Abstract
BACKGROUND: The role of stereotactic radiosurgery (SRS) for recurrent glioblastoma and the radionecrosis risk in this setting remain unclear.
OBJECTIVE: To perform a large retrospective study to help inform proper indications, efficacy, and anticipated complications of SRS for recurrent glioblastoma.
METHODS: We retrospectively analyzed patients who underwent Gamma Knife SRS between 1991 and 2013. We used the partitioning deletion/substitution/addition algorithm to identify potential predictor covariate cut points and Kaplan-Meier and proportional hazards modeling to identify factors associated with post-SRS and postdiagnosis survival.
RESULTS: One hundred seventy-four glioblastoma patients (median age, 54.1 years) underwent SRS a median of 8.7 months after initial diagnosis. Seventy-five percent had 1 treatment target (range, 1-6), and median target volume and prescriptions were 7.0 cm3 (range, 0.3-39.0 cm3) and 16.0 Gy (range, 10-22 Gy), respectively. Median overall survival was 10.6 months after SRS and 19.1 months after diagnosis. Kaplan-Meier and multivariable modeling revealed that younger age at SRS, higher prescription dose, and longer interval between original surgery and SRS are significantly associated with improved post-SRS survival. Forty-six patients (26%) underwent salvage craniotomy after SRS, with 63% showing radionecrosis or mixed tumor/necrosis vs 35% showing purely recurrent tumor. The necrosis/mixed group had lower mean isodose prescription compared with the tumor group (16.2 vs 17.8 Gy; P = .003) and larger mean treatment volume (10.0 vs 5.4 cm3; P = .009).
CONCLUSION: Gamma Knife may benefit a subset of focally recurrent patients, particularly those who are younger with smaller recurrences. Higher prescriptions are associated with improved post-SRS survival and do not seem to have greater risk of symptomatic treatment effect.
Keywords: Gamma knife, Glioblastoma, Radionecrosis, Recurrent, Stereotactic radiosurgery, SRS
ABBREVIATIONS
- EBRT
external beam radiotherapy
- GK
Gamma Knife
- OS
overall survival
- partDSA
partitioning deletion/ substitution/addition
- SRS
stereotactic radiosurgery
Surgery, temozolomide, and fractionated radiotherapy are standards of care for newly diagnosed glioblastoma;1 how-ever, the approach for recurrence is considerably more heterogeneous.2 Options include repeat surgery,3-9 chemotherapy (eg, temozolo-mide,10-18 irinotecan,19 or nitrosoureas20), bevacizumab,21,22 experimental agents, or reirradiation.23 Numerous reirradiation strategies exist, including conventional fractionated external beam radiotherapy (EBRT),24,25 fractionated stereotactic radiotherapy,23,26 hypofractionated stereotactic radiotherapy,27 high-dose-rate brachytherapy,28-30 low-dose-rate brachytherapy,31-33 or single-fraction stereotactic radiosurgery (SRS). Despite numerous options, median survival after recurrence remains poor at 9 to 20 months.34
Single-fraction SRS has been considered for recurrent glioblastoma for >2 decades. Because histopathological analysis reveals diffuse tumor spread, critics argue that spatially targeted treatments like SRS are suboptimal. However, most patients progress within a few centimeters of the primary tumor site,4,35-39 so SRS might be sufficient for focal recurrences. One SRS modality, Gamma Knife (GK; Elekta, Stockholm, Sweden), has seen diverse use for glioblastoma, including as monotherapy, in combination with chemotherapy (eg, Park et al40), for newly diagnosed disease (eg, Nwokedi et al41), and for recurrence.
The literature supporting GK for glioblastoma is complex and evolving.42 Retrospective series report conflicting results regarding the efficacy of adding SRS to conventional radiation, but randomized trials have shown no clear benefit to dose escalation or boosts.41,43 Specifically, the only randomized phase III glioblastoma trial investigating GK (RTOG 93-05) found no benefit of an initial SRS boost before standard fractionated radiotherapy plus carmustine after initial resection vs conventional radiotherapy and carmustine alone.44,45 However, RTOG 93-05 patients were not treated with temozolomide, affecting contemporary applicability.
Use of single-fraction SRS for select glioblastoma recurrences seems promising; however, there is no consensus recommendation. We sought to contribute by analyzing our treatment experience over 20 years. Our main objectives were to summarize outcomes, to clarify target patients, and to better understand radionecrosis risk.
METHODS
We retrospectively reviewed patients who underwent GK for recurrent glioblastoma at our center between 1991 and 2013. Our center used the Leksell Gamma Knife 4C machine (Elekta) between 1991 and 2007 and the Leksell Gamma Knife Perfexion model after 2007. This study was approved by our Committee for Human Research.
We included patients with pathologically confirmed glioblastoma/gliosarcoma who received comprehensive or radiosurgical care at our institution. Our center had no standardized GK patient selection criteria except for some trial participants.46 SRS was recommended by a multidisciplinary neuro-oncology tumor board. For patients with multiple targets treated in the same GK session, we focused on parameters from the largest volumetric lesion. We focused on patients' first GK session.
To assess radionecrosis risk, we identified patients who underwent post-GK salvage craniotomy. These patients were divided into 2 cohorts depending on whether post-GK pathology revealed necrosis or tumor recurrence.
Radiosurgical Technique
Patients are fitted with a stereotactic head frame before 1.5-T magnetic resonance imaging (MRI) acquisition with intravenous gadolinium contrast. Treatment plans are prepared jointly by a neurosurgeon and a radiation oncologist with target volumes based largely on the T1 postcontrast sequence. Fluid-attenuated inversion-recovery sequences are used to demarcate the full extent of tumor cellularity when T1 imaging is insufficient. No additional margin is added. Targets were prescribed marginal doses of 10 to 22 Gy on the basis of institutional protocol to minimize potential adverse radiation effect, adopted from brain metastasis treatment, with decreasing dose used as an inverse square function of increasing target volume. Dose planning was performed with multiple isocenters to maximize dose-gradient index.
Statistics
Primary end points were overall survival (OS) after initial glioblastoma diagnosis and OS from date of the first GK procedure. Given the radiographic challenge of distinguishing progression from radionecrosis, particularly in early post-GK serial imaging, we did not specifically analyze time to disease progression to minimize interpretation bias. To calculate OS, patients were censored at the last date of follow-up if they were alive or could not be definitively identified in the Death Index. Patients were included regardless of follow-up because a Social Security Death Index was used. Median OS was estimated with Kaplan-Meier methods.
