of several amino acids, Glu, Asp, Ser, Tyr, Arg, and Cit were increased significantly in LivTg-HE suggesting that the increased BCAA oxidation did influence the metabolism/ synthesis of other amino acids. As stated above, BCAAs provide nitrogen for the amination of α-ketoglutarate to form Glu, which is a key nitrogen donor for synthesis of other indispensable amino acids such as Ala and Asp [50,51]. No changes in Ala concentrations were observed, which would be consistent with the glucose/Ala cycle where Ala synthesis occurs primarily outside the liver and mainly in skeletal muscle. Liver BCAA transamination was likely the major source of nitrogen for the increased Glu concentrations in the liver. Glutamate dehydrogenase (GDH), which catalyzes the reversible oxidative deamination of glutamate to α-ketoglutarate and ammonia [52], could provide ammonia for the Urea Cycle as well as α-ketoglutarate that can enter the TCA cycle. Ammonia could also be produced by the formation of a complex between BCATm and GDH which yields NADH and free ammonia [53]. Although other Urea Cycle metabolites were not measured, the increases in Asp, Glu, Arg, and Cit (all Urea Cycle metabolites) though not Orn, which is formed by arginase catalyzed cleavage to Cit and urea, suggest that increased Urea Cycle activity may have occurred in LivTg-HE animals. Nevertheless, LivTg-HE did not show changes in liver Gln concentrations. Because Gln synthesis occurs in perivenous hepatocytes, whereas the Urea cycle is found primarily in periportal hepatocytes [54], this suggests that the metabolic changes may be occurring more in the periportal hepatocyte population.
Low plasma BCAAs are found in patients with liver cirrhosis with hyperammonemia being implicated in the pathogenesis of this disorder [55,56]. BCAA supplementation is commonly used as a treatment option in humans as it may enhance detoxification of ammonia via skeletal muscle Gln synthesis [56,57]. With a functional liver, increased BCAA metabolism in the transgenic animals did not exceed the capacity of the liver to metabolize the nitrogen from BCAA metabolism. Because plasma amino acids were largely unaffected by the BCATm transgene, it implies that systemic metabolism may have adjusted to the increased hepatic BCAA metabolism thus preventing changes in the circulating plasma amino acids.
BCAAs are elevated in human obesity and alterations in BCAA metabolism in liver and adipose tissue, but not skeletal muscle, have been linked to the rise in plasma BCAAs in obesity [19]. Feeding LivTg-HE animals with a high fat diet, however, did not change or normalize the concentrations of plasma BCAAs. Leu remained significantly lower in the plasma of LivTg-HE as compared to WT mice during the high fat diet feeding. The observed lower plasma Leu concentrations correlated with decreased phosphorylation of 4E-BP1 and S6K in the liver consistent with Leu role in activating mTORC1 [58] and indicating attenuation of the mTORC1 signaling pathway in the liver. Creation of a liver-specific deletion of mTORC1 (Raptor) did not result in developmentally abnormal mice as these animals had body weight comparable to that of their WT littermates [59]. However, when challenged with a western diet (high fat and cholesterol diet), the animals failed to elevate their liver triglycerides and plasma cholesterol [59]. The role of mTORC1 signaling pathway in lipogenesis, although important, was only partial as signaling through mTORC2 signaling pathway is also essential for lipogenesis [60]. Although, LivTg-HE mice did not phenotypically differ from their WT littermates when fed a high fat diet, they exhibited higher blood glucose concentrations. Attenuation in the hepatic Leu signaling via mTORC1 pathway may interfere with the utilization of glucose during liver glycolysis since suppressing mTORC1 activity could lead to decreased expression of metabolic genes including the gene encoding for glucokinase [61]. However, higher blood glucose concentrations in the transgenic animals were associated with impaired glucose tolerance suggesting possible reduction in insulin/glucagon ratio. A lower insulin/glucagon ratio is also hypothesized to play a key role in disturbing the balance between anabolism and catabolism during liver failure leading to a decreased BCAA/AAA ratio [62]. Interestingly, plasma AAAs were elevated significantly in LivTg-HE mice fed a high fat diet, leading to a lower plasma BCAA/AAA ratio. A lower plasma BCAA/AAA ratio is consistent with the known competition of BCAAs with other large neutral amino acids for uptake with the system L-amino acid transporter (LAT1) [63] and having in mind that these animals had completely functional liver, it is unlikely to assume that liver failure and/or lower insulin/glucagon ratio were accountable for the lower BCAA/AAA ratio. During hepatic failure, plasma levels of BCAAs are decreased and the AAAs are increased as a result of increased BCAA catabolism in the muscle and decreased AAA breakdown in the failing liver [62].
