Abstract
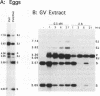
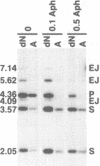
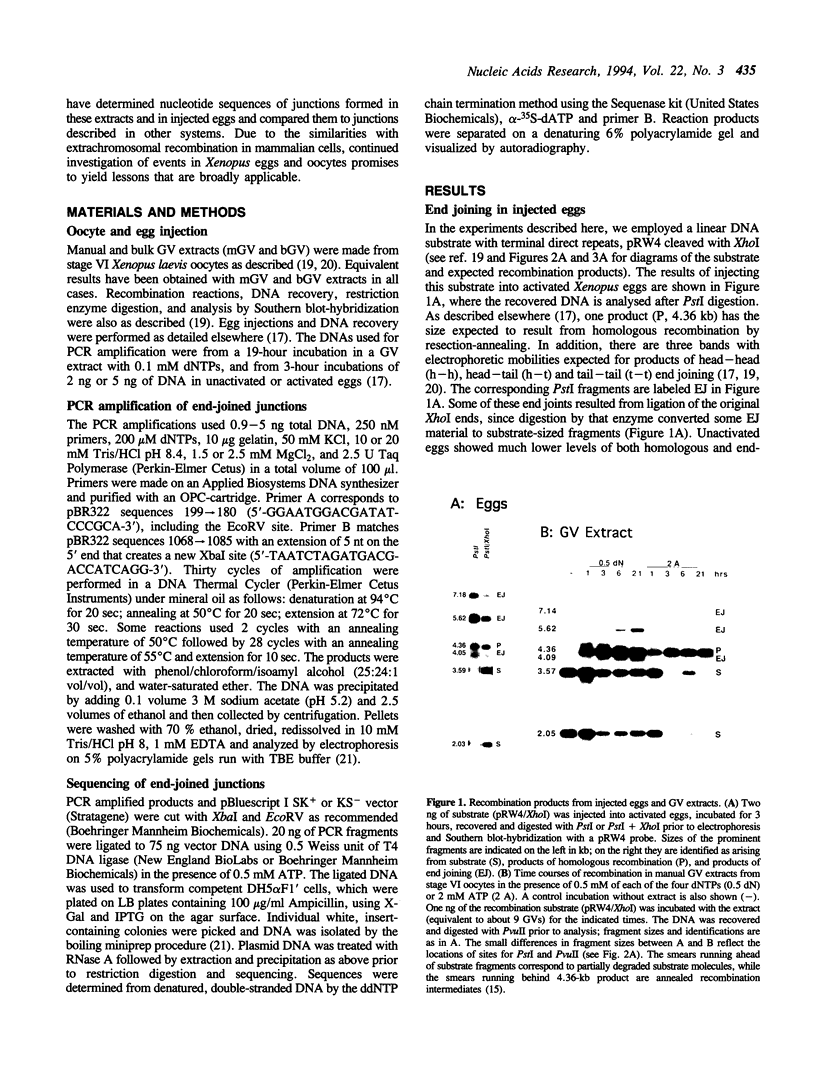
When linear DNAs are injected into Xenopus laevis eggs, they are converted into several different kinds of recombination products. Some molecules undergo homologous recombination by a resection-annealing mechanism; some ends are precisely ligated; and some ends are joined by illegitimate means. The homologous and illegitimate products are also generated in nuclear extracts from stage VI Xenopus oocytes. In order to gain insight into the mechanism(s) of illegitimate end joining, we amplified, cloned and sequenced a number of junctions from eggs and from oocyte extracts. The egg junctions fell into three categories: some with no homology at the join point that may have been produced by blunt-end ligation; some based on small, but significant homologies (5-10 bp); and some with matches of only 1 or 2 nucleotides at the joint. Junctions made in oocyte extracts were largely of the latter type. In the extracts, formation of illegitimate joints required the addition of all four deoxyribonucleoside triphosphates and was inhibited by aphidicolin. This indicates that this process involves DNA synthesis, and mechanisms incorporating this feature are considered. The spectrum of recombination products formed in Xenopus eggs is very reminiscent of those produced from DNA introduced into mammalian cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Carroll D., Wright S. H., Wolff R. K., Grzesiuk E., Maryon E. B. Efficient homologous recombination of linear DNA substrates after injection into Xenopus laevis oocytes. Mol Cell Biol. 1986 Jun;6(6):2053–2061. doi: 10.1128/mcb.6.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedecke W., Vielmetter W., Pfeiffer P. Activation of a system for the joining of nonhomologous DNA ends during Xenopus egg maturation. Mol Cell Biol. 1992 Feb;12(2):811–816. doi: 10.1128/mcb.12.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiuk E., Carroll D. Recombination of DNAs in Xenopus oocytes based on short homologous overlaps. Nucleic Acids Res. 1987 Feb 11;15(3):971–985. doi: 10.1093/nar/15.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T. M., Saxena J. K., Kumar A., Wilson S. H., Ackerman E. J. DNA polymerase beta and DNA synthesis in Xenopus oocytes and in a nuclear extract. Science. 1992 Oct 16;258(5081):475–478. doi: 10.1126/science.1411545. [DOI] [PubMed] [Google Scholar]
- Jeong-Yu S. J., Carroll D. Test of the double-strand-break repair model of recombination in Xenopus laevis oocytes. Mol Cell Biol. 1992 Jan;12(1):112–119. doi: 10.1128/mcb.12.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong-Yu S., Carroll D. Effect of terminal nonhomologies on homologous recombination in Xenopus laevis oocytes. Mol Cell Biol. 1992 Dec;12(12):5426–5437. doi: 10.1128/mcb.12.