We used the log-rank test and univariate proportional hazards modeling to assess the impact of various covariates on OS, including demographics (sex, age), pre-GK treatment specifics (surgical resection extent, multiple craniotomies, surgery-to-GK interval, upfront chemotherapy), and GK treatment parameters (number of targets, treatment volume, marginal dose, concurrent/adjuvant chemotherapy). The partitioning deletion/substitution/addition (partDSA) algorithm for creating survival risk groups47 was used for continuous covariates to identify specific categorical cut points for OS hazard analysis to help clarify the target patient profile for this intervention. These cut points were assessed for association with OS with the use of log-rank and proportional hazards. All proportional hazard models were assessed for validity according to weighted residual assumptions.48 All covariates were initially assessed in our multivariable model and then removed via a stepwise variable selection process if they failed to achieve a value of P < .1.
Clinical and treatment differences between the salvage craniotomy cohorts (eg, radionecrosis vs tumor) were evaluated with the Student 2-tailed t test for continuous variables or the χ2 for categorical variables. The threshold of significance was P < .05. We used GraphPad Prism (GraphPad, La Jolla, CA), SPSS (IBM, Armonk, NY), and R (http://www.R-project.org).
RESULTS
Patient Demographics and Pre-GK Treatment
We analyzed 174 patients (59% male; Table 1). Histopathologically, 162 patients (93%) had glioblastoma and 12 patients (7%) had gliosarcoma. Median age at GK was 54.1 years (women, 52.7 years; men, 54.8 years) with a range of 21.8 to 85.3 years. Extent of initial resection as determined by postoperative MRI was gross total resection for 37%, near-total resection for 6%, subtotal resection for 40%, and biopsy for 11%. Surgical extent was unavailable for 6%. Sixty-three patients (36%) had multiple pre-GK craniotomies.
TABLE 1.
Demographic and Clinical Criteriaa
| Patients, n | 174 |
| Male, n (%) | 102 (59) |
| Histopathological diagnosis, n (%) | |
| Glioblastoma | 162 (93) |
| Gliosarcoma | 12 (7) |
| Age at GK procedure, y | |
| Median for full cohort | 54.1 |
| Range for full cohort | 21.8-85.3 |
| Median for men | 54.8 |
| Median for women | 52.7 |
| Extent of first surgical procedure, n (%) | |
| Biopsy | 19 (11) |
| Subtotal | 69 (40) |
| Near total | 10 (6) |
| Gross total | 65 (37) |
| Not available | 11 (6) |
| Prior intracranial brachytherapy, n (%) | 27 (16) |
| Upfront chemotherapy regimen, n (%) | |
| Regimen contained temozolomide | 48 (28) |
| Regimen did not contain temozolomide | 23 (13) |
| No upfront chemotherapy | 51 (29) |
| Upfront chemotherapy history not available | 52 (30) |
| GK characteristics | |
| Duration of time between initial diagnosis and GK (median), mo | 8.7 |
| Single lesion targeted with GK, n (%) | 131 (75) |
| Multiple lesions targeted with GK, n (%) | 43 (25) |
| GK total treatment volume, cm3 | |
| Median volume | 7.0 |
| Minimum volume | 0.3 |
| Maximum volume | 39 |
| GK prescription dose, Gy | |
| Median marginal prescription dose | 16 |
| Minimum marginal prescription dose | 10 |
| Maximum marginal prescription dose | 22 |
| Concurrent/adjuvant chemotherapy with GK, n (%) | |
| Received concurrent or adjuvant chemotherapy | 77 (44) |
| Did not receive concurrent/adjuvant chemo | 46 (26) |
| Adjuvant chemotherapy history not available | 51 (29) |
aGK, Gamma Knife.
One hundred seventy-one patients (98%) received EBRT before GK with a median dose of 60 Gy (range, 40-72 Gy); 1 patient had distant EBRT for optic nerve glioma; 1 patient had brachytherapy but no EBRT; and radiotherapy history was unavailable for 1 patient. Twenty-seven patients (16%) had intracranial iodine-125 brachytherapy before GK.
Upfront chemotherapy history was available for 70% of our cohort. Of these patients, 39% received temozolomide or temozolomide-containing regimens, 19% received chemotherapy with nontemozolomide regimens, and 42% received no upfront chemotherapy.
GK Treatment
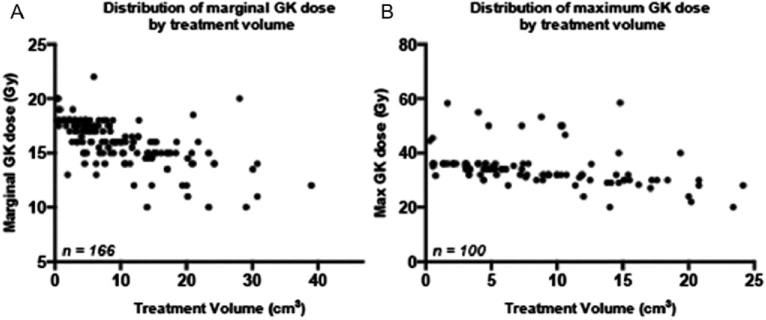
Patients received single-session GK SRS a median of 8.7 months (range, 0.4-195.8 months) after diagnosis. One hundred thirty-one patients (75%) had 1 GK target, and 43 (25%) had multiple concurrent lesions (range, 2-6). Median total GK treatment volume was 7.0 cm3 (range, 0.3-39 cm3). Median marginal prescription dose was 16 Gy (range, 10-22 Gy) for the entire treatment volume (if single target) or the largest lesion (if multiple targets). Prescriptions were available for approximately 60% of patients, and of this group, 83% were prescribed to the 50% isodose line (range, 25%-60%). Figure 1 shows the relationship of the treatment volume compared with its marginal dose (Figure 1A) and estimated maximal dose (Figure 1B).
FIGURE 1.
Distribution of A marginal Gamma Knife (GK) dose and B maximal GK dose (Dmax) vs total GK treatment volume.
We reviewed chemotherapy records for 123 patients (71% of cohort); the remainder were unavailable because they were in destroyed paper charts. Of this subgroup with available records, 77 patients (63%) received concurrent/adjuvant chemotherapy with GK. There was significant heterogeneity; common agents included temozolomide (n = 20), CCNU (n = 13), BCNU (n = 11), and thalidomide (n = 10). Three patients received bevacizumab as additional adjuvant therapy.