Lower plasma Leu concentrations and impaired glucose tolerance in the animals with the highest level of hepatic expression of BCATm (LivTg-HE) was opposite to the elevated plasma BCAAs and improvements in glucose tolerance following diet induced obesity found in the global BCATm−/− animals [20].This is consistent with other findings showing that dietary BCAA supplements can decrease fat mass, body weight, and improve glucose metabolism [34]. As far as global or tissue specific alterations in BCAA metabolism can contribute to obesity-related changes in plasma BCAA concentrations, proper regulation of BCAA metabolism may prove beneficial in controlling obesity.
In summary, expression of BCATm in the mouse liver shifted BCAA metabolism to the liver without large fluctuations in either plasma amino acids or extrahepatic BCAA metabolism. Diet induced obesity, however, did have an impact on blood glucose and aromatic amino acid concentrations of the transgenic animals highlighting the importance of liver BCAA catabolic regulation under disease conditions. The study also demonstrates the adaptable role of the liver in maintaining the homeostasis of BCAAs so that the net whole body BCAA metabolism remains unaltered.
Acknowledgments
We thank Dr. Adele Addington for technical assistance and critical evaluation of the manuscript and Janet Rinehart for assistance with plasma amino acid measurements. E. A. A. designed, conducted experiments, analyzed data, and wrote the manuscript. C. G. V. H. created the transgenic animals and conducted experiments on mice fed a standard rodent chow. M. R. J. designed and performed the high fat study. S. M. H. designed experiments, participated in data interpretation, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Sources of financial support:
This work was supported in whole or in part by the National Institute of Health (grant DK 34738 to SMH) and Virginia Tech, USA (startup grant to SMH)
List of Abbreviations
- AAAs
aromatic amino acids
- BCAAs
branched chain amino acids
- BCKAs
branched chain keto acids
- BCATm
mitochondrial branched chain aminotransferase
- BCKDC
branched chain α-keto acid dehydrogenase enzyme complex
- KIC
α-ketoisocaproate
- KIV
α-ketoisovalerate
- WT
wildtype
- mTOR
mammalian target of rapamycin
- mTORC1
complex 1 of mTOR pathway
- α-KG
α-ketoglutarate
- E1α
α-subunit of the branched chain α-keto acid dehydrogenase
- E2
dihydrolipoamide acyltransferase
- E3
dihydrolipoamide dehydrogenase
- BDK
branched chain keto acid dehydrogenase kinase
- LivTg mice
liver transgenic mice
- LivTg-LE
BCATm low expresser
- LivTg-HE
BCATm high expresser
- ApoE
apolipoprotein E
- Vldlr
very-low-density-lipoprotein receptor
- 4E-BP1
eukaryotic translation initiation factor 4E binding protein 1
- S6K
70-kDa ribosomal protein S6 kinase
- GTT
glucose tolerance test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
References
- 1.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 2.Garber AJ, Karl IE, Kipnis DM. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem. 1976;251:836–843. [PubMed] [Google Scholar]
- 3.Garber AJ, Karl IE, Kipnis DM. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J Biol Chem. 1976;251:826–835. [PubMed] [Google Scholar]