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. S., Valcarcel E. R., Rufer J. T., Phillips J. W., Morgan W. F. Noncomplementary DNA double-strand-break rejoining in bacterial and human cells. Nucleic Acids Res. 1993 Mar 11;21(5):1055–1059. doi: 10.1093/nar/21.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman C. W., Carroll D. Homologous recombination catalyzed by a nuclear extract from Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10840–10844. doi: 10.1073/pnas.88.23.10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman C. W., Carroll D. Isolation of large quantities of functional, cytoplasm-free Xenopus laevis oocyte nuclei. Anal Biochem. 1993 Jun;211(2):311–319. doi: 10.1006/abio.1993.1275. [DOI] [PubMed] [Google Scholar]
- Lehman C. W., Clemens M., Worthylake D. K., Trautman J. K., Carroll D. Homologous and illegitimate recombination in developing Xenopus oocytes and eggs. Mol Cell Biol. 1993 Nov;13(11):6897–6906. doi: 10.1128/mcb.13.11.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Maryon E., Carroll D. Characterization of recombination intermediates from DNA injected into Xenopus laevis oocytes: evidence for a nonconservative mechanism of homologous recombination. Mol Cell Biol. 1991 Jun;11(6):3278–3287. doi: 10.1128/mcb.11.6.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryon E., Carroll D. Degradation of linear DNA by a strand-specific exonuclease activity in Xenopus laevis oocytes. Mol Cell Biol. 1989 Nov;9(11):4862–4871. doi: 10.1128/mcb.9.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryon E., Carroll D. Involvement of single-stranded tails in homologous recombination of DNA injected into Xenopus laevis oocyte nuclei. Mol Cell Biol. 1991 Jun;11(6):3268–3277. doi: 10.1128/mcb.11.6.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P., Vielmetter W. Joining of nonhomologous DNA double strand breaks in vitro. Nucleic Acids Res. 1988 Feb 11;16(3):907–924. doi: 10.1093/nar/16.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont-Kingdon G., Dawson R. J., Carroll D. Intermediates in extrachromosomal homologous recombination in Xenopus laevis oocytes: characterization by electron microscopy. EMBO J. 1993 Jan;12(1):23–34. doi: 10.1002/j.1460-2075.1993.tb05628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker J., Chalk J., Ganesh A., North P. A mechanism for deletion formation in DNA by human cell extracts: the involvement of short sequence repeats. Nucleic Acids Res. 1992 Dec 11;20(23):6183–6188. doi: 10.1093/nar/20.23.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thode S., Schäfer A., Pfeiffer P., Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990 Mar 23;60(6):921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]
- Wang T. S. Eukaryotic DNA polymerases. Annu Rev Biochem. 1991;60:513–552. doi: 10.1146/annurev.bi.60.070191.002501. [DOI] [PubMed] [Google Scholar]
- Winegar R. A., Lutze L. H., Rufer J. T., Morgan W. F. Spectrum of mutations produced by specific types of restriction enzyme-induced double-strand breaks. Mutagenesis. 1992 Nov;7(6):439–445. doi: 10.1093/mutage/7.6.439. [DOI] [PubMed] [Google Scholar]