Post-GK Outcomes
Median follow-up (for posttreatment imaging or clinic visit) was 8.7 months (range, 0-120.1 months) after GK and 19.1 months after diagnosis (range, 2.3-206.2 months). Nine patients (5%) were lost to follow-up immediately after GK, but death dates were obtained. Death dates were available for 94% of patients at the time of analysis. Estimated median OS was 10.6 months (range, 1.4-157.6 months) after GK and 21.1 months (range, 3.8-206.2 months) after diagnosis. For gliosarcoma patients, estimated median OS was 10.8 months after GK and 22.6 months after diagnosis.
PartDSA was used for several continuous variables to identify numerical cut points with maximal chance for significance in subsequent hazard analysis to create high- and low-risk subgroups. Table 2 shows partDSA cut points for both outcomes. For 2 variables (surgery-to-GK interval and GK treatment volume), we identified 2 cut points.
TABLE 2.
Results of the Partitioning Deletion/Substitution/Addition Algorithm to Identify Potential Categorical Variable Risk Cut Points for Subsequent Hazard Analysisa
| Cut Points Established by partDSA Algorithm | ||||
|---|---|---|---|---|
| OS From First | OS From Initial | |||
| Continuous Covariate | Cohort Median | Cohort Range | GK Procedure | Glioblastoma Diagnosis |
| Age at first GK procedure, y | 54.1 | 21.8-85.3 | 53.7 | 45.4 |
| Duration between first surgery and first GK procedure, mo | 8.7 | 0.4-195.8 | 14.8, 20.3b | 10.3, 20.3b |
| GK treatment volume, cm3 | 7.2 | 0.3-39.0 | 5.1, 5.8b | 6.2, 11.4b |
| GK marginal dose, Gy | 16 | 10-22 | 15.5 | 17.5 |
aGK, Gamma Knife; OS, overall survival; partDSA, partitioning deletion/substitution/addition.
bPartDSA analysis identified 2 analytical cut points.
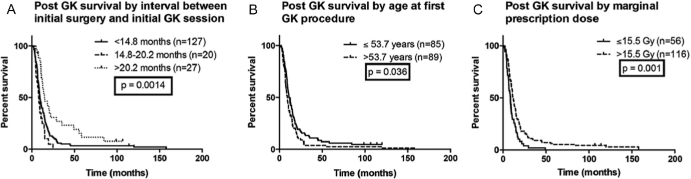
The partDSA-determined cut points and the categorical variables were then evaluated for association with post-GK OS. We evaluated the impact of the surgery-to-GK time interval (Figure 2A) and found that the longest interval (>20.2 months) had the best OS (median OS, 15.1 months). The poorest survival was a small subcohort of patients (n = 20) who underwent GK roughly 15 to 20 months after surgery (median OS, 8.3 months). The patients with the shortest interval had intermediate OS (median OS, 9.7 months), and subcohort differences were highly significant (P = .001 by log-rank test).
FIGURE 2.
Kaplan-Meier OS estimates with a median follow-up time of 8.7 months after Gamma Knife (GK) with various subgroups, including A duration between initial pathological diagnosis and first GK session, B age at first GK session, and C marginal dose of the largest treated lesion.
Patient age is significantly associated with post-GK outcomes (Figure 2B). Specifically, median OS for younger patients (age ≤53.7 years) is 11.5 months vs 9.6 months (P = .04) for older patients. Patients prescribed a higher marginal dose of >15.5 Gy (Figure 2C) had improved post-GK OS (11.5 vs 8.7 months; P = .001). Differences in other clinical and treatment variables did not significantly affect OS.
All potential covariates, including the risk groups identified by partDSA, were further analyzed in univariate proportional hazard models for post-GK OS (Table 3) and postdiagnosis OS (Table 4). Note that for postdiagnosis OS, several models did not meet our proportional hazard assumptions, and results are not reported. Again, patients with the longest surgery-to-GK interval (>20.2 months) had improved postprocedural survival (hazard ratio = 0.51; P = .003) compared with the shortest interval. Younger age was associated with significantly improved post-GK and postdiagnosis OS. Patents with increased marginal dose (>15.5 Gy) also had improved outcomes.
TABLE 3.
Univariate Cox Proportional Hazards for Overall Survival From the Time of the Gamma Knife Procedurea
| Hazard Ratio | |||
|---|---|---|---|
| Clinical Parameter | n | (95% Confidence Interval) | P |
| Male sex (vs female) | 174 | 0.84 (0.61-1.15) | .27 |
| Gliosarcoma (vs glioblastoma) | 174 | 0.85 (0.45-1.62) | .62 |
| Age at glioblastoma diagnosis | 174 | 1.02 (1.01-1.03) | .005 |
| Extent of first surgical resection (vs gross total resection) | |||
| Gross total | 65 | 1.00 | Referent |
| Near total | 10 | 0.80 (0.38-1.81) | .64 |
| Subtotal | 69 | 0.99 (0.70-1.41) | .97 |
| Biopsy | 19 | 1.42 (0.84-2.39) | .19 |
| Multiple pre-GK craniotomies | 174 | 1.23 (0.89-1.70) | .21 |
| Concurrent chemotherapy with initial fractionated radiotherapy | 122 | ||
| No chemotherapy with initial radiotherapy | 51 | 1.00 | Referent |
| Chemotherapy with initial radiotherapy | 71 | 0.77 (0.59-1.01) | .06 |
| Time from initial surgery to GK, mo | |||
| Continuous variable | 174 | 0.99 (0.98-1.00) | .07 |
| ≤14.8 | 127 | 1.00 | Referent |
| 14.8-20.2 (vs ≤14.8) | 20 | 1.66 (1.03-2.68) | .004 |
| >20.2 (vs ≤14.8) | 27 | 0.51 (0.32-0.79) | .003 |
| Age at GK procedure, y | |||
| Continuous variable | 174 | 1.02 (1.01-1.03) | .007 |
| ≤53.7 | 85 | 1.00 | Referent |
| >53.7 | 89 | 1.54 (1.13-2.11) | .007 |
| Multiple GK treatment targets (vs single lesion) | 174 | 1.23 (0.89-1.70) | .21 |
| Total treatment volume, cm3 | |||
| Continuous variable | 167 | 1.02 (1.00-1.04) | .11 |
| ≤5.10 | 64 | 1.00 | Referent |
| 5.10-5.77 (vs ≤5.10) | 6 | 0.33 (0.12-0.92) | .03 |
| >5.77 (vs ≤5.10) | 97 | 1.24 (0.90-1.72) | .19 |
| Marginal treatment prescription dose, Gy | |||
| Continuous variable | 172 | 0.89 (0.82-0.96) | .002 |
| ≤15.5 | 56 | 1.00 | Referent |
| >15.5 (vs ≤15.5) | 116 | 0.57 (0.41-0.80) | .001 |
| Concurrent or adjuvant chemotherapy | 123 | ||
| No chemotherapy after GK | 46 | 1.00 | Referent |
| Concurrent/adjuvant chemotherapy | 77 | 0.85 (0.58-1.25) | .42 |
aGK, Gamma Knife.
