Abstract
Selective receptor-targeting peptide based agents have attracted considerable attention in molecular imaging of tumor cells that overexpress corresponding peptide receptors due to their unique properties such as rapid clearance from circulation as well as high affinities and specificities for their targets. The rapid growth of chemistry modification techniques has enabled the design and development of various peptide-based imaging agents with enhanced metabolic stability, favorable pharmacokinetics, improved binding affinity and selectivity, better imaging ability as well as biosafety. Among them, many radiolabeled peptides have already been translated into the clinic with impressive diagnostic accuracy and sensitivity. This review summarizes the current status in the development of peptide-based imaging agents with an emphasis on the consideration of probe design including the identification of suitable peptides, the chemical modification of probes and the criteria for clinical translation. Specific examples in clinical trials have been provided as well with respect to their diagnostic capability compared with other FDA approved imaging agents.
Keywords: Peptide, Molecular imaging, Chemical modification, Clinical experience
1. Introduction
Molecular imaging visualizes and measures biological processes at the cellular and subcellular levels within living systems. Targeted molecular imaging, which can quantify the target expression, is an indispensable tool in diagnosing and managing diseases [1–3]. A targeted imaging probe is generally composed of a targeting ligand (such as peptide, aptamer, protein or antibody), an imaging moiety (such as radioisotope for positron emission tomography (PET) or single photon emission computed tomography (SPECT), magnetic nanoparticle for magnetic resonance imaging (MRI) and organic fluorescent dye for optical imaging) and a linker to connect these two [4–6]. An ideal imaging probe should have high binding affinity and specificity for the particular receptor, and can be rapidly cleared from non-targets in order to ensure an adequate target-to-background ratio. In addition, high stability and integrity under physiological condition, low immunogenicity and toxicity for human exposure as well as easy production are all necessary for clinical translation.
With the help of sophisticated molecular biology, a great number of disease targets and corresponding target ligands have been discovered [7–9]. Given their unique advantages, peptides have attracted much attention for targeted imaging [10–12]. Peptides play important roles in cellular functions and intercellular communication. They are composed of amino acid monomers connected by amide bonds and typically have a low molecular weight (less than 100 amino acid residues according to the United States Food and Drug Administration (FDA) definition) which enables fast clearance from the blood as well as non-target tissue. Selected peptides generally have high affinities and specificities for their receptors and are active at concentration down to nanomolar level, therefore, resulting in desirable target-to-non-target ratios. An increasing number of peptides, such as somatostatin (SST) peptide, vasoactive intestinal peptide (VIP), Arg-Gly-Asp (RGD) peptide, and bombesin/gastrin-releasing peptide (BBN/GRP), have been successfully characterized for tumor receptor imaging [13–17].
The FDA typically handles peptides as conventional drugs instead of biological products with a focus on their compound structure [18]. Peptides normally are susceptible to chemical modification. After determining the amino acid residues for specific targeting, chemical modifications (such as cyclization, PEGylation, introduction of unnatural amino acid) are utilized to engineer the peptides for enhanced metabolic stabilities and favorable pharmacokinetics. Imaging labels are directly or indirectly conjugated to the peptides for in vivo imaging application. Accompanied by the structure modification is the possibility to lose the binding affinity and biological activity of peptides. Thus, design of chemical structure (such as insertion of appropriate linker) is explored to minimize the interaction between active binding site and unnatural modification.
In general, construction of peptide-based imaging probes involves three steps: (1) identification of the receptor and its targeting peptide; (2) design and preparation of the peptide analogs with the aim to optimize the biological activity and metabolic behavior; and (3) chemical conjugation of an imaging functionality to the peptide. Despite the great progress made in the development of peptide-based probes, their application in diagnostic imaging and monitoring therapeutic efficacy is still in its infancy. The clinical application of peptide-based agents will greatly rely on the evaluation of in vivo selectivity (whether the probe can specifically bind to its target and not to the non-target tissues); the in vivo stability (whether the probe can reach the target in an intact state); the pharmacokinetic profile (the rate and extent of the probes clears from the body) and the toxicological studies. In this review, we make a summary of the major progress in the development of peptide-based imaging agents for disease detection with a focus on demonstrating the design concept for improving the performance of imaging probes.
2. Peptides targeting receptors overexpressed in specific cancers
Targeting peptide sequences can be selected mainly in three different ways: (1) derivatization from natural proteins [19]; (2) chemical synthesis and structure-based rational engineering [20,21]; and (3) screening of peptide libraries [22]. Each method has its own strength and weakness, and is a review within itself. Among them, phage display technology is a conventional but most widely used method with many advantages such as ease of handling and large number of different peptides can be screened effectively [23].
2.1. Peptide selection/identification by phage display
Phage display technology is based on the principle of screening for specific peptides that bind to the desired target from a library of phage particles. It was introduced by George P. Smith in 1985 [24]. Since then, thousands of peptides have been screened out via phage display. In a typical in vitro phage display process, the phage surface is exposed to the foreign peptide libraries composed of small peptides varying from 5 to 45 amino acids to allow the peptides to incorporate into the phage [25,26]. Every phage clone displays one single peptide while the whole library can display up to 109 peptides in total. The phage display library is passed through the targeting molecules. The unbound phage is then washed off and those with desired binding activity is captured and later recovered by competitive elution. A variety of affinity selection methods have been reported to increase the chance to obtain peptides binding to the targeting molecules with good affinity. Usually, at least four rounds of iterative selection are needed to enrich phage with desired binding ability [23]. For in vivo phage display, library phages are injected into animals. Unbound phages are removed via vascular perfusion as well as additional ex vivo washing. The phages with binding activity are rescued from target organs, amplified and purified [27]. Potential candidates obtained via this identification step will be further subjected to chemical and biological evaluations for in vivo molecular imaging.
2.2. Representative peptides for in vivo imaging
The biological activities of peptides are regulated through binding with corresponding receptors. Those with receptors overexpressed on tumor cells rather than on normal cells are excellent candidates for in vivo tumor imaging. To date, many peptides and their analogs have been identified and used for disease detection (Table 1). Some representative ones are discussed below.
Table 1.
Some representative peptides, receptors and corresponding imaging applications.
| Peptide designation | Key sequence | Receptor | Indication | Subject studied | Reference |
|---|---|---|---|---|---|
| RGD | Arg-Gly-Asp | Integrin | Angiogenesis | In vitro Rodents Humans |
[28–32] |
| Bombesin/gastrin-releasing peptide | Share sequence of Trp-Ala-Val-Gly-His-Leu-Met | GRPR | Prostate cancer; breast cancer; glioma | In vitro Rodents Humans |
[33–36] |
| CCK/gastrin | Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe | CCK2R | Medullary thyroid cancer | In vitro Rodents |
[37–39] |
| VIP | His-Ser-Asp-Ala-Val-Phe-Thr-Asp-Asn-Tyr-Thr-Arg-Leu-Arg- Lys-Gln-Met-Ala-Val-Lys-Lys-Tyr-Leu-Asn-Ser-Ile-Leu-Asn | VPAC1/2 | Primary and metastatic lesions of breast, ovarian, prostate, colon and urinary bladder carcinomas, and meningiomas | In vitro Rodents Humans |
[13,40,41] |
| NT | pGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu | NTSR1 | Tumor progression (lung cancer/breast cancer/prostate cancer) | In vitro Rodents |
[42,43] |
| Somatostatin | Ala-Gly-Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys (Disulfide bridge Cys3-Cys14) | SSTR1–5 | Neuroendocrine tumors | In vitro Rodents Humans |
[14,44–46] |
| α-MSH | Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val | Melanocortin-1 receptor | Melanogenesis | In vitro Rodents |
[47,48] |
| T140 | Arg-Arg-Nal-Cys-Tyr-Arg-Lys-D-Lys-Pro-Tyr-Arg-Cit-Cys-Arg | CXCR4 | Tumor aggressiveness, invasiveness, and metastasis formation | In vitro Rodents |
[49,50] |
| Exendin-4 | His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met- Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn- Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser | GLP-1R | Insulinomas | In vitro Rodents Humans |
[51–53] |
| NPY | Tyr-Pro-Ser-Lys-Pro-Asp-Asn-Pro-Gly-Glu-Asp-Ala-Pro-Ala- Glu-Asp-Leu-Ala-Arg-Tyr-Tyr-Ser-Ala-Leu-Arg-His-Tyr-Ile- Asn-Leu-Ile-Thr-Arg-Gln-Arg-Tyr | NPY1R | Breast cancer; sarcomas | In vitro Rodents |
[54,55] |
| Substance P | Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met | NK1R | Glioblastoma | In vitro Rodents |
[56,57] |
| TMTP1 | Asn-Val-Val-Arg-Gln | Unclear | Highly metastatic cancer cells | In vitro Rodents |
[58,59] |
2.2.1. Arg-Gly-Asp (RGD) peptide
The tripeptide RGD is specifically binding to integrin receptors [60]. Integrins constitute two subunits (α and β subunits). The integrin family, especially αVβ3, is significant for tumor angiogenesis and metastasis. They are overexpressed on endothelial cells during angiogenesis, but barely detectable in most normal organs. Therefore, they are widely used for diagnostic imaging.
2.2.2. Bombesin (BBN)/gastrin-releasing peptide (GRP)
Amphibian BBNs and their related peptides consist a family of neuropeptides exhibiting various physiological effects such as exocrine and endocrine secretions, thermoregulation, sucrose regulations as well as cell growth [35]. The bombesin-like peptide receptors have 4-subtypes: the neuromedin B receptor, the bombesin 3 receptor, the GRP receptor, and the bombesin 4 receptor. These receptors are overexpressed in many tumors such as breast cancer, ovarian cancer and gastrointestinal stromal tumors.
2.2.3. Somatostatin (SST) peptide
SSTs are naturally occurring cyclopeptide hormones with either 14 or 28 amino acids [61]. They can inhibit the secretion of insulin, glucagon and some other hormones. Their biological effects are mediated via binding to specific high-affinity somatostatin receptors (SSTRs) which are overexpressed in many tumors including but not limited to the gliomas, neuroendocrine tumors and breast tumor. Till now, five subtypes of SSTR (SSTR1–SSTR5) have been discovered.
2.2.4. Vasoactive intestinal peptide (VIP)
VIP is a neuropeptide with 28 amino acids [13]. It not only can promote vasodilation, but also can promote growth and proliferation of cells via cell-surface receptor mediated growth. Its action is mainly controlled by two receptor subtypes (VPAC1 and VPAC2). A large amount of VIP receptors are expressed on many tumors including but not limited to brain tumors, adenocarcinomas of the pancreas and neuroendocrine tumors. It is worth mentioning that VIP is also a potential drug and doses at submicrogram level can cause toxic effect.
2.2.5. Cholecystokinin (CCK)/gastrin peptide
CCK and gastrin are structurally and functionally similar peptides that exert a variety of physiological actions in the gastrointestinal tract as well as the central nervous system [62]. They have an identical sequence at the biologically active part but differ in sulfation position of the tyrosine (position 6 for gastrin and position 7 for CCK). Till now, three types of receptors for CCK (CCK1, CCK2 and CCK2i4sv) have been identified, which all belong to the superfamily of GPCRs. Among them, CCK2/gastrin receptors have been frequently found in human cancers such as stromal ovarian cancers and astrocytomas.
2.2.6. α-Melanocyte-stimulating hormone (α-MSH)
α-MSHs are linear tridecapeptides, mainly responsible for skin pigmentation regulation [63]. α-MSHs and their analogs exhibit impressive binding affinities to melanocortin-1 receptors (MC-1r) which are expressed in over 80% of human melanoma metastases, and thus, are widely used as vehicles for melanoma-targeted imaging and radiotherapy.
2.2.7. Neutrotensin (NT)
NT is a peptide with 13 amino acids, targeting NT receptor which has been identified in various tumors such as ductal pancreatic adenocarcinomas, small cell lung cancer, and medullary thyroid cancer [64]. Therefore, it is an attractive candidate for cancer imaging.