- 4.Kanekawa T, Nagai H, Kanayama M, Sumino Y. Importance of branched-chain amino acids in patients with liver cirrhosis and advanced hepatocellular carcinoma receiving hepatic arterial infusion chemotherapy. Cancer Chemother Pharmacol. 2014 doi: 10.1007/s00280-014-2564-z. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi G, Marzocchi R, Agostini F, Marchesini G. Update on nutritional supplementation with branched-chain amino acids. Curr Opin Clin Nutr Metab Care. 2005;8:83–87. doi: 10.1097/00075197-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 7.Chuang DT, Chuang JL, Wynn RM. Lessons from genetic disorders of branched-chain amino acid metabolism. J Nutr. 2006;136:243S–249S. doi: 10.1093/jn/136.1.243S. [DOI] [PubMed] [Google Scholar]
- 8.Harper AE, Benevenga NJ, Wohlhueter RM. Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev. 1970;50:428–558. doi: 10.1152/physrev.1970.50.3.428. [DOI] [PubMed] [Google Scholar]
- 9.Hutson SM, Fenstermacher D, Mahar C. Role of mitochondrial transamination in branched chain amino acid metabolism. J Biol Chem. 1988;263:3618–3625. [PubMed] [Google Scholar]
- 10.Ichihara A, Koyama E. Transaminase of branched chain amino acids. I. Branched chain amino acids-alpha-ketoglutarate transaminase. J Biochem. 1966;59:160–169. doi: 10.1093/oxfordjournals.jbchem.a128277. [DOI] [PubMed] [Google Scholar]
- 11.Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab. 2004;286:E64–E76. doi: 10.1152/ajpendo.00276.2003. [DOI] [PubMed] [Google Scholar]
- 12.Lee HY, Hall TB, Kee SM, Tung HY, Reed LJ. Purification and properties of branched-chain alpha-keto acid dehydrogenase kinase from bovine kidney. Biofactors. 1991;3:109–112. [PubMed] [Google Scholar]
- 13.Harris RA, Popov KM, Zhao Y, Shimomura Y. Regulation of branched-chain amino acid catabolism. J Nutr. 1994;124:1499S–1502S. doi: 10.1093/jn/124.suppl_8.1499S. [DOI] [PubMed] [Google Scholar]
- 14.Hawes JW, Schnepf RJ, Jenkins AE, Shimomura Y, Popov KM, Harris RA. Roles of amino acid residues surrounding phosphorylation site 1 of branched-chain alpha-ketoacid dehydrogenase (BCKDH) in catalysis and phosphorylation site recognition by BCKDH kinase. J Biol Chem. 1995;270:31071–31076. doi: 10.1074/jbc.270.52.31071. [DOI] [PubMed] [Google Scholar]
- 15.Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, Vary TC. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab. 2002;283:E503–E513. doi: 10.1152/ajpendo.00084.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hutson SM, Berkich D, Drown P, Xu B, Aschner M, LaNoue KF. Role of branched-chain aminotransferase isoenzymes and gabapentin in neurotransmitter metabolism. J Neurochem. 1998;71:863–874. doi: 10.1046/j.1471-4159.1998.71020863.x. [DOI] [PubMed] [Google Scholar]
- 17.Donato J, Jr, Pedrosa RG, Cruzat VF, Pires IS, Tirapegui J. Effects of leucine supplementation on the body composition and protein status of rats submitted to food restriction. Nutrition. 2006;22:520–527. doi: 10.1016/j.nut.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN, Thomas AP, Oort PJ, Kieffer DA, Amin R, Bettaieb A, Haj FG, Permana P, Anthony TG, Adams SH. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–E1187. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–E1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vianna D, Resende GF, Torres-Leal FL, Pantaleao LC, Donato J, Jr, Tirapegui J. Long-term leucine supplementation reduces fat mass gain without changing body protein status of aging rats. Nutrition. 2012;28:182–189. doi: 10.1016/j.nut.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelking LJ, Kuriyama H, Hammer RE, Horton JD, Brown MS, Goldstein JL, Liang G. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J Clin Invest. 