TABLE 4.
Univariate Cox Proportional Hazards for Overall Survival From the Time of Glioblastoma Diagnosisa
| Hazard Ratio | |||
|---|---|---|---|
| Clinical Parameter | n | (95% Confidence Interval) | P |
| Male sex (vs female) | 174 | 0.93 (0.67-1.27) | .63 |
| Gliosarcoma (vs glioblastoma) | 174 | 0.84 (0.44-1.60) | .60 |
| Age at glioblastoma diagnosis | 174 | 1.03 (1.02-1.04) | <.001 |
| Extent of first surgical resection (vs gross total resection) | |||
| Gross total | 65 | 1.00 | Referent |
| Near total | 10 | 1.18 (0.54-2.59) | .68 |
| Subtotal | 69 | 1.00 (0.70-1.41) | .98 |
| Biopsy | 19 | 1.50 (0.89-2.53) | .12 |
| Multiple pre-GK craniotomies | 174 | 0.78 (0.57-1.08) | .13 |
| Concurrent chemotherapy with initial fractionated radiotherapyb | — | — | |
| Time from initial surgery to GKb | — | — | |
| Age at GK procedure, y | |||
| Continuous variable | 174 | 1.029 (1.016-1.042) | <.001 |
| ≤45.4 | 43 | 1.00 | Referent |
| >45.4 | 131 | 2.33 (1.60-3.40) | <.001 |
| Multiple GK treatment targets (vs single lesion)b | — | — | |
| Total treatment volume, cm3 | |||
| Continuous variable | 167 | 1.02 (1.00-1.04) | .11 |
| ≤6.20 | 76 | 1.00 | Referent |
| 6.20-11.40 (vs ≤6.20) | 42 | 1.67 (1.12-2.49) | .01 |
| >11.40 (vs ≤6.20) | 49 | 1.06 (0.74-1.50) | .76 |
| Marginal treatment prescription dose, Gy | |||
| Continuous variable | 172 | 0.93 (0.87-1.00) | .04 |
| ≤17.5 | 124 | 1.00 | Referent |
| >17.5 (vs ≤17.5) | 48 | 0.65 (0.46-0.92) | .02 |
| Concurrent or adjuvant chemotherapyb | — | — |
aGK, Gamma Knife.
bCovariate failed to meet the a priori proportional hazard assumption for the model per Grambsch and Therneau,48 so results are not reported.
Age at diagnosis was linearly correlated with age at GK, so only the categorical age-at-GK partDSA cut points were included for multivariable hazards modeling. All 3 covariates that achieved univariate significance remained significant in multivariable modeling for post-GK OS (Table 5), except for the small cohort with intermediate surgery-to-GK interval. Our multivariable model for postdiagnosis OS failed to meet the proportional hazard validity assumptions, so no results are reported.
TABLE 5.
Multivariate Cox Proportional Hazards Model Results for Overall Survival After the Gamma Knife Procedurea
| Hazard Ratio | ||
|---|---|---|
| (95% Confidence | ||
| Clinical Parameter (n = 172)* | Interval) | P |
| Time from initial surgery to GK, mo | ||
| 14.8-20.3 (vs ≤14.8) | 1.55 (0.96-2.51) | .07 |
| >20.3 (vs ≤14.8) | 0.57 (0.36-0.90) | .02 |
| Age at GK, y | ||
| >53.7 (vs ≤53.7) | 1.49 (1.09-2.05) | .01 |
| Margin treatment prescription dose, Gy | ||
| >15.5 (vs ≤15.5) | 0.57 (0.41-0.80) | .001 |
aGK, Gamma Knife.
Post-GK Salvage Craniotomy
After GK, 46 patients (26.4%) underwent craniotomy at our center for recurrence or symptomatic radionecrosis. Median GK-to-salvage surgery interval was 6.6 months (range, 1.1-83.6 months). Histopathological assessment of the 46 salvage craniotomy patients revealed that 16 (35%) had purely recurrent glioblastoma, 23 (50%) had tumor plus necrosis, 6 (13%) had only necrosis, and 1 had indeterminate pathology. We created 2 subcohorts of patients: those with recurrent glioblastoma (tumor cohort, n = 16) and those found to have tumor plus radionecrosis or only radionecrosis (necrosis cohort, n = 29).
We compared multiple demographic and treatment parameters between the 2 post-GK craniotomy cohorts (Table 6). Compared with the necrosis cohort, patients in the tumor cohort had smaller mean treatment volumes (5.4 vs 10.0 cm3; P = .009) and higher mean marginal doses (17.8 vs 16.2 Gy; P = .003). There was no significant difference in OS.
TABLE 6.
Clinical Comparison Between Patients Undergoing Post–Gamma Knife Salvage Craniotomya
| Tumor | Necrosis | ||
|---|---|---|---|
| Group | Group | ||
| Clinical Criteria | (n = 16) | (n = 29) | P |
| Mean duration of time between glioblastoma diagnosis and GK, mo | 24.2 | 12.6 | .07 |
| Mean age at time of GK, y | 48.1 | 47.5 | .86 |
| Mean total GK treatment volume, cm3 | 5.4 | 10.0 | .009 |
| Mean prescription GK treatment dose, Gy | 17.8 | 16.2 | .003 |
| Mean maximum GK dose, Gy | 34.4 | 35.1 | .77 |
| Mean OS, mo | 33.2 | 30.5 | .81 |
aGK, Gamma Knife; OS, overall survival.
DISCUSSION
Role of SRS for Recurrent Glioblastoma
Management of recurrent glioblastoma remains challenging, and decision making is highly individualized. Highly conformal approaches like SRS have been explored because most patients will develop recurrence proximal to the original tumor. Recent glioblastoma practice guidelines stated that Level III data support all reirradiation modalities, including SRS, for recurrence, but specific patients likely to benefit remain undefined.49 To the best of our knowledge, this report from >20 years of institutional experience is the largest single-institution retrospective series of single-fraction SRS for recurrent glioblastoma.
We report post-GK OS of 10.6 months and postdiagnosis OS of 19.1 months. These data are consistent with outcomes from growing literature (Table 7) that tends to conclude that GK is safe and modestly effective for recurrent disease.72,73 Across these series, median post-GK OS ranges from 3.9 to 30 months;53,65 however, most report a median OS of 9 to 13 months.
TABLE 7.