2.2.8. T140
T140, a peptide with 14 amino acids and one disulfide bridge, is an inverse agonist of chemokine receptor type 4 (CXCR4) [65]. Its derivatives are widely used as CXCR4 imaging agents.
2.2.9. Exendin-4
Exendin-4 is a 39-amino acid peptide hormone [66]. It has 50% homology in the amino acid composition with native glucagon-like peptide 1 (GLP-1) and is found to be an agonist for GLP-1 receptor. Exendin-4 has been clinically used for the treatment of patients with insulinoma and type 2 diabetes.
2.2.10. Neuropeptide Y (NPY)
NPY is a peptide with 36 amino acids and belongs to the pancreatic polypeptide family [67]. NPY receptors are overexpressed in various tumors including neuroblastomas, sarcomas, and breast cancers. Many NPY analogs have been evaluated in animal models.
2.2.11. Substance P
Substance P is an undecapeptide belonging to a family of neuropeptides known as tachykinins [68]. Substance P is a specific endogenous ligand known for neurokinin 1 receptor (NK1R) which is found to be expressed on various cancer cells. Substance P analogs are synthesized and used for NK1R positive tumor detection.
2.2.12. Tumor molecular targeted peptide 1 (TMTP1)
TMTP1 is a 5-amino acid peptide that has been found to specifically bind to highly metastatic cancer cells, especially those from a typical liver micrometastasis [69]. However, high concentration of TMTP1 could mediate tumor cell apoptosis. Therefore, suitably labeled TMTP1 has been tested pre-clinically for imaging and therapeutic applications.
2.3. Agonists vs. antagonists
Distinguishing the concept of “antagonist” from “agonist” is vital for the design of peptide based imaging probes. Most of the peptides mentioned above could cause physiological changes with receptor binding and are called “agonists”. Conversely, the peptides that bind to the receptors without activation changes are termed “antagonists”. It was originally believed that receptor agonists will be internalized and retained in the cell, therefore, more appropriate than antagonists for imaging. However, more and more studies have showed the advantages of antagonist for in vivo imaging. For example, Ginj et al. developed a radiolabeled SSTR2 antagonist (111In-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-[4-NO2-Phe-c(DCys-Tyr-DTrp-Lys-Thr-Cys)-DTyr-NH2]) and compared the in vivo tumor targeting properties with a highly potent radiolabeled SSTR2 agonist (111Indiethylenetriaminepentaacetic acid (DTPA)–[Tyr3,Thr8]-octreotide) [45]. Although the antagonist showed a lower cellular receptor affinity than that of the agonist, the in vivo tumor retention of the antagonist is nearly twice that of the agonist. In another study, Cescato et al. compared the in vitro and in vivo tumor targeting properties of a bombesin agonist (N4-[Pro1, Tyr4, Nle14]Bombesin) with a bombesin antagonist (N4-[D-Phe6, Leu-NHEt13, des-Met14]Bombesin) [34]. Compared with the agonist, the antagonist exhibits similar binding affinity, no active internalization and a three-fold higher tumor uptake. They ascribed this enhanced tumor uptake of antagonist to its binding ability to more receptor sites, slower dissociation rate from the receptor as well as its stronger resistance to membrane-bound enzymes. Besides, since the antagonists exhibit much less side-effects than agonists, they are more clinically preferred.
3. Chemical modification of a peptide
Despite their specific targeting ability and desirable pharmacokinetics, native peptides are seldom directly used for in vivo imaging. Peptides usually have a very short in vivo biological half-life of around several minutes. Suffering from enzymatic degradation as well as fast renal clearance, they may lose their bioactivity even before reaching the intended target.
However, peptides are susceptible to chemical modification which can greatly enhance their proteolytic stability, improve their water solubility and reduce their renal clearance [70,71]. The strategies to develop metabolically stable peptides include cyclization, the use of more stable amino acids, modification of peptide C- and N-termini and the use of pseudo-peptide. To prevent the peptides from fast renal clearance, synthetic strategies like polymer fusion and attachment to long-lived proteins are explored. Furthermore, the synthetic strategies of dimers, tetramers and heterodimers are employed to increase the receptor affinity of monomeric peptides.
3.1. Strategies to enhance the metabolic stability of peptides
Cyclization often increases the metabolic stability of peptides [72]. The prolonged biological activity is mainly due to two reasons: first, cyclization can help avoid degradation by reducing the formation of conformers susceptible to proteolytic enzymes [73]. Second, cyclization protects the N- or C-terminus of peptide sequence which is the cleavage site of exoproteases [74]. Bogdanowich-Knipp et al. showed that the cyclic RGD peptide (cyclo-(1,6)-Ac-CRGDF-Pen-NH2) was much more stable than the linear one at biological pH [75]. Their hypothesis was that cyclization of RGD peptides via disulfide bond can induce structural rigidity and prevent the degradation regulated by the aspartic acid residue. The ring size of the cyclic peptide also matters. Octreotide is a clinically used SST like peptide drug. Compared with native SST-14, octreotide has used more stable D-Trp for L-Trp and the ring size has been decreased from 12 to 6 [76]. When maintaining the receptor binding affinity of somatostatin, octreotide has a prolonged plasma half-life of about 100 min in humans.
Incorporation of more stable amino acids, such as D-amino acids [77] and β-Ala [78], is also widely used to improve enzymatic stability [77]. Substituting D-tyrosine by D-phenylalanine in cyclo(RGDyK) results in cyclo(RGDfK). Compared with cyclo(RGDyK), cyclo(RGDfK) is more stable during various treatments such as heating, pH adjustment and serum incubation. This greatly benefits development of RGD based imaging probes which may require heating and may cause oxidation of the tyrosine but not phenylalanine.
Modification of a peptide bond to obtain non-peptidic structure which is not cleavable by peptidases, is another way to alter the physicochemical characteristics of a peptide [70]. Jenson’s group has reduced the CO-NH linkage of bombesin to CH2-NH during the solid-phase synthesis. This approach highly improved the resistance towards enzymatic hydrolysis in the modified position. What’s more, the [Leu14-ψ-CH2NH-Leu13]Bombesin they synthesized exhibited a 100-fold improvement in binding affinity than the corresponding bombesin receptor antagonists [79]. However, not all the bond modifications can maintain or improve the biological activity. Elimination of a peptide bond CO group probably causes the loss of potential intramolecular hydrogen bonding point and increases the rotation of C—N bond.
3.2. Strategies to prolong the blood circulation of peptides
PEGylation can improve the pharmacokinetic properties of peptides significantly by increasing their water solubility and reducing their reticuloendothelial system (RES) or renal clearance [71]. One or multiple polyethyleneglycol (PEG) chains with molecular weight ranging from several hundreds to several thousands have been attached to different peptides. The PEGylated dimeric RGD has a much lower liver uptake around 2.25 ± 0.26%ID/g compared with 4.38 ± 0.39%ID/g of dimeric RGD [80]. In another study, linkage of a 10 kDa PEG to HM-3 (an RGD modified endostatin-derived synthetic peptide) elongates its half-life in the male SD rats by a factor of 5.86 (from 27.66 ± 7.37 min to 162.08 ± 36.57 min) [81]. Glycosylation is also investigated to increase the hydrophilicity of peptides or peptide derivatives, and thus decrease their hepatic accumulation. [18F]Galacto-RGD, designed by attaching a sugar amino acid to the cyclic peptide c(RGDfK), showed a liver uptake of around 0.8%ID/g in U87MG tumor bearing mice 60 min post-injection which is much less than that of corresponding RGD (around 1.6%ID/g) [82]. Human serum albumin, a 65 kDa protein that is abundant in the circulation system with a half-life of around 20 days, has been used to improve the blood circulation of peptides as well. Chen et al. have covalently conjugated IRDye800 (organic fluorescent dye) and RGD peptide with human serum albumin. This probe exhibits a prolonged circulation half-life and has been successfully used for tumor imaging [83]. An alternative to the direct attachment with albumin is to use the albumin-binding molecules. Albumin-binding molecules, such as phenol red, fatty acid and Evans blue (EB) dye, can indirectly tether the peptides to the long-lived serum protein [84]. Recently, Chen’s group conjugated truncated EB (tEB) to exendin-4 peptide, resulting in an albumin binding drug candidate, denoted as Abextide [85]. The tEB conjugation did not comprise the binding affinity of exendin-4, however, greatly increased its half-life from 5.16 ± 5.23 h to 36.28 ± 7.01 h.
3.3. Strategies to increase the binding affinity of peptides
The introduction of the dimeric or multimeric peptide system is expected to enhance receptor targeting. Although specific targeting ability of each individual peptide is weak, through cooperative interaction, the overall binding affinity of multimeric system is expected to be greatly enhanced. Janssen et al. proved that the 99mTc modified RGDfK dimer (99mTc-HYNIC-E-[c(RGDfK)]2) has 10 times higher binding affinity than 99mTc modified RGDfK monomer (99mTc-HYNIC-c(RGDfK)) [86]. Wu et al. further demonstrated that the binding avidity of RGDfK tetramer is two times higher than RGDfK dimer [87]. The 64Cu labeled tetrameric RGD peptide appears to be an excellent ligand for in vivo integrin targeting with a rapid and high U87MG tumor accumulation around 9.93 ± 1.05%ID/g at 30 min.
To optimize the polyvalent effect of multimeric peptide, a large variety of linkers are incorporated between the peptides and the imaging moieties, the length, flexibility and hydrophilicity of which all need to be considered. Guo et al. synthesized three 18F labeled dimeric RGD peptides: 18F-AlF-NOTA-E[c(RGDfK)]2 without PEGylation, 18F-AlF-NOTA-PEG4-E[c(RGDfK)]2 with a PEG4 linker between radio-chelator NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) and RGD dimer, and 18F-AlF-NOTA-E[PEG4-c(RGDfk)]2 with PEG4 linkers between two RGDfK [88]. It is understandable that radiochelator somewhat reduces the receptor binding of RGDfK and the PEGylation will restore the binding affinity to some extent. The existence of two PEG4 linkers further provides a suitable distance between two RGDfK for simultaneous binding in a bivalent fashion. Thus, the binding affinity of these peptides followed the order of NOTA-E[PEG4-c(RGDfk)]2 > NOTA-PEG4-E[c(RGDfK)]2 > NOTA-E[c(RGDfK)]2. The 18F-AlF-NOTA-E[PEG4-c(RGDfK)]2 shows its superior capability for in vivo integrin αvβ3 imaging with relatively low liver accumulation and high tumor uptake. Liu et al. found that by increasing the length of linker between two cyclic RGD motifs, the binding affinity of RGD dimer was highly improved [89]. They inserted the Gly3 or PEG4 linkers between each RGD. The distances between two cyclic RGD motifs are 6 bonds in NOTA-E[c(RGDfK)]2, 26 bonds in NOTA-E[Gly3-c(RGDfK)2] and 38 bonds in NOTA-E[PEG4-c(RGDfK)2]. Although no direct evidence to prove the simultaneous binding of two RGD motifs after inserting the linkers, the binding affinity of NOTA-E[Gly3-c(RGDfK)2] and NOTA-E[PEG4-c(RGDfK)2] is significantly increased compared with NOTA-E[c(RGDfK)]2. In vivo tumor uptake further confirmed the increased binding affinity with tumor uptake around 9.04 ± 2.05%ID/g, 10.13 ± 1.81%ID/g and 5.28 ± 1.03%ID/g for 68Ga-NOTA-E[Gly3-c(RGDfK)2], 68Ga-NOTA-E[PEG4-c(RGDfK)2] and 68Ga-NOTA-E[c(RGDfK)]2 at 30 min post-injection respectively in the integrin αvβ3-positive U87MG tumor model.