2004;113:1168–1175. doi: 10.1172/JCI20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananieva EA, Patel CH, Drake CH, Powell JD, Hutson SM. Cytosolic Branched Chain Aminotransferase (BCATc) Regulates mTORC1 Signaling and Glycolytic Metabolism in CD4+ T Cells. J Biol Chem. 2014;289:18793–18804. doi: 10.1074/jbc.M114.554113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole JT, Sweatt AJ, Hutson SM. Expression of mitochondrial branched-chain aminotransferase and alpha-keto-acid dehydrogenase in rat brain: implications for neurotransmitter metabolism. Front Neuroanat. 2012;6:18. doi: 10.3389/fnana.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweatt AJ, Garcia-Espinosa MA, Wallin R, Hutson SM. Branched-chain amino acids and neurotransmitter metabolism: expression of cytosolic branched-chain aminotransferase (BCATc) in the cerebellum and hippocampus. J Comp Neurol. 2004;477:360–370. doi: 10.1002/cne.20200. [DOI] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu G, Knabe DA. Free and protein-bound amino acids in sow's colostrum and milk. J Nutr. 1994;124:415–424. doi: 10.1093/jn/124.3.415. [DOI] [PubMed] [Google Scholar]
- 30.Simonet WS, Bucay N, Lauer SJ, Taylor JM. A far-downstream hepatocyte-specific control region directs expression of the linked human apolipoprotein E and C-I genes in transgenic mice. J Biol Chem. 1993;268:8221–8229. [PubMed] [Google Scholar]
- 31.Harris RA, Joshi M, Jeoung NH, Obayashi M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J Nutr. 2005;135:1527S–1530S. doi: 10.1093/jn/135.6.1527S. [DOI] [PubMed] [Google Scholar]
- 32.Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Furnsinn C, Promintzer M, Anderwald C, Bischof M, Roden M. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–1607. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- 33.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 34.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:319S–323S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 36.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- 37.Lynch CJ, Halle B, Fujii H, Vary TC, Wallin R, Damuni Z, Hutson SM. Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab. 2003;285:E854–E863. doi: 10.1152/ajpendo.00153.2003. [DOI] [PubMed] [Google Scholar]
- 38.Wahren J, Felig P, Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976;57:987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutson SM, Cree TC, Harper AE. Regulation of leucine and alpha-ketoisocaproate metabolism in skeletal muscle. J Biol Chem. 1978;253:8126–8133. [PubMed] [Google Scholar]
- 40.Hutson SM, Wallin R, Hall TR. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues. J Biol Chem. 1992;267:15681–15686. [PubMed] [Google Scholar]
- 41.Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun. 2004;313:391–396. doi: 10.1016/j.bbrc.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Islam MM, Wallin R, Wynn RM, Conway M, Fujii H, Mobley JA, Chuang DT, Hutson SM. A novel branched-chain amino acid metabolon. Protein-protein interactions in a supramolecular complex. J Biol Chem. 2007;282:11893–11903. doi: 10.1074/jbc.M700198200. [DOI] [PubMed] [Google Scholar]
- 43.DeSantiago S, Torres N, Suryawan A, Tovar AR, Hutson SM. Regulation of branched-chain amino acid metabolism in the lactating rat. J Nutr. 1998;128:1165–1171. doi: 10.1093/jn/128.7.1165. [DOI] [PubMed] [Google Scholar]
- 44.Trottier NL. Nutritional control of amino acid supply to the mammary gland during lactation in the pig. Proc Nutr Soc. 1997;56:581–591. doi: 10.1079/pns19970059. [DOI] [PubMed] [Google Scholar]