Literature Review for the Use of Single-Fraction Stereotactic Radiosurgery for Recurrent Glioblastomaa
| Study Name | Recurrent Glioblastoma Patients, n | Radiation Modality | Median (Range) Margin Dose, Gy | Adjuvant Agent | Median Treatment Volume, cm3 | Median OS After SRS, mo | Factors Associated With Improved OS (Multivariate) |
|---|---|---|---|---|---|---|---|
| Mahajan et al50 | 86 | LINAC | 13 (6-20) | 10.1 | 10.2 | Younger age, smaller volume | |
| Hall et al51 | 26 | LINAC | 20b (7.5-40) | 28b | 8 | Younger age | |
| Larson et al52 | 66 | GK | 16 (5-37.5)c | 6.5b | 9.2 or 13.1d | ||
| Kondziolka et al53 | 19 | GK | 15.5 (12-25) | 6.5b | 30 | ||
| DoDoo et al54 | 27 | 17 | 9 | ||||
| Cho et al55 | 27 | LINAC | 17 (9-40)c | 30c | 7.1 | ||
| Sanghavi et al56 | 30 | LINAC | 12 | 7.2 | 8 | ||
| Park et al57 | 23 | LINAC/GK | 15 (12-20) | 9.9 | 10.3 | ||
| Larson et al46 | 14 | GK | 15 (12-17.5) | Marimastat | 17.2 | 8.7 | |
| Larson et al46 | 39 | GK | 16 (10-20) | 13.6 | 10.1 | ||
| Hsieh et al58 | 26 | GK | 12 | 21.6 | 16.7 | Karnofsky Performance Score >90; adjuvant chemotherapy | |
| Mahajan et al50 | 41 | LINAC | 4.7 | 11 | |||
| Combs et al26 | 32 | LINAC | 15 (10-20) | 10 | 10 | ||
| Kong et al59 | 65 | GK | 16 (12-50)c | 10.6c | 13 | ||
| Pouratian et al60 | 26 | GK | 6 (3-15) | 21.3 | 9.4 | No corticosteroid requirement | |
| Patel et al61 | 26 | LINAC | 18 (12-20) | 10.4 | 8.4 | Imaging response to SRS | |
| Biswas et al62 | 18 | Novalis | 15 (9-20) | 8.4 | 5.3 | ||
| Elliott et al63 | 16 | GK | 15 (12-18) | 1.35 | 12.9 | Karnofsky Performance Score >90, interval between surgery and recurrence, smaller tumor volume | |
| Marazano et al64 | 13 | LINAC | 17 (14-22) | 5.3 | 11 | ||
| Cuneo et al65 | 49 | LINAC | 15 (12.5-25) | Bevacizumab | 4.8 | 11.2 (+bevacizumab), 3.9 (−bevacizumab) | Bevacizumab, Karnofsky Performance Score >70, and age <50 y |
| Skeie et al66 | 51e | GK | 12.2 (8-20) | 12.4 (mean) | 12 | GK treatment (vs surgery) | |
| Park et al40 | 11 | GK | 16 (13-18) | Bevacizumab + irinotecan/TMZ | 13.6 | 18 | Bevacizumab |
| Koga et al67 | 15f | GK | 20 | 15 (conventional); 13 (extended field) | 10.5 (conventional); 9 (extended field) | ||
| Conti et al16 | 11 | Cyberknife | 20 | 15.1 | 7 | ||
| Conti et al16 | 12 | Cyberknife | 20 | TMZ | 13.8 | 12 | |
| Cabrera et al68 | 8 | Novalis | 18 | Bevacizumab | 14.4b | ||
| Dodoo et al54 | 35 | GK | 20 (14-22) | 4.8 | 11.3 | ||
| Martínez-Carrillo et al69 | 46 | LINAC | 18 (14-20) | 6 | 7.5 | ||
| Niranjan et al70 | 153 | GK | 15 (9-25) | 14g | 10.2g | Age >60 y, tumor volume >14 cm3 | |
| Kim et al71 | 29 | GK | 15 (9-30) | 11 | 9.2 | ||
| Kim et al71 | 28 | GK | 15 (5-20) | TMZ | 9.8 | 15.5 | |
| Imber et al (present study) | 174 | GK | 16 (10-22) | 7.0 | 10.6 | Age >53.7 y, interval between surgery and GK, marginal dose |
aGK, Gamma Knife; LINAC, linear accelerator; OS, overall survival; SRS, stereotactic radiosurgery; TMZ, temozolomide.
bIncludes anaplastic astrocytoma and glioblastoma.
cIncludes other histopathologies besides glioblastoma.
d13.1 months if tumor satisfies brachytherapy criteria; 9.2 if it did not.
eCompared GK with reoperation for recurrent disease.
fSeven patients underwent extended field SRS and 8 underwent conventional SRS.
gCohort includes mixture of patients who received adjuvant GK for residual unresectable disease and those who received radiosurgery for recurrent tumors.
Identifying SRS Candidates
We show that younger GK patients had significantly improved survival, consistent with other reports.65,70 Younger patients may have improved procedural tolerance, but they also have better glioblastoma prognosis, making the assessment of true GK treatment impact challenging. However, age independently predicted improved post-GK survival on multivariable modeling, which suggests that younger patients may be better candidates. Univariate modeling also showed that patients with higher marginal doses had improved post-GK OS. Patients who received higher GK doses likely had smaller recurrences; therefore, their improved survival is unsurprising.
However, the impact of treatment volume on OS was insignificant. We performed sensitivity testing with various size cutoffs but were unable to identify a threshold for significantly poorer outcomes. Numerous reports50,59,63,70,74 assert that SRS may be most suitable for small, focal, or nodular glioblastoma recurrences. Specifically, a large recent study found post-SRS OS to be significantly better for patients with <14 cm3 of treatment volume.70 Despite our findings, emerging consensus is that larger treatment targets pose greater risk.
We also report a significant association between the surgery-to-GK interval and post-GK OS. Patients with the longest intervals (>20.2 months) had improved survival that was significant in univariate and multivariable modeling. These patients may have more indolent disease with slower-growing recurrences. Interestingly, there was also a small subcohort who underwent GK 15 to 20 months after initial surgery who had significantly poorer survival than patients with the shortest intervals. This seems counterintuitive because patients with shorter durations might have residual disease or more aggressive tumors. We did not identify any significant differences in demographic or treatment characteristics between this small subcohort and the other 2 subcohorts, so it is difficult to comment on the generalizability of this finding.