Besides using the same type of peptide ligands to construct homodimers or homomultimers, different types of peptide ligands can be put together with suitable linkers to form heterodimers for targeting multi-receptor over-expressed tumor cells. The understanding of receptor expression pattern on cells is of great importance for the design of heterodimer with high affinity and specificity. As androgen-independent prostate cancer cells overexpress both gastrin-releasing peptide receptor (GRPR) and integrin αvβ3, radiolabeled BBN-RGD peptide heterodimers with BBN motif for GRPR targeting and RGD motif for integrin targeting have been developed to improve the imaging results over both radiolabeled BBN and RGD monopeptide. Liu et al. linked bombesin and RGD via a glutamate linker and further conjugated NOTA for 64Cu radiolabeling [90]. Due to the synergistic effect, the 64Cu-NOTA-RGD-BBN showed a much higher tumor uptake than 64Cu-NOTA-RGD, 64Cu-NOTA-BBN and even 64Cu-NOTA-RGD plus 64Cu-NOTA-BBN in PC-3 tumor at 4 h post-injection. The in vivo integrin and GRPR dual-receptor binding ability were further proved by the in vivo blocking studies. The tumor uptake of these probes can be totally blocked with both RGD and bombesin. Either RGD or bombesin will only partially inhibit the tumor uptake. In a later study, they modified the heterodimer by inserting a PEG3 spacer, 11-amino-3,6,9-trioxaundecanoic acid, onto the glutamate group and labeled it with 18F-FB (18F-fluorobenzoyl) prosthetic labeling group [91]. The PEG3 greatly increased the hydrophilicity of the heterodimer, leading to a faster renal clearance and more favorable pharmacokinetic behavior. Other heterodimers have been reported including but not limited to MSH/CCK [92], RGD/octreotate [93], and RGD/ATWLPPR [94].
The linkers, either rigid or flexible, between the two different peptides are of great concern for heterodimer since it has to present ligands simultaneously to the receptors with the lowest entropy and optimized conformation. Vagner et al. used short flexible ethylene glycol (PEG) spacers together with semirigid Pro-Gly (PG) repeats as linkers to connect MSH and Delt-II (Deltorphin-II) binding moieties [95]. Among 8 different linkers, -PGn- (n = 3, 6, 9, 12, 15) and -PEG–[PG]m–PEG-(m = 6, 12, 18) with different lengths from 13 to 96 Å, the cell binding experiment proved that the optimal linker length to span both receptors (melanocortin-4 for MSH and δ-opioid for Delt-II) is around 20–50 Å. The result is consistent with the modeling study.
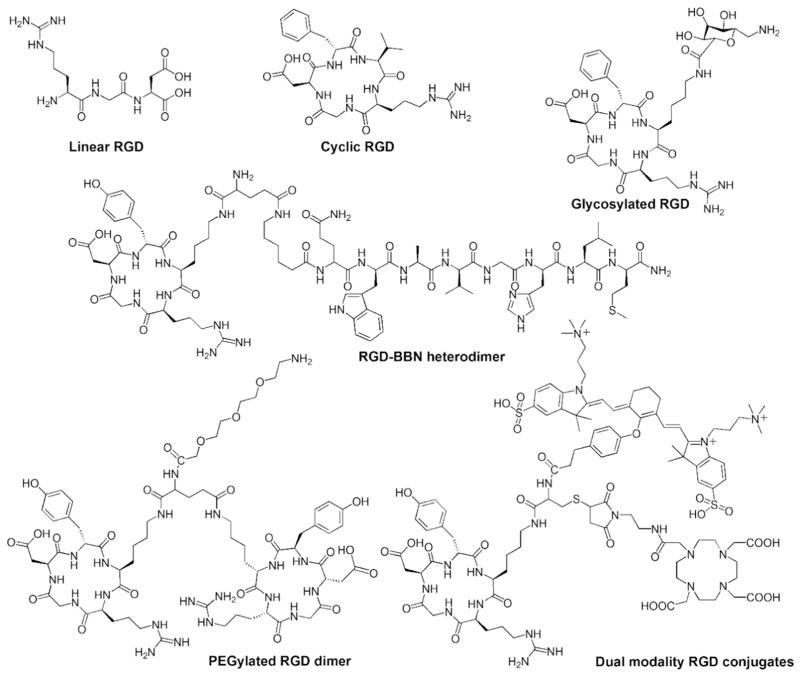
It is worth mentioning that chemical modification also could improve the binding selectivity of peptides. For example, cyclization could constrain the geometries of linear peptides to certain isoforms for targeted receptors. Pfaff et al. found that compared with linear GRGDS, the cyclic pentapeptide (RGDFV) was 10-fold more active to αvβ3 but equal in activity to α5β1 [96] (Fig. 1).
Fig. 1.
Examples of chemical modification of RGD peptides. Cyclization of RGD sequence increases the biostability and binding selectivity for integrin (modified from reference [97]). Glycosylation (modified from reference [82]) and PEGylation (modified from reference [88]) improve the blood half-life of RGD. Formation of homodimeric (modified from reference [88]) or heterodimeric (modified from reference [98]) peptide enhances the receptor targeting ability. Introduction of hybrid derivatives containing both a fluorescent and radioactive label allows the dual modality imaging (modified from reference [83]).
4. Imaging functionality
4.1. Traditional imaging functionality
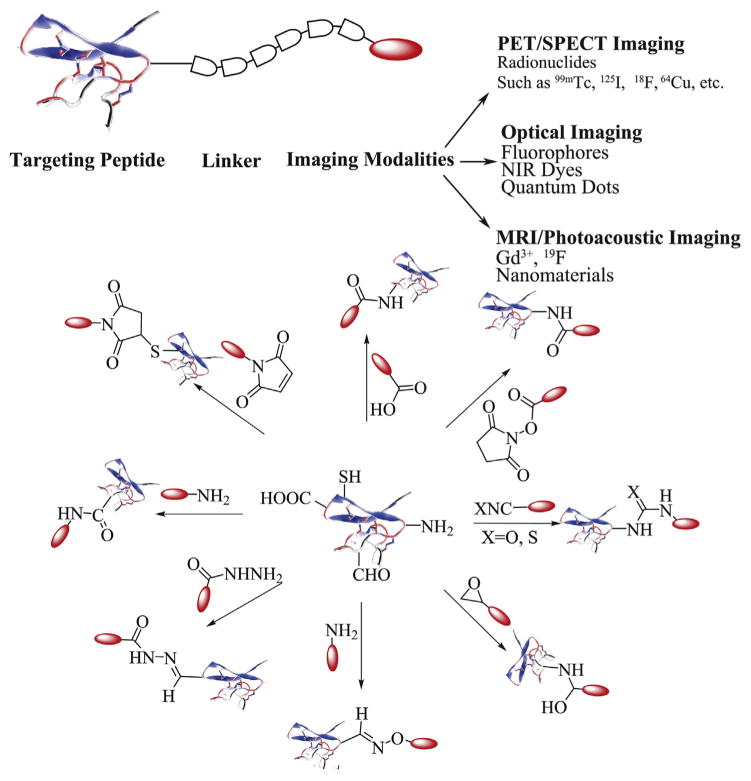
The selected peptides and peptide analogs need to be labeled with imaging moieties in order to serve as in vivo imaging probes. Some widely used imaging moieties include organic dyes for optical imaging, radionuclides for PET and SPECT imaging and Gd3+ chelators for MR imaging [11]. The functional groups of peptides available for conjugation include but are not limited to the ε-amino group on lysine side chains, the guanidinium group on arginine side chains, the carboxyl groups on aspartic acid or glutamic acid, the cysteine thiol, and the phenol on tyrosine [99]. The most common conjugation reactions are carbodiimide/N-hydroxysuccinimidyl (EDC/NHS) mediated carboxyl and amine coupling, maleimide conjugation to thiol groups, and diazonium modification of the phenol on tyrosine. Fig. 2 highlights the representative chemistries to couple peptides with imaging moieties. Details can be found in a good number of reviews [100,101].
Fig. 2.
A general schematic diagram of functionalization chemistries of peptide based imaging probes (modified from reference [11] and reference [100]).
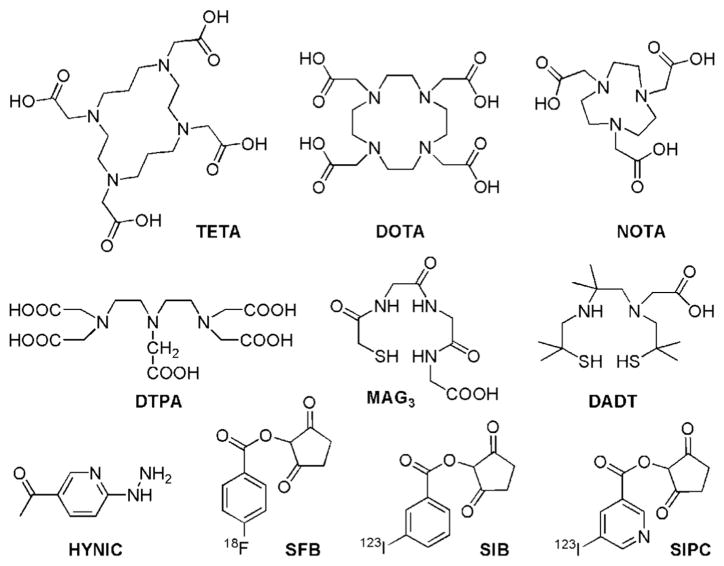
Radiolabeled peptides are the most widely used peptide-based imaging agents [102]. Radionuclide based PET/SPECT imaging could detect the imaging agents at micromolar to picomolar concentration. Therefore, it is possible to minimize the usage of peptides in order to reduce any adverse biological effect. To develop peptide based probes for PET/SPECT imaging, radionuclides should be labeled onto targeting peptides. Several radionuclides employed for peptide labeling are 99mTc, 123I, and 111In for SPECT imaging and 18F, 64Cu and 68Ga for PET imaging [103]. Generally, these radionuclides are attached to the peptides via chelators or post-synthetic radiolabeling groups. Some widely-used chelators are listed in Fig. 3.
Fig. 3.
Selected labeling chelators and post-synthetic groups: 1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid (TETA), 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), diethylenetriaminepentaacetic acid (DTPA), mercaptoacetylglycilgylcilgylicine (MAG3), diaminedithiols (DADT), 2-hydrazidonicotinic acid (HYNIC), N-succinimidyl-4-18F-fluorobenzoate (18FSFB), N-succinimidyl-5-iodo-3-pyridinecarboxylate (SIB) and N-succinimidyl-3-iodobenzoate (SIPC).
The radiochemical stability is of critical importance for PET/SPECT imaging probe development [104]. The choice of radionuclides is very significant for peptides: due to their small size, the receptor binding affinity and pharmacokinetic patterns can be strongly affected with the attachment of different radionuclides. Liu et al. labeled RGD-BBN with three different radioisotopes: 68Ga and 64Cu via NOTA chelation and 18F via FB prosthetic labeling group [105]. The metal isotopes (68Ga, 64Cu) labeled RGD-BBN showed higher tumor uptake than 18F labeled RGD-BBN in both T47D (GRPR+/integrin αvβ3 −) and MDA-MB-435 (GRPR−/integrin αvβ3 +) breast cancer models while the 18F labeled RGD-BBN showed a faster wash out than the former two. The radiolabeling site also plays an important role to maintain the biological activity of the peptides. It is necessary to understand the active sequence of peptides so that the radiolabeling can be directed at a site away from the receptor binding site. Usually, the sequence conferring the binding affinity is at the carboxy terminus while the chelator is modified to the amino terminal group. If necessary, spacers like PEG are used to further extend the distance between the receptor binding site and chelation site [106]. Specific examples have been discussed in Section 3.3.