- 45.Holecek M, Sprongl L, Tilser I. Metabolism of branched-chain amino acids in starved rats: the role of hepatic tissue. Physiol Res. 2001;50:25–33. [PubMed] [Google Scholar]
- 46.Holecek M. Effect of starvation on branched-chain alpha-keto acid dehydrogenase activity in rat heart and skeletal muscle. Physiol Res. 2001;50:19–24. [PubMed] [Google Scholar]
- 47.Holecek M, Tilser I, Skopec F, Sprongl L. Leucine metabolism in rats with cirrhosis. J Hepatol. 1996;24:209–216. doi: 10.1016/s0168-8278(96)80031-6. [DOI] [PubMed] [Google Scholar]
- 48.Hutson SM, Harper AE. Blood and tissue branched-chain amino and alpha-keto acid concentrations: effect of diet, starvation, and disease. Am J Clin Nutr. 1981;34:173–183. doi: 10.1093/ajcn/34.2.173. [DOI] [PubMed] [Google Scholar]
- 49.Abumrad NN, Wise KL, Williams PE, Abumrad NA, Lacy WW. Disposal of alpha-ketoisocaproate: roles of liver, gut, and kidneys. Am J Physiol. 1982;243:E123–E131. doi: 10.1152/ajpendo.1982.243.2.E123. [DOI] [PubMed] [Google Scholar]
- 50.Parrilla R, Goodman MN. Nitrogen metabolism in the isolated perfused rat liver. Nitrogen balance, redox state and rates of proteolysis. Biochem J. 1974;138:341–348. doi: 10.1042/bj1380341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krebs HA. The role of chemical equilibria in organ function. Adv Enzyme Regul. 1975;13:449–472. doi: 10.1016/0065-2571(75)90030-8. [DOI] [PubMed] [Google Scholar]
- 52.Spanaki C, Plaitakis A. The role of glutamate dehydrogenase in mammalian ammonia metabolism. Neurotox Res. 2012;21:117–127. doi: 10.1007/s12640-011-9285-4. [DOI] [PubMed] [Google Scholar]
- 53.Islam MM, Nautiyal M, Wynn RM, Mobley JA, Chuang DT, Hutson SM. Branched-chain amino acid metabolon: interaction of glutamate dehydrogenase with the mitochondrial branched-chain aminotransferase (BCATm) J Biol Chem. 2010;285:265–276. doi: 10.1074/jbc.M109.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haussinger D, Sies H, Gerok W. Functional hepatocyte heterogeneity in ammonia metabolism. The intercellular glutamine cycle. J Hepatol. 1985;1:3–14. doi: 10.1016/s0168-8278(85)80063-5. [DOI] [PubMed] [Google Scholar]
- 55.Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013;28:580–588. doi: 10.1177/0884533613496432. [DOI] [PubMed] [Google Scholar]
- 56.Holecek M. Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition. 2013;29:1186–1191. doi: 10.1016/j.nut.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 57.Holecek M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition. 2010;26:482–490. doi: 10.1016/j.nut.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 58.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamming DW, Sabatini DM. A Central role for mTOR in lipid homeostasis. Cell Metab. 2013;18:465–469. doi: 10.1016/j.cmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai W, Panserat S, Mennigen JA, Terrier F, Dias K, Seiliez I, Skiba-Cassy S. Post-prandial regulation of hepatic glucokinase and lipogenesis requires the activation of TORC1 signalling in rainbow trout (Oncorhynchus mykiss) J Exp Biol. 2013;216:4483–4492. doi: 10.1242/jeb.091157. [DOI] [PubMed] [Google Scholar]
- 62.Dejong CH, van de Poll MC, Soeters PB, Jalan R, Olde Damink SW. Aromatic amino acid metabolism during liver failure. J Nutr. 2007;137:1579S–1585S. doi: 10.1093/jn/137.6.1579S. discussion 1597S–1598S. [DOI] [PubMed] [Google Scholar]
- 63.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]