One strength of our large cohort is our ability to confirm previously reported observations. We feel that there is now a growing body of evidence to suggest that SRS may offer salvage outcomes similar to those of reoperation plus chemotherapy7 or more novel approaches such as stereotactic laser interstitial thermal therapy.75 Compared with laser interstitial thermal therapy, GK may offer the additional benefits of less invasiveness and potential for larger treatment volumes.
Selecting appropriate GK patients remains challenging. We advise repeat surgical resection if the patient has adequate performance status, and we feel that >80% resection can be achieved, given data that suggest that this is the OS advantage threshold.76 If the patient is nonoperative, we use MRI to assess whether the recurrence is focal or diffuse. MR spectroscopy is used only if there is conflicting opinion as to whether imaging reflects recurrence or treatment effect. Patients with diffuse disease are offered systemic chemotherapy, in a trial if possible. If patients have small recurrences (typically <5 cm3), particularly nodular/focal deep tumors, we consider GK or convection-enhanced delivery trials. Our study suggests that it may be beneficial to consider GK for younger patients with small, delayed recurrences.
Many groups conclude that chemotherapy is a valuable adjunctive to GK; however, it has rarely been significantly associated with improved OS in multivariable models. We also did not find a significant correlation, and our institution does not have a standardized approach. A small retrospective review of patients who received bevacizumab after GK reported a median OS of 18 months.40 Compared with matched historical controls who did not receive bevacizumab, the bevacizumab arm had significantly improved progression-free survival and OS. Adding bevacizumab also increased OS in a hypofractionated stereotactic regimen.77 Thus, bevacizumab may be a valuable adjuvant to SRS. In our cohort, no patients received concurrent bevacizumab, but some received it for additional salvage treatment. Another proposal to increase the efficacy of GK is extended treatment field. Koga et al67 reported that adding a 0.5- to 1-cm margin to SRS planning for small recurrent lesions was superior to conventional SRS for local control but had no impact on survival.
Risks of Post-SRS Necrosis
Another objective was to clarify postprocedural radionecrosis risk. We focused on patients whose necrosis warranted reresection because these patients either were symptomatic or had imaging concerning for recurrence. Twenty-six percent of patients had post-GK salvage craniotomy, most with some pathological evidence of necrosis. Given that most patients receive full neurosurgical care at our institution, our data suggest that the estimated rate of symptomatic necrosis requiring surgery after GK is 15% to 25%. Compared with patients with post-GK tumor recurrence, those with radionecrosis had larger treatment volumes and lower marginal doses. Our finding of larger radiation fields as a necrosis risk factor is consistent with prior observations.78 Patients with radionecrosis had a shorter mean interval between their original radiotherapy and GK.
Evaluating asymptomatic necrosis was challenging because MRI was often equivocal. One group estimates that normal brain becomes necrotic after a normalized cumulative dose of approximately 100 Gy.79 SRS is theoretically preferable to repeat EBRT because of the high conformality with limited dose to surrounding structures.80 Nevertheless, GK patients remain at risk for symptomatic treatment effect. In 1 series of patients with glioma (57% glioblastoma) receiving SRS after standard fractionated radiotherapy, radiation necrosis occurred in 24.4%.59
Adjuvant bevacizumab may reduce the development of SRS-associated radionecrosis, with 1 study reporting a 46% to 9% risk reduction.40 This mirrors the experience of Boothe et al81 treating brain metastases in which bevacizumab reduced steroid requirements and stabilized/improved clinical symptoms of SRS-associated radionecrosis. At present, the radioprotective characteristics of bevacizumab are intriguing, but more prospective evidence is needed.
Limitations
This retrospective study has limitations, including selection bias because SRS is offered to patients with higher performance status and inherent propensity for longer survival. Furthermore, because of the conformal nature of SRS, smaller recurrences were more likely to be considered. Many cohort patients had “nodular enhancement” on pre-SRS MRI, and the natural history of diffuse recurrences may be inherently worse. Except for some patients embedded in our series who were part of a protocoled trial,46 there were no strict patient selection criteria. As described, this series included a heterogeneous population, and some patients had several therapies, including systemic chemotherapy, before receiving SRS. Many patients also received additional salvage therapies after their GK. It would be incorrect to attribute the full survival benefit to GK alone. Finally, molecular/genetic biomarkers were unavailable for most patients; therefore, it is possible that some outcome variance is due to inherent tumor biology differences. We feel our cohort is generalizable because it mirrors the significant heterogeneity present in the current management of glioblastoma.
CONCLUSION
Single-fraction SRS may be appropriate for select glioblastoma recurrences. Many patients can anticipate post-GK survival of about 1 year, which is consistent with or superior to other contemporary therapeutic options. In the absence of randomized data, we assert that the modality may be best for younger patients with delayed recurrences or small, focal tumors or patients for whom surgery or clinical trials are contraindicated. Our data suggest that if a patient's recurrent tumor burden is small and the patient can tolerate a higher SRS marginal prescription dose, the patient has the best chances of improved OS. All patients must be counseled on the 15% to 25% radionecrosis risk.