The design of fluorophore labeled peptides is similar to radiolabeled peptides except that fluorophores are used to replace radionuclides. Various dyes are commercially available (for examples, Cyanine dyes from GE Healthcare and Alexa Fluor dyes from Invitrogen). Cheng et al. have conjugated Cy5.5 to RGD monomer, dimer and tetramer for in vivo optical imaging [107]. The binding affinities showed the same trend as those for radiolabeled RGD. The Cy5.5 labeled RGD tetramer showed the highest tumor-to-normal tissue ratio at 4 h post-injection. Although the fluorescence imaging has the advantages of high resolution, non-invasive and safe detection, the use of fluorophores in vivo is limited by the light penetration and tissue autofluorescence. The introduction of hybrid derivatives containing both a fluorescent tag and a radioactive label opens a new avenue to the field of image-guided surgery. Zhu et al. have conjugated cyclic RGD peptide with both DOTA for 64Cu labeling and a near-infrared ZW-1 dye [83]. The c(RGDyK) peptides did not lose their biological activity after dual-labeling. The tumor region in preclinical xenograft models can be clearly seen via both optical imaging and PET imaging with high tumor-to-background contrast. In another example, by taking the advantage of the strong interaction between streptavidin (SAv) and biotin, Kang et al. conjugated 64Cu-labeled Alexa Fluor 680-streptavidin with biotin-PEG-dimeric cyclic RGD peptide [108]. IC50 value of this probe (DOTA-(AF)SAv/biotin-PEG-RGD2) was 3.1-fold higher than that of RGD2. This imaging agent provided specific and sensitive PET/optical dual modality images for monitoring integrin αVβ3 expression. For the design of peptide-based probes for multimodality imaging, a major concern is that the relatively large size and complicated structure of the tags may strongly affect the original binding affinity and biological activity of peptides.
4.2. Nanoplatform based imaging functionality
The emerging nanotechnology provides new platforms for peptides [109–112]. Nanomaterials with at least one dimension within 1 to 100 nm have large surface areas allowing the efficient modification of multiple targeting peptides as well as imaging modalities. This could greatly improve the binding affinity of single peptide via a polyvalent effect. Further, nanoparticles themselves have unique physicochemical properties for theranostic applications. For example, semiconducting quantum dots (QDs) have higher fluorescence quantum yield and better photostability than traditional fluorescent dyes for optical imaging; superparamagnetic iron oxide nanoparticles are FDA approved MRI agents. Furthermore, the blood half-lives of nanomaterials are highly dependent on their size, shape and surface modifications [113,114]. By engineering the nanoplatforms, especially the surface coating, it is possible to improve the blood half-life and targeting efficiency of peptides.
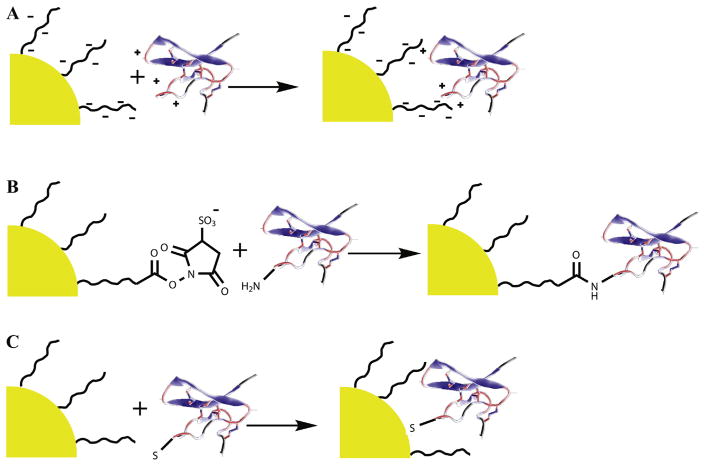
Generally, peptides are attached to the nanomaterials via three strategies: covalent conjugation, electrostatic assembly, and selective noncovalent binding interactions (Fig. 4) [111]. Covalent conjugation can provide a rigid attachment. The conjugation chemistries for nanomaterials are similar to those for small molecules. Details have been discussed in Section 4.1. Cai et al. conjugated SH-c(RGDyK) peptide to poly(ethylene glycol) coated quantum dots via maleimide-thiol coupling [115]. Each QD was estimated to be coated with around 90 copies of c(RGDyk). Meanwhile, the QD-RGD conjugate was coupled with DOTA for 64Cu labeling. These DOTA-QD-RGD nanoparticles exhibited 60-fold-higher integrin αVβ3 avidity than c(RGDyK). The in vivo dual PET/NIRF imaging was successfully obtained in mice bearing U87MG tumor (Fig. 5). In a similar way, Lee et al. have conjugated polyaspartic acid coated iron oxide nanoparticles with DOTA and SH-c(RGDyK) for PET/MRI dual modality tumor imaging [116]. Liu et al. have modified single-walled carbon nanotubes (SWNTs) with PEGylated phospholipids and further conjugated with c(RGDyK) for integrin targeting and DOTA for 64Cu labeling [117]. In both cases, the tumor uptake of nanoparticles with active targeting RGD peptides was much higher than those without RGD modification. Although conjugation of different peptides to the surface of nanoparticles provides an effective way to develop target-specific multimodal imaging agents, the formation of chemical bonds may interfere with the targeting ability [106,118].
Fig. 4.
Three general schemes used for the construction of peptide-nanocomplex: (A) electrostatic interaction between opposite charges on the NP surface and the peptide; (B) covalent chemical attachment using classical bioconjugation chemistry like amine-carboxyl coupling; and (C) direct interaction between certain peptide motif and NP surface with high affinity. (modified from [111]).
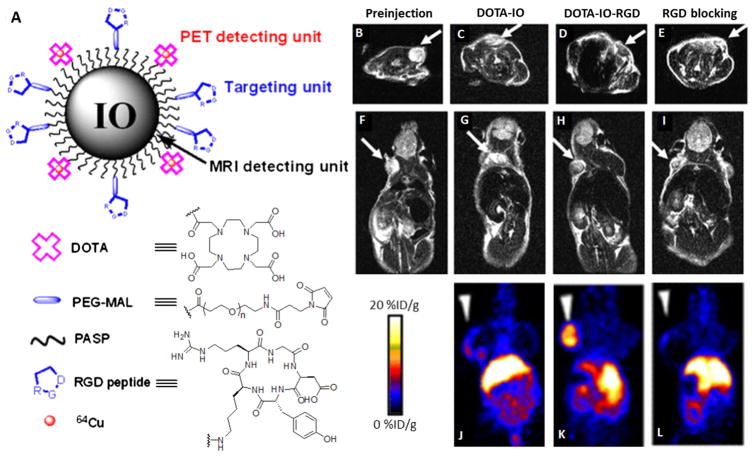
Fig. 5.
(A) A representative peptide-targeting MRI/PET dual modality nanoprobe. (B–I) T2-weighted MR images and (J–L) decay-corrected whole-body coronal PET images of nude mice bearing U87MG tumor before (B, F) and 4 h after injection of 64Cu-DOTA-iron oxide (IO) nanoparticles (C, G, J) 64Cu-DOTA-IO-RGD (D, H, K) and 64Cu-DOTA-IO-RGD with a blocking of RGD (E, I, L) [116].
Electrostatic attachment is the simplest method for NP bioconjugation by making use of the attraction between the charged nanomaterials and the oppositely charged peptides. Wagner et al. used positively charged peptide VW05 (H2N-Abz-LERKLKELERKLKELERKLKELERKL-COOH) as a template for the assembly of negative charged 11-mercaptoundecanoic acid modified Au nanoparticles [119]. Since the nanoparticle–peptide interaction is dependent on electrostatic forces, the assembly and disassembly of colloids and peptides can be controlled by pH of the solution. However, the concerns that the peptide can be easily dissociated from the complex due to the interruption of the pH and ionic strength hinder the application of this kind of probes for in vivo imaging.
An alternative strategy to maintain the bioactivity of peptides without sacrificing the rigid interaction between peptides and nanocomplex is to utilize some specific noncovalent binding between peptides and nanomaterials. A representative example is to form stable coordination complexes between the electron-donating amino acids, especially histidine, and the transition metals such as Ni(II) and Zn(II). Choi et al. used a peptide which consists of a triplicate tandem repeat of ZnO binding motif (RPHRK) and flexible linker (GGDA) to modify Fe3O4/ZnO core/shell nanoparticles [120]. The binding constant of this peptide to ZnO surface is around 1.4 × 106 M−1. Fused with this peptide, an over 10 times CEA (a tumor antigen) loading was achieved with the same amount of nanoparticles. This nanocomplex provides a simple way for ex vivo antigen loading of dendritic cells and for in vivo tracking. Bioconjugation of Au or semiconductor NPs with thiolated peptides is another typical example of dative bonds: the sulfur atom of thiol can contribute a lone pair of electrons to the empty orbital of gold atom or semiconductor atom at the interface. Pinaud et al. have synthesized a phytochelatin-related peptide as an organic coating for CdSe/ZnS core/shell nanoparticles [121]. The peptide was designed to directly bind to the nanocrystals via multiple repeats of cysteine pairs. A flexible hydrophilic linker was followed to promote the colloidal stability. Shi et al. have modified the CdSe/ZnS QDs with tetrapeptide RGDC and further labeled the peptide molecules with rhodamine dyes as energy acceptor [122]. Due to the fluorescence resonance energy transfer (FRET) between the QDs and the attached rhodamine molecules, the emission color of QDs changed from green to orange. Cleavage of the peptide by extracellular matrix metalloproteinases (MMPs) resulted in a decrease in FRET efficiency and the recovery of the green emission. This design enables monitoring the activity of specific proteolytric enzymes. This selective non-covalent binding strategy in general is highly dependent on the discovery of peptidyl sequences which can specifically bind to NP with high affinity. However, although stronger than electrostatic adsorption, dative bonds still face the challenges of pH change, oxidation as well as competition from other similar biomolecules.
Recent development in biotechnology and nanotechnology has offered various exciting possibilities for the development of multifunctional nanoparticles to target-specific delivery of theranostic agents [110,123,124]. To construct nanoplatforms with peptides as targeting agents, the unique properties of peptides (such as the low molecular weight and relative metabolic instability) place extra criteria for optimal construction: (1) minimizing the resulting hydrodynamic size of nanocomplex; (2) controlling the peptide display orientation and peptide separation distance; (3) avoiding access to non-natural residues and limiting chemical diversity during preparation; and (4) demanding purification before administration [109]. Some construction strategies for other purposes like encapsulating peptides inside hollow or mesoporous nanomatrix for therapeutic application are referred to other reviews [125,126].
5. Clinical application
An imaging probe suitable for clinical use must satisfy the following criteria: it must dominantly accumulate in the target tissue but not in the normal tissues; it should be stable enough to reach its target in an intact state but also can be fast cleared from the circulation to minimize the background and to avoid long-term exposure; all the toxicological studies, pharmacokinetics studies and preclinical tests must be passed. Due to the chemical complication and unknown toxicology, till now, very few peptide modified nanoplatforms have been translated into the clinic, although some nanocomplexes do show impressive tumor targeting and imaging capacity preclinically. Radiolabeled peptides have been applied in a wide range of hospitals continuously with their favorable pharmacokinetic behavior.
To date, several tens of radiolabeled peptides have been developed and clinically used for diagnosis and therapy. For example, 111In-DTPA-octreotide (OctreoScan; Mallinckrodt Inc.) has been used as the gold standard for the diagnosis and staging of SSTR positive tumors since the 1980s [127]. 99mTc-RP527, a bombesin analog based probe, has shown specific tumor localization and excellent imaging characteristics in humans with prostate and breast cancer [128]. 111In-labeled derivatives of the insulinotropic 39-mer peptide exendin-4 were proven to be beneficial in both pre- and intraoperative localization of benign lesions [129].
Peptides have been chemically engineered with the aim for enhanced metabolic stabilities and more favorable pharmacokinetics. Take RGD peptide as an example, radiolabeled derivatives which have been used in the clinical trials include but not limited to 99mTc-αP2, [18F]Galacto-RGD, [18F]Fluciclatide (also known as [18F]AH111585), [18F]RGD-K5, 18F-FPPRGD2, [18F]Alfatide, [68Ga]NOTA-PRGD2, and 99mTc-3PRGD2 [130]. In 1998, Sivolapenko et al. first reported the results of fourteen patients diagnosed with metastatic melanoma using 99mTc-αP2, which is a linear decapeptide with two RGD motifs [131]. Due to the fast removal of synthetic peptide from the circulation, the imaging quality is not satisfactory. Various chemical modifications, such as cyclization, glucolysation, PEGylation and multimerization, have been made to optimize the imaging quality. [18F]Galacto-RGD, which contains one cyclic RGD motif and a glucosamine moiety, was the first RGD based PET tracer tested in human [132,133]. The biodistribution of [-18F]Galacto-RGD demonstrated specific receptor binding and rapid renal clearance in cancer patients. [18F]Fluciclatide, a compound optimized by the cyclization, PEGylation and introduction of two disulfide bonds, was developed by GE Healthcare. Kenny et al. reported that the phase I trial of [18F]Fluciclatide in breast cancer patients with all 18 tumors was visible on the PET images and confirmed by CT [134]. [-18F]FPPRGD2, a PEGylated dimeric RGD peptide, has been developed and approved by the FDA as the first dimeric RGD peptide for human trial [135]. It has stable kinetics for imaging integrin αvβ3 expression, feasible early assessment of response to anti-angiogenic treatment and demonstrates encouraging results in patients with glioblastoma multiforme (GBM).
A simple, automatic, high-yield synthetic procedure is of great help to clinical translation. Especially for procedures involving radioisotopes, saving time is important due to their decay characteristics. Radiolabeling of [18F]Galacto-RGD requires coupling of 4-nitrophenyl-2-[18F]fluoropropionate to precursor of glycopeptide. The whole process is around 200 min, involving 4 steps of radiosynthesis and 3 rounds of HPLC purification [132]. Thus, [18F]RGD-K5, a compound having a similar structure with that of [18F]Galacto-RGD but suitable for a simplified radiofluorination method via click chemistry, was developed [136]. The reaction time was decreased to 90 min with the possibility for (semi)automated synthesis. Since direct labeling of 18F suffers from the problems like multistep and time-consuming, [18F]fluoride-aluminum complexes have been developed for peptide labeling. Chen’s group have successfully synthesized [18F]AlF-NOTA-PRGD2 (donated as [18F]Alfatide) [137] and subsequently, a more stable tracer [18F]NOTA-E[PEG4-c(RGDfk)]2 (denoted as [18F]Alfatide II) [138]. 18F-fluoride strongly binds to the aluminum which is chelated by NOTA chelator. The whole labeling process can be accomplished within 40 min. They further simplified the reagents into lyophilized kit formulation and reduced the reaction time to 20 min including cartridge purification for wide-spread use in the clinic.
The possibility to obtain the isotopes (either from its own radiochemistry facility or transport from other centers) is another barrier for translating radiolabeled peptides to the clinical setting. Isotopes have been widely used in clinical trials including 18F and 68Ga for PET imaging, 99mTc and 111In for SPECT imaging and 90Y and 177Lu for radiotherapy. 111In-DTPA-octreotide serves as an effective prognostic standard for well-differentiated malignant endocrine tumors [139]. However, 111In is not ideal for clinical use due to its unfavorable nuclear physical properties, and limited availability. 99mTc labeled somatostatin analogs, such as 99mTc labeled depreotide (99mTc-NeoTect, Diatide, Inc.) [140] and 99mTc-N4-[Tyr3]octreotate [141], become more and more important because of their better imaging quality and the wide availability of 99mTc. Compared with SPECT imaging, PET imaging with 68Ga and 18F labeled somatostatin analogs has higher sensitivity and reduced scanning time for neuroendocrine tumor (NET) detection. Somatostatin peptides have been radiolabeled with 18F and 68Ga via different chemical methods [142–145]. 68Ga labeled tracers have certain advantages over 18F labeled ones since the generator based 68Ga production makes it possible to prepare compounds easily even in centers without an on-site cyclotron. 68Ga labeled DOTA conjugated [Tyr3]octreotide (TOC) [142], [Tyr3, Thr8]octreotide (TATE) [146], and [1-NaI3]octreotide (NOC) [147] are the most popular 68Ga-labeled somatostatin analogs clinically used for NETs. A great number of studies reported a higher tumor-to-tissue contrast and a higher sensitivity for PET/CT detection compared with CT and somatostatin receptor scintigraphy (SRS). Although this review focuses on the imaging application, it is worth to note that peptides are also excellent candidates for peptide receptor targeted radiotherapy (PRTR) due to their fast clearance ability and specific binding ability which leads to minimal exposure of health tissues [14].
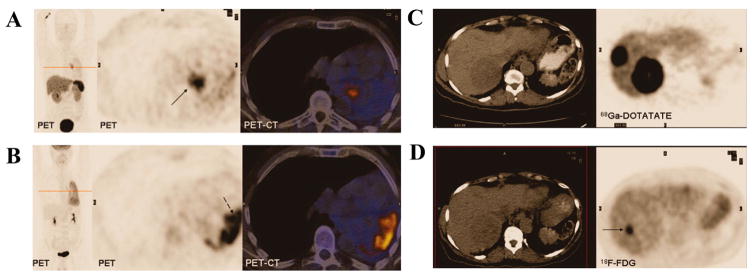
Many radiolabeled peptides exhibit diagnostic advantages over other imaging procedures in clinical trials. For example, 68Ga-DOTATATE (68Ga-DOTA-(Tyr3)-octreotate) PET/CT has shown superiority in neuroendocrine tumor diagnosis than some currently FDA approved imaging methods [148]. 123I-MIBG is used as a standard functional imaging for initial evaluation of neuroendocrine tumors. 68Ga-DOTATATE is superior to 123I-MIBG in detecting lesions in all anatomical locations, especially bony lesions. In one study of 15 recruited patients, the lesions in 4 patients were picked up by 68Ga-DOTATATE but missed by 123I-MIBG [149]. Schmid-Tannwald et al. proved that the detection rate of pancreatic NET with 68Ga-DOTATATE PET/CT was significantly higher than that with MRI. The NETs were detected in 8/23 (34.8%) and 9/23 (39.1%) patients on T2w imaging by observers but with 100% on PET/CT [150]. Goel et al. found that 68Ga-DOTATATE PET detected bone metastases at a higher rate than CT: out of a total of 225 lesions detected by 68Ga-DOTATATE PET, only 84 lesions could be detected by CT scans [151]. Besides, compared with well-established clinical PET imaging agent, 18F-FDG and 68Ga-DOTATATE could provide additional tumor information [152–154]. When NETs are in the initial stage, they have a slow metabolic activity and a high SSTR2 expression, resulting in a low uptake of 18F-FDG but high uptake of 68Ga-DOTATATE. When the tumor characteristics change into poorly differentiated ones, they have high 18F-FDG uptake but low 68Ga-DOTATATE uptake. In a study of 38 patients with primary or recurrent NET [153], the standard uptake value of 68Ga-DOTATATE and 18F-FDG was 29 vs. 2.9 in low-grade NET but 4.4 vs. 11.7 in high-grade NET (Fig. 6). It is generally believed that 18F-FDG PET reflects the glucose metabolism and is more sensitive for tumor staging, while radiolabeled peptides target the receptor and have more potential value for assessing tumor grade and selection of patients suitable for receptor-targeted therapies.
Fig. 6.
(A) 68Ga-DOTATATE and (B) 18F-FDG PET/CT images from a patient with well-differentiated bronchial carcinoid tumor. Histology showed that the left lung had postcollapse pneumonitis that was negative for 68Ga-DOTATATE but positive for 18F-FDG. (C) 68Ga-DOTATATE and (D) 18F-FDG PET/CT images in a 54-year-old female patient with metastatic carcinoid tumor. The lesions show 68Ga-DOTATATE uptake along the whole circumference of the lesion, whereas there is only focal 18F-FDG uptake at the margin [153].
6. Conclusion
The development of peptide based imaging agents relies on the cooperative efforts from biologists to discover new disease-related targets and corresponding targeting peptides, chemists to synthesize and characterize the peptide-based probes, engineers and medical physicists to improve imaging quality, as well as clinicians to assess the clinical value.
In recent years, numerous peptides have been discovered and designed for molecular imaging, including PET, SPECT, MRI, optical imaging, photoacoustic imaging and Raman imaging. Among these, radiolabeled peptides for PET and SPECT imaging are considered as the best candidates for clinical translation. Optimization of radiolabeled peptides can be achieved via chemical modification. First, cyclization of peptides can help restrict their three-dimensional conformation and improve receptor binding specificity and stability. Second, incorporation of an appropriate functional group such as glycosylation, PEGylation and human albumin may result in improved pharmacokinetic properties. Third, multimerization of targeting peptides (homo or hetero) can enhance the binding affinity due to the multivalency effect. Fourth, site-specific labeling or insertion of an appropriate linker between targeting peptides and radioisotopes helps retain the binding affinity and imaging activity. Fifth, a simple, automatic, high-yield labeling method is significant for clinical use. It is also worth to mention that development of multifunctional imaging probes, especially nanomaterial based imaging probes, which combines the advantages of different imaging modalities is of great interest, yet the pharmacokinetics and toxicities need careful investigation before possible use in the clinic. One typical example is the clinical trial of Bradbury group’s 124I-cRGDY-PEG-C dots [155].
Many radiolabeled peptides have been introduced into the clinic with impressive sensitivity and accuracy for tumor assessment. However, only several have been approved by FDA till now. One major challenge is how to obtain a significant foothold in the clinic, especially compared with well-established clinical imaging agents such as 18F-FDG. Efforts are still needed in developing more sensitive, specific, and stable peptide-based diagnostics without unwanted tissue uptake and possible side effect. The clinical potential of existing probes on tumor grading also needs to be evaluated with more comprehensive information based on large scale clinical trials. What’s more, the development of radionuclide based peptides can greatly benefit individualized theranostics, where imaging and the radiotherapy are carried out using the same vector by exchanging the imaging and therapeutic radionuclides. Quantifying the receptor density and distribution via PET/SPECT imaging would help design the therapeutics based on the same ligand and the same target. It is anticipated that these efforts can assist personalized therapy including early detection, staging, therapy planning and monitoring.
Acknowledgments
This study was financially supported by the National Key Basic Research Program of China (2014CB744503) and National Natural Science Foundation of China (21271030, 51502251, 81471707, and 81571743).
Abbreviation
- BBN
bombesin
- CCK
cholecystokinin
- CXCR4
chemokine receptor type 4
- DADT
diaminedithiols
- Delt-II
Deltorphin-II
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- DTPA
diethylenetriaminepentaacetic acid
- EB
Evans blue
- EDC
carbodiimide
- FDA
Food and Drug Administration
- FDG
fludeoxyglucose
- 18F-FB
18F-fluorobenzoyl
- FRET
fluorescence resonance energy transfer
- 18FSFB
N-succinimidyl-4-18F-fluorobenzoate
- 68Ga-DOTATATE
68Ga-DOTA-(Tyr3)-octreotate
- GBM
glioblastoma multiforme
- GLP-1
glucagon-like peptide 1
- GRP
gastrin-releasing peptide
- GRPR
gastrin-releasing peptide receptor
- HYNIC
2-hydrazidonicotinic acid
- MAG3
mercaptoacetylglycilgylcilgylicine
- MC-1r
melanocortin-1 receptors
- MIBG
metaiodobenzylguanidine
- MMPs
metalloproteinases
- MRI
magnetic resonance imaging
- α-MSH
α-melanocyte-stimulating hormone
- NET
neuroendocrine tumor
- NHS
N-hydroxysuccinimidyl
- NK1R
neurokinin 1 receptor
- NIRF
near infrared fluorescence
- NOTA
1,4,7-triazacyclononane-1,4,7-triacetic acid
- NT
neutrotensin
- PEG
polyethyleneglycol
- PET
positron emission tomography
- PRTR
peptide receptor targeted radiotherapy
- QDs
quantum dots
- RGD
Arg-Gly-Asp
- SIB
N-succinimidyl-5-iodo-3-pyridinecarboxylate
- SIPC
N-succinimidyl-3-iodobenzoate
- SPECT
single photon emission computed tomography
- SRS
somatostatin receptor scintigraphy
- SST
somatostatin
- SWNTs
single-walled carbon nanotubes
- TETA
1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid
- TMTP1
tumor molecular targeted peptide 1
- VIP
vasoactive intestinal peptide
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Peptides and peptide conjugates in medicine”.