Disclosures
Dr Imber was supported by UCSF-CTSI grant TL1 TR000144. Dr Molinaro was supported by National Institutes of Health grant R01 CA163687. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 2. Niyazi M, Siefert A, Schwarz SB et al. Therapeutic options for recurrent malignant glioma. Radiother Oncol. 2011;98(1):1-14. [DOI] [PubMed] [Google Scholar]
- 3. Young B, Oldfield EH, Markesbery WR et al. Reoperation for glioblastoma. J Neurosurg. 1981;55(6):917-921. [DOI] [PubMed] [Google Scholar]
- 4. Ammirati M, Galicich JH, Arbit E, Liao Y. Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery. 1987;21(5):607-614. [DOI] [PubMed] [Google Scholar]
- 5. Harsh GR, Levin VA, Gutin PH, Seager M, Silver P, Wilson CB. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21(5):615-621. [DOI] [PubMed] [Google Scholar]
- 6. Dirks P, Bernstein M, Muller PJ, Tucker WS. The value of reoperation for recurrent glioblastoma. Can J Surg. 1993;36(3):271-275. [PubMed] [Google Scholar]
- 7. Barker FG, Chang SM, Gutin PH et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42(4):709-720; discussion 720-723. [DOI] [PubMed] [Google Scholar]
- 8. Bloch O, Han SJ, Cha S et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117(6):1032-1038. [DOI] [PubMed] [Google Scholar]
- 9. Park CK, Kim JH, Nam DH et al. A practical scoring system to determine whether to proceed with surgical resection in recurrent glioblastoma. Neuro Oncol. 2013;15(8):1096-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol. 2002;4(1):39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brandes AA, Tosoni A, Cavallo G et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from Gruppo Italiano Cooperativo Di Neuro-Oncologia (GICNO). Br J Cancer. 2006;95(9): 1155-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wick A, Felsberg J, Steinbach JP et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25(22):3357-3361. [DOI] [PubMed] [Google Scholar]
- 13. Kong DS, Lee JI, Kim JH et al. Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol. 2010;12(3):289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perry JR, Bélanger K, Mason WP et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010; 28(12):2051-2057. [DOI] [PubMed] [Google Scholar]
- 15. Abacioglu U, Caglar HB, Yumuk PF, Akgun Z, Atasoy BM, Sengoz M. Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):585-593. [DOI] [PubMed] [Google Scholar]
- 16. Conti A, Pontoriero A, Arpa D et al. Efficacy and toxicity of CyberKnife reirradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir (Wien). 2012;154(2):203-209. [DOI] [PubMed] [Google Scholar]
- 17. Norden AD, Lesser GJ, Drappatz J et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013;15(7):930-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Omuro A, Chan TA, Abrey LE et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013;15 (2):242-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS. Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol. 2009;11(1):80-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolff JEA, Berrak S, Koontz Webb SE, Zhang M. Nitrosourea efficacy in high-grade glioma: a survival gain analysis summarizing 504 cohorts with 24193 patients. J Neurooncol. 2008;88(1):57-63. [DOI] [PubMed] [Google Scholar]
- 21. Kreisl TN, Kim L, Moore K et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2008;27(5):740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733-4740. [DOI] [PubMed] [Google Scholar]
- 23. Combs SE, Debus J, Schulz-Ertner D. Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer. 2007;7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bauman GS, Sneed PK, Wara WM et al. Reirradiation of primary CNS tumors. Int J Radiat Oncol Biol Phys. 1996;36(2):433-441. [DOI] [PubMed] [Google Scholar]
- 25. Veninga T, Langendijk HA, Slotman BJ et al. Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol. 2001; 59(2):127-137. [DOI] [PubMed] [Google Scholar]
- 26. Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23(34): 8863-8869. [DOI] [PubMed] [Google Scholar]
- 27. Fogh SE, Andrews DW, Glass J et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010; 28(18):3048-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sneed PK, McDermott MW, Gutin PH. Interstitial brachytherapy procedures for brain tumors. Semin Surg Oncol. 1997;13(3):157-166. [DOI] [PubMed] [Google Scholar]
- 29. Tselis N, Kolotas C, Birn G et al. CT-guided interstitial HDR brachytherapy for recurrent glioblastoma multiforme. Long-term results. Strahlenther Onkol. 2007; 183(10):563-570. [DOI] [PubMed] [Google Scholar]
- 30. Archavlis E, Tselis N, Birn G, Ulrich P, Baltas D, Zamboglou N. Survival analysis of HDR brachytherapy versus reoperation versus temozolomide alone: a retrospective cohort analysis of recurrent glioblastoma multiforme. BMJ Open. 2013;3(3): e002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halligan JB, Stelzer KJ, Rostomily RC, Spence AM, Griffin TW, Berger MS. Operation and permanent low activity 125I brachytherapy for recurrent high-grade astrocytomas. Int J Radiat Oncol Biol Phys. 1996;35(3):541-547. [DOI] [PubMed] [Google Scholar]
- 32. Patel S, Breneman JC, Warnick RE et al. Permanent iodine-125 interstitial implants for the treatment of recurrent glioblastoma multiforme. Neurosurgery. 2000;46(5):1123-1128; discussion 1128-1130. [DOI] [PubMed] [Google Scholar]
- 33. Julow J, Viola A, Bálint K, Szeifert GT. Image fusion-guided stereotactic iodine-125 interstitial irradiation of inoperable and recurrent gliomas. Prog Neurol Surg. 2007;20:303-311. [DOI] [PubMed] [Google Scholar]
- 34. Villavicencio AT, Burneikienė S, Romanelli P et al. Survival following stereotactic radiosurgery for newly diagnosed and recurrent glioblastoma multiforme: a multicenter experience. Neurosurg Rev. 2009;32(4):417-424. [DOI] [PubMed] [Google Scholar]
- 35. Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907-911. [DOI] [PubMed] [Google Scholar]
- 36. Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16(6):1405-1409. [DOI] [PubMed] [Google Scholar]
- 37. Masciopinto JE, Levin AB, Mehta MP, Rhode BS. Stereotactic radiosurgery for glioblastoma: a final report of 31 patients. J Neurosurg. 1995;82(4):530-535. [DOI] [PubMed] [Google Scholar]
- 38. Dobelbower MC, Burnett OL III, Nordal RA et al. Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide. J Med Imaging Radiat Oncol. 2011;55(1):77-81. [DOI] [PubMed] [Google Scholar]
- 39. Gebhardt BJ, Dobelbower MC, Ennis WH, Bag AK, Markert JM, Fiveash JB. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat Oncol. 2014;9(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park KJ, Kano H, Iyer A et al. Salvage Gamma Knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol. 2012;107(2):323-333. [DOI] [PubMed] [Google Scholar]
- 41. Nwokedi EC, DiBiase SJ, Jabbour S, Herman J, Amin P, Chin LS. Gamma Knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002;50(1):41-46; discussion 46-47. [DOI] [PubMed] [Google Scholar]
- 42. Elaimy AL, Mackay AR, Lamoreaux WT et al. Clinical outcomes of Gamma Knife radiosurgery in the salvage treatment of patients with recurrent high-grade glioma. World Neurosurg. 2013;80(6):872-878. [DOI] [PubMed] [Google Scholar]