References
- 1.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 2.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 3.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Chen X. Design and development of molecular imaging probes. Curr Top Med Chem. 2010;10:1227. doi: 10.2174/156802610791384225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin VS, Chen W, Xian M, Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem Soc Rev. 2015;44:4596–4618. doi: 10.1039/c4cs00298a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 8.Hoelder S, Clarke PA, Workman P. Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol Oncol. 2012;6:155–176. doi: 10.1016/j.molonc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong H, Goel S, Zhang Y, Cai W. Molecular imaging with nucleic acid aptamers. Curr Med Chem. 2011;18:4195. doi: 10.2174/092986711797189691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Xie J, Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem Rev. 2010;110:3087–3111. doi: 10.1021/cr900361p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Xie J, Chen X. Peptide-based probes for targeted molecular imaging. Biochemistry. 2010;49:1364–1376. doi: 10.1021/bi901135x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fani M, Maecke H, Okarvi S. Radiolabeled peptides: valuable tools for the detection and treatment of cancer. Theranostics. 2012;2:481–501. doi: 10.7150/thno.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi H, Fujimori N, Ito T, Nakamura T, Oono T, Nakamura K, Suzuki K, Jensen RT, Takayanagi R. Vasoactive intestinal peptide (VIP) and VIP receptors—elucidation of structure and function for therapeutic applications. Int J Clin Med. 2011;2:500–508. [Google Scholar]
- 14.De Jong M, Breeman WA, Kwekkeboom DJ, Valkema R, Krenning EP. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc Chem Res. 2009;42:873–880. doi: 10.1021/ar800188e. [DOI] [PubMed] [Google Scholar]
- 15.Tweedle MF. Peptide-targeted diagnostics and radiotherapeutics. Acc Chem Res. 2009;42:958–968. doi: 10.1021/ar800215p. [DOI] [PubMed] [Google Scholar]
- 16.Laverman P, Sosabowski JK, Boerman OC, Oyen WJ. Radiolabelled peptides for oncological diagnosis. Eur J Nucl Med Mol Imaging. 2012;39:78–92. doi: 10.1007/s00259-011-2014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schottelius M, Wester H-J. Molecular imaging targeting peptide receptors. Methods. 2009;48:161–177. doi: 10.1016/j.ymeth.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Uhlig T, Kyprianou T, Martinelli FG, Oppici CA, Heiligers D, Hills D, Calvo XR, Verhaert P. The emergence of peptides in the pharmaceutical business: from exploration to exploitation. EuPA Open Proteom. 2014;4:58–69. [Google Scholar]
- 19.Nagpal R, Behare P, Rana R, Kumar A, Kumar M, Arora S, Morotta F, Jain S, Yadav H. Bioactive peptides derived from milk proteins and their health beneficial potentials: an update. Food Funct. 2011;2:18–27. doi: 10.1039/c0fo00016g. [DOI] [PubMed] [Google Scholar]
- 20.Andersson L, Blomberg L, Flegel M, Lepsa L, Nilsson B, Verlander M. Large-scale synthesis of peptides. Pept Sci. 2000;55:227–250. doi: 10.1002/1097-0282(2000)55:3<227::AID-BIP50>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Merrifield R. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 2006;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- 22.Gray BP, Brown KC. Combinatorial peptide libraries: mining for cell-binding peptides. Chem Rev. 2013;114:1020–1081. doi: 10.1021/cr400166n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deutscher SL. Phage display in molecular imaging and diagnosis of cancer. Chem Rev. 2010;110:3196–3211. doi: 10.1021/cr900317f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 25.Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 26.Cutler CS, Chanda N, Shukla R, Sisay N, Cantorias M, Zambre A, McLaughlin M, Kelsey J, Upenandran A, Robertson D. Theranostics, Gallium-68, and Other Radionuclides. Springer; 2013. Nanoparticles and phage display selected peptides for imaging and therapy of cancer; pp. 133–147. [DOI] [PubMed] [Google Scholar]
- 27.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 28.Schottelius M, Laufer B, Kessler H, Wester H., Jr Ligands for mapping αvβ3-integrin expression in vivo. Acc Chem Res. 2009;42:969–980. doi: 10.1021/ar800243b. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I, Bading JR, Laug WE, Conti PS. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor αvβ3-integrin expression. J Nucl Med. 2004;45:1776–1783. [PubMed] [Google Scholar]
- 30.Craig WS, Cheng S, Mullen DG, Blevitt J, Pierschbacher MD. Concept and progress in the development of RGD-containing peptide pharmaceuticals. Biopolymers. 1995;37:157–175. doi: 10.1002/bip.360370209. [DOI] [PubMed] [Google Scholar]
- 31.Gaertner F, Kessler H, Wester H-J, Schwaiger M, Beer A. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39:126–138. doi: 10.1007/s00259-011-2028-1. [DOI] [PubMed] [Google Scholar]
- 32.Cai H, Conti PS. RGD-based PET tracers for imaging receptor integrin αvβ3 expression. J Label Compd Radiopharm. 2013;56:264–279. doi: 10.1002/jlcr.2999. [DOI] [PubMed] [Google Scholar]
- 33.Sancho V, Di Florio A, Moody TW, Jensen RT. Bombesin receptor-mediated imaging and cytotoxicity: review and current status. Curr Drug Deliv. 2011;8:79–134. doi: 10.2174/156720111793663624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cescato R, Maina T, Nock B, Nikolopoulou A, Charalambidis D, Piccand V, Reubi JC. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med. 2008;49:318–326. doi: 10.2967/jnumed.107.045054. [DOI] [PubMed] [Google Scholar]
- 35.Ohki-Hamazaki H, Iwabuchi M, Maekawa F. Development and function of bombesin-like peptides and their receptors. Int J Dev Biol. 2005;49:293–300. doi: 10.1387/ijdb.041954ho. [DOI] [PubMed] [Google Scholar]
- 36.Yu Z, Ananias HJ, Carlucci G, Hoving HD, Helfrich W, Dierckx RA, Wang F, Jong IJd, Elsinga PH. An update of radiolabeled bombesin analogs for gastrin-releasing peptide receptor targeting. Curr Pharm Des. 2013;19:3329–3341. doi: 10.2174/1381612811319180015. [DOI] [PubMed] [Google Scholar]
- 37.Ananias H, De Jong I, Dierckx R, de Wiele Cv, Helfrich W, Elsinga P. Nuclear imaging of prostate cancer with gastrin-releasing-peptide-receptor targeted radiopharmaceuticals. Curr Pharm Des. 2008;14:3033–3047. doi: 10.2174/138161208786404335. [DOI] [PubMed] [Google Scholar]
- 38.Varvarigou A, Bouziotis P, Zikos C, Scopinaro F, De Vincentis G. Gastrin-releasing peptide (GRP) analogues for cancer imaging. Cancer Biother Radiopharm. 2004;19:219–229. doi: 10.1089/108497804323072002. [DOI] [PubMed] [Google Scholar]
- 39.Roosenburg S, Laverman P, van Delft FL, Boerman OC. Radiolabeled CCK/gastrin peptides for imaging and therapy of CCK2 receptor-expressing tumors. Amino Acids. 2011;41:1049–1058. doi: 10.1007/s00726-010-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang B, Yong X, Xie R, Li Q-W, Yang S-M. Vasoactive intestinal peptide receptor-based imaging and treatment of tumors (review) Int J Oncol. 2014;44:1023–1031. doi: 10.3892/ijo.2014.2276. [DOI] [PubMed] [Google Scholar]
- 41.Igarashi H, Ito T, Mantey SA, Pradhan TK, Hou W, Coy DH, Jensen RT. Development of simplified vasoactive intestinal peptide analogs with receptor selectivity and stability for human vasoactive intestinal peptide/pituitary adenylate cyclase-activating polypeptide receptors. J Pharmacol Exp Ther. 2005;315:370–381. doi: 10.1124/jpet.105.088823. [DOI] [PubMed] [Google Scholar]
- 42.Morgat C, Mishra AK, Varshney R, Allard M, Fernandez P, Hindié E. Targeting neuropeptide receptors for cancer imaging and therapy: perspectives with bombesin, neurotensin, and neuropeptide-Y receptors. J Nucl Med. 2014;55:1650–1657. doi: 10.2967/jnumed.114.142000. [DOI] [PubMed] [Google Scholar]
- 43.Alshoukr F, Prignon A, Brans L, Jallane A, Mendes S, Talbot J-N, Tourwé D, Barbet J, Gruaz-Guyon A. Novel DOTA-neurotensin analogues for 111In scintigraphy and 68Ga PET imaging of neurotensin receptor-positive tumors. Bioconjug Chem. 2011;22:1374–1385. doi: 10.1021/bc200078p. [DOI] [PubMed] [Google Scholar]
- 44.Maecke HR, Reubi JC. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. Nucl Med. 2011;52:841–844. doi: 10.2967/jnumed.110.084236. [DOI] [PubMed] [Google Scholar]
- 45.Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, Erchegyi J, Rivier J, Mäcke HR, Reubi JC. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103:16436–16441. doi: 10.1073/pnas.0607761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259–2277. doi: 10.2217/fon.14.139. [DOI] [PubMed] [Google Scholar]
- 47.Quinn T, Zhang X, Miao Y. Targeted melanoma imaging and therapy with radiolabeled alpha-melanocyte stimulating hormone peptide analogues. G Ital Dermatol Venereol. 2010;145:245–258. [PMC free article] [PubMed] [Google Scholar]
- 48.Miao Y, Quinn TP. Alpha-melanocyte stimulating hormone peptide-targeted melanoma imaging. Front Biosci. 2006;12:4514–4524. doi: 10.2741/2406. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson O, Weiss ID, Szajek LP, Niu G, Ma Y, Kiesewetter DO, Farber JM, Chen X. PET imaging of CXCR4 using copper-64 labeled peptide antagonist. Theranostics. 2011;1:251–262. doi: 10.7150/thno/v01p0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobson O, Weiss I, Farber J, Chen X. PET imaging of tumor CXCR4 expression with 18F-T140. J Nucl Med. 2010;51:282–282. doi: 10.2967/jnumed.110.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wild D, Wicki A, Mansi R, Béhé M, Keil B, Bernhardt P, Christofori G, Ell PJ, Mäcke HR. Exendin-4-based radiopharmaceuticals for glucagonlike peptide-1 receptor PET/CT and SPECT/CT. Nucl Med. 2010;51:1059–1067. doi: 10.2967/jnumed.110.074914. [DOI] [PubMed] [Google Scholar]
- 52.Wu H, Liang S, Liu S, Pan Y, Cheng D, Zhang Y. 18F-radiolabeled GLP-1 analog exendin-4 for PET/CT imaging of insulinoma in small animals. Nucl Med Commun. 2013;34:701–708. doi: 10.1097/MNM.0b013e3283614187. [DOI] [PubMed] [Google Scholar]
- 53.Hubalewska-Dydejczyk A, Sowa-Staszczak A, Tomaszuk M, Stefańska A. GLP-1 and exendin-4 for imaging endocrine pancreas. A review. Labelled glucagon-like peptide-1 analogues: past, present and future. Q J Nucl Med Mol Imaging. 2015;59:152–160. [PubMed] [Google Scholar]
- 54.Li J, Tian Y, Wu A. Neuropeptide Y receptors: a promising target for cancer imaging and therapy. Regen Biomater. 2015;2:215–219. doi: 10.1093/rb/rbv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langer M, La Bella R, Garcia-Garayoa E, Beck-Sickinger AG. 99mTc-labeled neuropeptide Y analogues as potential tumor imaging agents. Bioconjug Chem. 2001;12:1028–1034. doi: 10.1021/bc015514h. [DOI] [PubMed] [Google Scholar]
- 56.Cordier D, Forrer F, Kneifel S, Sailer M, Mariani L, Mäcke H, Müller-Brand J, Merlo A. Neoadjuvant targeting of glioblastoma multiforme with radiolabeled DOTAGA–substance P—results from a phase I study. J Neuro-Oncol. 2010;100:129–136. doi: 10.1007/s11060-010-0153-5. [DOI] [PubMed] [Google Scholar]
- 57.Mozaffari S, Erfani M, Beiki D, Daha FJ, Kobarfard F, Balalaie S, Fallahi B. Synthesis and preliminary evaluation of a new 99mTc labeled substance P analogue as a potential tumor imaging agent. Iran J Pharm Res. 2015;14:97. [PMC free article] [PubMed] [Google Scholar]
- 58.Shi J, Fan D, Zhang X, Yang L, Sun Y, Zhao H, Zhu Z, Jia B, Wang F. PET imaging of tumor metastasis using 68Ga-labeled a novel tumor-homing peptide TMTP1. J Nucl Med. 2014;55:1086–1086. [Google Scholar]
- 59.Li F, Cheng T, Dong Q, Wei R, Zhang Z, Luo D, Ma X, Wang S, Gao Q, Ma D. Evaluation of 99m Tc-HYNIC-TMTP1 as a tumor-homing imaging agent targeting metastasis with SPECT. Nucl Med Biol. 2015;42:256–262. doi: 10.1016/j.nucmedbio.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 61.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 62.Matsuno M, Matsui T, Iwasaki A, Arakawa Y. Role of acetylcholine and gastrin-releasing peptide (GRP) in gastrin secretion. J Gastroenterol. 1997;32:579–586. doi: 10.1007/BF02934105. [DOI] [PubMed] [Google Scholar]
- 63.Singh M, Mukhopadhyay K. Alpha-melanocyte stimulating hormone: an emerging anti-inflammatory antimicrobial peptide. Biomed Res Int. 2014;2014:874610. doi: 10.1155/2014/874610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tyler-McMahon BM, Boules M, Richelson E. Neurotensin: peptide for the next millennium. Regul Pept. 2000;93:125–136. doi: 10.1016/s0167-0115(00)00183-x. [DOI] [PubMed] [Google Scholar]
- 65.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, Kipps TJ, Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 66.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tatemoto K. Neuropeptide Y: history and overview, Neuropeptide Y and Related Peptides. Springer; 2004. pp. 1–21. [Google Scholar]
- 68.Strand FL. Neuropeptides: Regulators of Physiological Processes. MIT press; 1999. [Google Scholar]
- 69.Yang W, Luo D, Wang S, Wang R, Chen R, Liu Y, Zhu T, Ma X, Liu R, Xu G. TMTP1, a novel tumor-homing peptide specifically targeting metastasis. Clin Cancer Res. 2008;14:5494–5502. doi: 10.1158/1078-0432.CCR-08-0233. [DOI] [PubMed] [Google Scholar]
- 70.Gentilucci L, De Marco R, Cerisoli L. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr Pharm Des. 2010;16:3185–3203. doi: 10.2174/138161210793292555. [DOI] [PubMed] [Google Scholar]
- 71.Adessi C, Soto C. Converting a peptide into a drug: strategies to improve stability and bioavailability. Curr Med Chem. 2002;9:963–978. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 72.Davies JS. The cyclization of peptides and depsipeptides. J Pept Sci. 2003;9:471–501. doi: 10.1002/psc.491. [DOI] [PubMed] [Google Scholar]
- 73.Gilon C, Halle D, Chorev M, Selincer Z, Byk G. Backbone cyclization: a new method for conferring conformational constraint on peptides. Biopolymers. 1991;31:745–750. doi: 10.1002/bip.360310619. [DOI] [PubMed] [Google Scholar]
- 74.Bogdanowich-Knipp SJ, Chakrabarti S, Siahaan TJ, Williams TD, Dillman RK. Solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53:530–541. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 75.Bogdanowich-Knipp S, Jois D, Siahaan T. The effect of conformation on the solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53:523–529. doi: 10.1034/j.1399-3011.1999.00055.x. [DOI] [PubMed] [Google Scholar]
- 76.Grace CRR, Erchegyi J, Samant M, Cescato R, Piccand V, Riek R, Reubi JC, Rivier JE. Ring size in octreotide amide modulates differently agonist versus antagonist binding affinity and selectivity. J Med Chem. 2008;51:2676–2681. doi: 10.1021/jm701445q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong SY, Oh JE, Lee K-H. Effect of D-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem Pharmacol. 1999;58:1775–1780. doi: 10.1016/s0006-2952(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 78.Rathore N, Gellman SH, de Pablo JJ. Thermodynamic stability of β-peptide helices and the role of cyclic residues. Biophys J. 2006;91:3425–3435. doi: 10.1529/biophysj.106.084491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coy D, Lu-Hua W, Ning-Yi J, Jensen R. Short chain bombesin pseudopeptides with poterat bosnbesin receptor antagonist activity in rat and Guinea pig pancreatic acinar cells. Eur J Pharmacol. 1990;190:31–38. doi: 10.1016/0014-2999(90)94109-b. [DOI] [PubMed] [Google Scholar]
- 80.Shi J, Kim Y-S, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly3 and PEG4 linkers. Bioconjug Chem. 2009;20:750–759. doi: 10.1021/bc800455p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou K, Zheng X, Xu H-M, Zhang J, Chen Y, Xi T, Feng T. Studies of poly (ethylene glycol) modification of HM-3 polypeptides. Bioconjug Chem. 2009;20:932–936. doi: 10.1021/bc900070r. [DOI] [PubMed] [Google Scholar]
- 82.Maschauer S, Haubner R, Kuwert T, Prante O. 18F-Glyco-RGD peptides for PET imaging of integrin expression: efficient radiosynthesis by click chemistry and modulation of biodistribution by glycosylation. Mol Pharm. 2013;11:505–515. doi: 10.1021/mp4004817. [DOI] [PubMed] [Google Scholar]
- 83.Zhu L, Guo N, Li Q, Ma Y, Jacboson O, Lee S, Choi HS, Mansfield JR, Niu G, Chen X. Dynamic PET and optical imaging and compartment modeling using a dual-labeled cyclic RGD peptide probe. Theranostics. 2012;2:746–756. doi: 10.7150/thno.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Z, Chen X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem Soc Rev. 2016;45:1432–1456. doi: 10.1039/c5cs00158g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen H, Wang G, Lang L, Jacobson O, Kiesewetter O, Liu Y, Ma Y, Zhang X, Wu H, Zhu L. Chemical conjugation of Evans blue derivative: a strategy to develop long-acting therapeutics through albumin binding. Theranostics. 2016;6:143–153. doi: 10.7150/thno.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Janssen M, Oyen WJ, Massuger LF, Frielink C, Dijkgraaf I, Edwards DS, Radjopadhye M, Corstens FH, Boerman OC. Comparison of a monomeric and dimeric radiolabeled RGD-peptide for tumor targeting. Cancer Biother Radiopharm. 2002;17:641–646. doi: 10.1089/108497802320970244. [DOI] [PubMed] [Google Scholar]
- 87.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. microPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 88.Guo J, Lang L, Hu S, Guo N, Zhu L, Sun Z, Ma Y, Kiesewetter DO, Niu G, Xie Q. Comparison of three dimeric 18F-AlF-NOTA-RGD tracers. Mol Imaging Biol. 2014;16:274–283. doi: 10.1007/s11307-013-0668-1. [DOI] [PubMed] [Google Scholar]
- 89.Liu Z, Niu G, Shi J, Liu S, Wang F, Liu S, Chen X. 68Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin αvβ3 PET imaging. Eur J Nucl Med Mol Imaging. 2009;36:947–957. doi: 10.1007/s00259-008-1045-1. [DOI] [PubMed] [Google Scholar]
- 90.Liu Z, Li Z-B, Cao Q, Liu S, Wang F, Chen X. Small-animal PET of tumors with 64Cu-labeled RGD-bombesin heterodimer. J Nucl Med. 2009;50:1168–1177. doi: 10.2967/jnumed.108.061739. [DOI] [PubMed] [Google Scholar]
- 91.Liu Z, Yan Y, Chin FT, Wang F, Chen X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J Med Chem. 2009;52:425–432. doi: 10.1021/jm801285t. [DOI] [PubMed] [Google Scholar]
- 92.Josan JS, Vagner J, Handl HL, Sankaranarayanan R, Gillies RJ, Hruby VJ. Solid-phase synthesis of heterobivalent ligands targeted to melanocortin and cholecystokinin receptors. Int J Pept Res Ther. 2008;14:293–300. doi: 10.1007/s10989-008-9150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Capello A, Krenning EP, Bernard BF, Breeman WA, Erion JL, de Jong M. Anti-cancer activity of targeted proapoptotic peptides. J Nucl Med. 2006;47:122–129. [PubMed] [Google Scholar]
- 94.Wu H, Chen H, Pan D, Ma Y, Liang S, Wan Y, Fang Y. Imaging integrin αvβ3 and NRP-1 positive gliomas with a novel fluorine-18 labeled RGD-ATWLPPR heterodimeric peptide probe. Mol Imaging Biol. 2014;16:781–792. doi: 10.1007/s11307-014-0761-0. [DOI] [PubMed] [Google Scholar]
- 95.Vagner J, Xu L, Handl HL, Josan JS, Morse DL, Mash EA, Gillies RJ, Hruby VJ. Heterobivalent ligands crosslink multiple cell-surface receptors: the human melanocortin-4 and δ-opioid receptors. Angew Chem Int Ed Engl. 2008;120:1709–1712. doi: 10.1002/anie.200702770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfaff M, Tangemann K, Müller B, Gurrath M, Müller G, Kessler H, Timpl R, Engel J. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- 97.Zhou Y, Chakraborty S, Liu S. Radiolabeled cyclic RGD peptides as radiotracers for imaging tumors and thrombosis by SPECT. Theranostics. 2011;1:58–82. doi: 10.7150/thno/v01p0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Z-B, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49:453–461. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 99.Roberts M, Bentley M, Harris J. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2012;64:116–127. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 100.Erathodiyil N, Ying JY. Functionalization of inorganic nanoparticles for bioimaging applications. Acc Chem Res. 2011;44:925–935. doi: 10.1021/ar2000327. [DOI] [PubMed] [Google Scholar]
- 101.Takahashi H, Emoto K, Dubey M, Castner DG, Grainger DW. Imaging surface immobilization chemistry: correlation with cell patterning on non-adhesive hydrogel thin films. Adv Funct Mater. 2008;18:2079–2088. doi: 10.1002/adfm.200800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maecke H. Molecular Imaging. Springer; 2005. Radiolabeled peptides in nuclear oncology: influence of peptide structure and labeling strategy on pharmacology; pp. 43–72. [DOI] [PubMed] [Google Scholar]
- 103.Chatalic KLS, Kwekkeboom DJ, de Jong M. Radiopeptides for imaging and therapy: a radiant future. J Nucl Med. 2015;56:1809–1812. doi: 10.2967/jnumed.115.161158. [DOI] [PubMed] [Google Scholar]
- 104.Sun X, Cai W, Chen X. Positron emission tomography imaging using radiolabeled inorganic nanomaterials. Acc Chem Res. 2015;48:286–294. doi: 10.1021/ar500362y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Z, Yan Y, Liu S, Wang F, Chen X. 18F, 64Cu, and 68Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjug Chem. 2009;20:1016–1025. doi: 10.1021/bc9000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sosabowski JK, Mather SJ. Conjugation of DOTA-like chelating agents to peptides and radiolabeling with trivalent metallic isotopes. Nat Protoc. 2006;1:972–976. doi: 10.1038/nprot.2006.175. [DOI] [PubMed] [Google Scholar]