- 43. Kong DS, Nam DH, Lee JI, Park K, Kim JH. Preservation of quality of life by preradiotherapy stereotactic radiosurgery for unresectable glioblastoma multiforme. J Neurosurg. 2006;(105 suppl):139-143. [DOI] [PubMed] [Google Scholar]
- 44. Souhami L, Seiferheld W, Brachman D et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60(3):853-860. [DOI] [PubMed] [Google Scholar]
- 45. Tsao MN, Mehta MP, Whelan TJ et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. Int J Radiat Oncol Biol Phys. 2005;63(1): 47-55. [DOI] [PubMed] [Google Scholar]
- 46. Larson DA, Prados M, Lamborn KR et al. Phase II study of high central dose Gamma Knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 2002;54(5):1397-1404. [DOI] [PubMed] [Google Scholar]
- 47. Lostritto K, Strawderman RL, Molinaro AM. A partitioning deletion/ substitution/addition algorithm for creating survival risk groups. Biometrics. 2012;68(4):1146-1156. [DOI] [PubMed] [Google Scholar]
- 48. Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515-526. [Google Scholar]
- 49. Ryu S, Buatti JM, Morris A, Kalkanis SN, Ryken TC, Olson JJ. The role of radiotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):489-499. [DOI] [PubMed] [Google Scholar]
- 50. Mahajan A, McCutcheon IE, Suki D et al. Case-control study of stereotactic radiosurgery for recurrent glioblastoma multiforme. J Neurosurg. 2005;103(2): 210-217. [DOI] [PubMed] [Google Scholar]
- 51. Hall WA, Djalilian HR, Sperduto PW et al. Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol. 1995;13(7):1642-1648. [DOI] [PubMed] [Google Scholar]
- 52. Larson DA, Gutin PH, McDermott M et al. Gamma Knife for glioma: selection factors and survival. Int J Radiat Oncol Biol Phys. 1996;36(5):1045-1053. [DOI] [PubMed] [Google Scholar]
- 53. Kondziolka D, Flickinger JC, Bissonette DJ, Bozik M, Lunsford LD. Survival benefit of stereotactic radiosurgery for patients with malignant glial neoplasms. Neurosurgery. 1997;41(4):776-783; discussion 783-785. [DOI] [PubMed] [Google Scholar]
- 54. Dodoo E, Huffmann B, Peredo I et al. Increased survival using delayed Gamma Knife radiosurgery for recurrent high-grade glioma: a feasibility study. World Neurosurg. 2014;82(5):e623-e632. [DOI] [PubMed] [Google Scholar]
- 55. Cho KH, Hall WA, Gerbi BJ, Higgins PD, McGuire WA, Clark HB. Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999;45(5):1133-1141. [DOI] [PubMed] [Google Scholar]
- 56. Sanghavi S, Skrupy R, Badic B, Robins I, Tome W, Mehta M. Recurrent malignant gliomas treated with radiosurgery. J Radiosurg. 1999;2(3):119-125. [Google Scholar]
- 57. Park J, Suh J, Barnett G et al. Survival after stereotactic radiosurgery for recurrent glioblastoma multiforme. J Radiosurg. 2000;3(3):169-175. [Google Scholar]
- 58. Hsieh PC, Chandler JP, Bhangoo S et al. Adjuvant Gamma Knife stereotactic radiosurgery at the time of tumor progression potentially improves survival for patients with glioblastoma multiforme. Neurosurgery. 2005;57(4):684-692; discussion 684-692. [PubMed] [Google Scholar]
- 59. Kong DS, Lee JI, Park K, Kim JH, Lim DH, Nam DH. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer. 2008; 112(9):2046-2051. [DOI] [PubMed] [Google Scholar]
- 60. Pouratian N, Crowley RW, Sherman JH, Jagannathan J, Sheehan JP. Gamma Knife radiosurgery after radiation therapy as an adjunctive treatment for glioblastoma. J Neurooncol. 2009;94(3):409-418. [DOI] [PubMed] [Google Scholar]
- 61. Patel M, Siddiqui F, Jin JY et al. Salvage reirradiation for recurrent glioblastoma with radiosurgery: radiographic response and improved survival. J Neurooncol. 2009;92(2):185-191. [DOI] [PubMed] [Google Scholar]
- 62. Biswas T, Okunieff P, Schell MC et al. Stereotactic radiosurgery for glioblastoma: retrospective analysis. Radiat Oncol. 2009;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Elliott RE, Parker EC, Rush SC et al. Efficacy of Gamma Knife radiosurgery for small-volume recurrent malignant gliomas after initial radical resection. World Neurosurg. 2011;76(1-2):128-140; discussion 61-62. [DOI] [PubMed] [Google Scholar]
- 64. Maranzano E, Anselmo P, Casale M et al. Treatment of recurrent glioblastoma with stereotactic radiotherapy: long-term results of a mono-institutional trial. Tumori. 2011;97(1):56-61. [DOI] [PubMed] [Google Scholar]
- 65. Cuneo KC, Vredenburgh JJ, Sampson JH et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82(5):2018-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Skeie BS, Enger PØ, Brøgger J et al. γ Knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012;78(6):658-669. [DOI] [PubMed] [Google Scholar]
- 67. Koga T, Maruyama K, Tanaka M et al. Extended field stereotactic radiosurgery for recurrent glioblastoma. Cancer. 2012;118(17):4193-4200. [DOI] [PubMed] [Google Scholar]
- 68. Cabrera AR, Cuneo KC, Desjardins A et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol Biol Phys. 2013;86(5):873-879. [DOI] [PubMed] [Google Scholar]
- 69. Martínez-Carrillo M, Tovar-Martín I, Zurita-Herrera M et al. Salvage radio-surgery for selected patients with recurrent malignant gliomas. Biomed Res Int. 2014;2014:657953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Niranjan A, Kano H, Iyer A, Kondziolka D, Flickinger JC, Lunsford LD. Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era. J Neurosurg. 2015;122(4):757-765. [DOI] [PubMed] [Google Scholar]
- 71. Kim HR, Kim KH, Kong DS et al. Outcome of salvage treatment for recurrent glioblastoma. J Clin Neurosci. 2015;22(3):468-473. [DOI] [PubMed] [Google Scholar]
- 72. Roberge D, Souhami L. Stereotactic radiosurgery in the management of intracranial gliomas. Technol Cancer Res Treat. 2003;2(2):117-125. [DOI] [PubMed] [Google Scholar]
- 73. Crowley RW, Pouratian N, Sheehan JP. Gamma Knife surgery for glioblastoma multiforme. Neurosurg Focus. 2006;20(4):E17. [DOI] [PubMed] [Google Scholar]
- 74. Shrieve DC, Alexander E, Wen PY et al. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36(2):275-282; discussion 282-284. [DOI] [PubMed] [Google Scholar]
- 75. Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37(6):E1. [DOI] [PubMed] [Google Scholar]
- 76. Oppenlander ME, Wolf AB, Snyder LA et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014; 120(4):846-853. [DOI] [PubMed] [Google Scholar]
- 77. Gutin PH, Iwamoto FM, Beal K et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75(1):156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nedzi LA, Kooy H, Alexander E, Gelman RS, Loeffler JS. Variables associated with the development of complications from radiosurgery of intracranial tumors. Int J Radiat Oncol Biol Phys. 1991;21(3):591-599. [DOI] [PubMed] [Google Scholar]
- 79. Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys. 2008;70(5):1350-1360. [DOI] [PubMed] [Google Scholar]
- 80. Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10): 1725-1731. [DOI] [PubMed] [Google Scholar]
- 81. Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15(9):1257-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]