- 107.Cheng Z, Wu Y, Xiong Z, Gambhir SS, Chen X. Near-infrared fluorescent RGD peptides for optical imaging of integrin αvβ3 expression in living mice. Bioconjug Chem. 2005;16:1433–1441. doi: 10.1021/bc0501698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kang CM, Koo H-J, An GI, Choe YS, Choi JY, Lee K-H, Kim B-T. Hybrid PET/optical imaging of integrin αVβ3 receptor expression using a 64Cu-labeled streptavidin/biotin-based dimeric RGD peptide. EJNMMI Res. 2015;5:1–10. doi: 10.1186/s13550-015-0140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Field LD, Delehanty JB, Chen Y, Medintz IL. Peptides for specifically targeting nanoparticles to cellular organelles: quo vadis? Acc Chem Res. 2015;48:1380–1390. doi: 10.1021/ar500449v. [DOI] [PubMed] [Google Scholar]
- 110.Lee D-E, Koo H, Sun I-C, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41:2656–2672. doi: 10.1039/c2cs15261d. [DOI] [PubMed] [Google Scholar]
- 111.Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev. 2013;113:1904–2074. doi: 10.1021/cr300143v. [DOI] [PubMed] [Google Scholar]
- 112.An L, Gilani HS, Rehan M, Liang G. Peptide-based nanostructures for cancer diagnosis and therapy. Curr Med Chem. 2014;21:2453–2466. doi: 10.2174/0929867321666140205140600. [DOI] [PubMed] [Google Scholar]
- 113.Wang B, He X, Zhang Z, Zhao Y, Feng W. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acc Chem Res. 2012;46:761–769. doi: 10.1021/ar2003336. [DOI] [PubMed] [Google Scholar]
- 114.Setyawati MI, Tay CY, Docter D, Stauber RH, Leong DT. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem Soc Rev. 2015;44:8174–8199. doi: 10.1039/c5cs00499c. [DOI] [PubMed] [Google Scholar]
- 115.Cai W, Shin D-W, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 116.Lee H-Y, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. PET/MRI dual-modality tumor imaging using arginine–glycine–aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 117.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 118.Perez Espitia PJ, de Fátima Ferreira Soares N, Coimbra JSdR, de Andrade NJ, Cruz RS, Medeiros A, Antonio E. Bioactive peptides: synthesis, properties, and applications in the packaging and preservation of food. Compr Rev Food Sci Food Saf. 2012;11:187–204. doi: 10.1111/j.1541-4337.2011.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wagner SC, Roskamp M, Colfen H, Bottcher C, Schlecht S, Koksch B. Switchable electrostatic interactions between gold nanoparticles and coiled coil peptides direct colloid assembly. Org Biomol Chem. 2009;7:46–51. doi: 10.1039/b813429d. [DOI] [PubMed] [Google Scholar]
- 120.Cho N-H, Cheong T-C, Min JH, Wu JH, Lee SJ, Kim D, Yang J-S, Kim S, Kim YK, Seong S-Y. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6:675–682. doi: 10.1038/nnano.2011.149. [DOI] [PubMed] [Google Scholar]
- 121.Pinaud F, King D, Moore H-P, Weiss S. Bioactivation and cell targeting of semiconductor CdSe/ZnS nanocrystals with phytochelatin-related peptides. J Am Chem Soc. 2004;126:6115–6123. doi: 10.1021/ja031691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shi L, De Paoli V, Rosenzweig N, Rosenzweig Z. Synthesis and application of quantum dots FRET-based protease sensors. J Am Chem Soc. 2006;128:10378–10379. doi: 10.1021/ja063509o. [DOI] [PubMed] [Google Scholar]
- 123.Rhyner MN, Smith AM, Gao X, Mao H, Yang L, Nie S. Quantum dots and multifunctional nanoparticles: new contrast agents for tumor imaging. Nanomedicine (London) 2006;1:209–217. doi: 10.2217/17435889.1.2.209. [DOI] [PubMed] [Google Scholar]
- 124.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6:865–878. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 125.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 126.Giri S, Trewyn BG, Lin VS. Mesoporous silica nanomaterials based biotechnological and biomedical delivery systems. Nanomedicine (London) 2007;2:99–111. doi: 10.2217/17435889.2.1.99. [DOI] [PubMed] [Google Scholar]
- 127.Krenning E, Breeman W, Kooij P, Lameris J, Bakker W, Koper J, Ausema L, Reubi J, Lamberts S. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;333:242–244. doi: 10.1016/s0140-6736(89)91258-0. [DOI] [PubMed] [Google Scholar]
- 128.Van de Wiele C, Dumont F, Dierckx RA, Peers SH, Thornback JR, Slegers G, Thierens H. Biodistribution and dosimetry of 99mTc-RP527, a gastrin-releasing peptide (GRP) agonist for the visualization of GRP receptor-expressing malignancies. J Nucl Med. 2001;42:1722–1727. [PubMed] [Google Scholar]
- 129.Wild D, Béhé M, Wicki A, Storch D, Waser B, Gotthardt M, Keil B, Christofori G, Reubi JC, Mäcke HR. [Lys40 (Ahx-DTPA-111In) NH2] exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med. 2006;47:2025–2033. [PubMed] [Google Scholar]
- 130.Chen H, Niu G, Wu H, Chen X. Clinical application of radiolabeled RGD peptides for PET imaging of integrin αvβ3. Theranostics. 2016;6:78–92. doi: 10.7150/thno.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sivolapenko GB, Skarlos D, Pectasides D, Stathopoulou E, Milonakis A, Sirmalis G, Stuttle A, Courtenay-Luck NS, Konstantinides K, Epenetos AA. Imaging of metastatic melanoma utilising a technetium-99m labelled RGD-containing synthetic peptide. Eur J Nucl Med. 1998;25:1383–1389. doi: 10.1007/s002590050312. [DOI] [PubMed] [Google Scholar]
- 132.Haubner R, Kuhnast B, Mang C, Weber WA, Kessler H, Wester H-J, Schwaiger M. [18F] Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug Chem. 2004;15:61–69. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- 133.Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, Becker K, Goebel M, Hein R, Wester H. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005;2:e70. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, McParland B, Cohen PS, Hui A-M, Palmieri C. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 135.Mittra ES, Goris ML, Iagaru AH, Kardan A, Burton L, Berganos R, Chang E, Liu S, Shen B, Chin FT. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology. 2011;260:182–191. doi: 10.1148/radiol.11101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doss M, Kolb HC, Zhang JJ, Bélanger M-J, Stubbs JB, Stabin MG, Hostetler ED, Alpaugh RK, von Mehren M, Walsh JC. Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J Nucl Med. 2012;53:787–795. doi: 10.2967/jnumed.111.088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wan W, Guo N, Pan D, Yu C, Weng Y, Luo S, Ding H, Xu Y, Wang L, Lang L. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–698. doi: 10.2967/jnumed.112.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mi B, Yu C, Pan D, Yang M, Wan W, Niu G, Chen X. Pilot prospective evaluation of 18F-alfatide II for detection of skeletal metastases. Theranostics. 2015;5:1115–1121. doi: 10.7150/thno.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Asnacios A, Courbon F, Rochaix P, Bauvin E, Cances-Lauwers V, Susini C, Schulz S, Boneu A, Guimbaud R, Buscail L. Indium-111-pentetreotide scintigraphy and somatostatin receptor subtype 2 expression: new prognostic factors for malignant well-differentiated endocrine tumors. Clin Oncol. 2008;26:963–970. doi: 10.1200/JCO.2007.12.7431. [DOI] [PubMed] [Google Scholar]
- 140.Blum J, Handmaker H, Lister-James J, Rinne N. A multicenter trial with a somato-statin analog 99mTc depreotide in the evaluation of solitary pulmonary nodules. Chest. 2000;117:1232–1238. doi: 10.1378/chest.117.5.1232. [DOI] [PubMed] [Google Scholar]
- 141.Maina T, Nock B, Nikolopoulou A, Sotiriou P, Loudos G, Maintas D, Cordopatis P, Chiotellis E. [99mTc] Demotate, a new 99mTc-based [Tyr3] octreotate analogue for the detection of somatostatin receptor-positive tumours: synthesis and preclinical results. Eur J Nucl Med Mol Imaging. 2002;29:742–753. doi: 10.1007/s00259-002-0782-9. [DOI] [PubMed] [Google Scholar]
- 142.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 143.Henze M, Schuhmacher J, Hipp P, Kowalski J, Becker DW, Doll J, Mäcke HR, Hofmann M, Debus J, Haberkorn U. PET imaging of somatostatin receptors using [68GA] DOTA-D-Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med. 2001;42:1053–1056. [PubMed] [Google Scholar]
- 144.Wild D, Mäcke HR, Waser B, Reubi JC, Ginj M, Rasch H, Müller-Brand J, Hofmann M. 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging. 2005;32:724–724. doi: 10.1007/s00259-004-1697-4. [DOI] [PubMed] [Google Scholar]
- 145.Wester H, Schottelius M, Scheidhauer K, Meisetschläger G, Herz M, Rau F, Reubi J, Schwaiger M. PET imaging of somatostatin receptors: design, synthesis and preclinical evaluation of a novel 18F-labelled, carbohydrated analogue of octreotide. Eur J Nucl Med Mol Imaging. 2003;30:117–122. doi: 10.1007/s00259-002-1012-1. [DOI] [PubMed] [Google Scholar]
- 146.Win Z, Rahman L, Murrell J, Todd J, Al-Nahhas A. The possible role of 68Ga-DOTATATE PET in malignant abdominal paraganglioma. Eur J Nucl Med Mol Imaging. 2006;33:506–506. doi: 10.1007/s00259-005-0035-9. [DOI] [PubMed] [Google Scholar]
- 147.Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AMM, Santini D, Ceccarelli C, Nori F, Franchi R, Corinaldesi R. Standardized uptake values of 68Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. 2010;51:353–359. doi: 10.2967/jnumed.109.066662. [DOI] [PubMed] [Google Scholar]
- 148.Mojtahedi A, Thamake S, Tworowska I, Ranganathan D, Delpassand ES. The value of (68) Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging. 2014;4:426–434. [PMC free article] [PubMed] [Google Scholar]
- 149.Antunes P, Ginj M, Zhang H, Waser B, Baum R, Reubi J-C, Maecke H. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–993. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 150.Schmid-Tannwald C, Schmid-Tannwald CM, Morelli JN, Neumann R, Haug AR, Jansen N, Nikolaou K, Schramm N, Reiser MF, Rist C. Comparison of abdominal MRI with diffusion-weighted imaging to 68Ga-DOTATATE PET/CT in detection of neuroendocrine tumors of the pancreas. Eur J Nucl Med Mol Imaging. 2013;40:897–907. doi: 10.1007/s00259-013-2371-5. [DOI] [PubMed] [Google Scholar]
- 151.Goel R, Shukla J, Bansal D, Sodhi K, Bhattacharya A, Marwaha RK, Mittal BR. 68Ga-DOTATATE positron emission tomography/computed tomography scan in the detection of bone metastases in pediatric neuroendocrine tumors. Indian J Nucl Med. 2014;29:13–17. doi: 10.4103/0972-3919.125762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kayani I, Conry BG, Groves AM, Win T, Dickson J, Caplin M, Bomanji JB. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J Nucl Med. 2009;50:1927–1932. doi: 10.2967/jnumed.109.066639. [DOI] [PubMed] [Google Scholar]
- 153.Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, Dickson J, Caplin M, Ell PJ. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–2455. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- 154.Von Falck C, Boerner A, Galanski M, Knapp W. Neuroendocrine tumour of the mediastinum: fusion of 18F-FDG and 68Ga-DOTATOC PET/CT datasets demonstrates different degrees of differentiation. Eur J Nucl Med Mol Imaging. 2007;34:812–812. doi: 10.1007/s00259-006-0350-9. [DOI] [PubMed] [Google Scholar]
- 155.Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gönen M, Kalaigian H, Schöder H. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med. 2014;6:260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]