Abstract
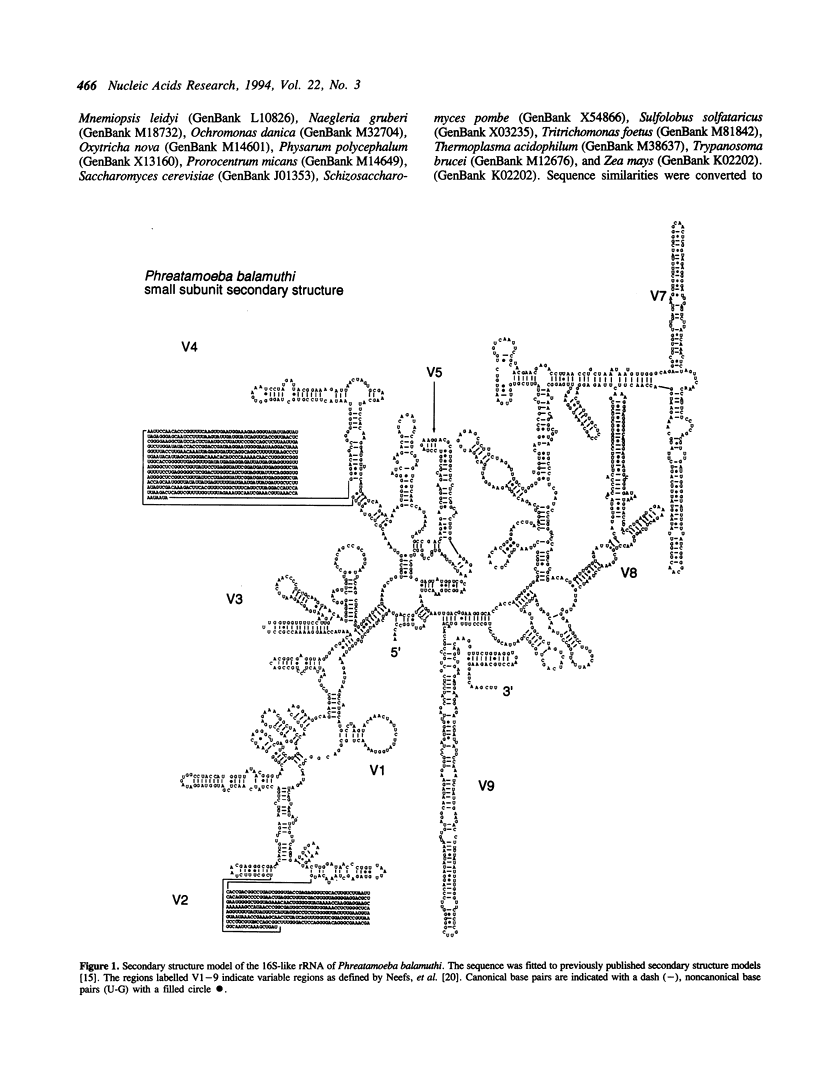
The small subunit ribosomal RNA (rRNA) of the anaerobic amoeba Phreatamoeba balamuthi is the longest 16S-like rRNA sequenced to date. Secondary structure analysis suggests that the additional sequence is incorporated in canonical eukaryotic expansion regions and is not due to the presence of introns. Reverse transcriptase sequencing of total RNA extracts confirmed that two uncommonly long expansion regions are present in native P. balamuthi 16S-like rRNA. Primary sequence comparison and similar secondary structure indicate a 61 base stem and loop repeat within an expansion region; a mechanism whereby the repeat may have been incorporated is presented. P. balamuthi provides further evidence that 16S-like rRNA length does not correlate with phylogenetic position.
Full text
PDF
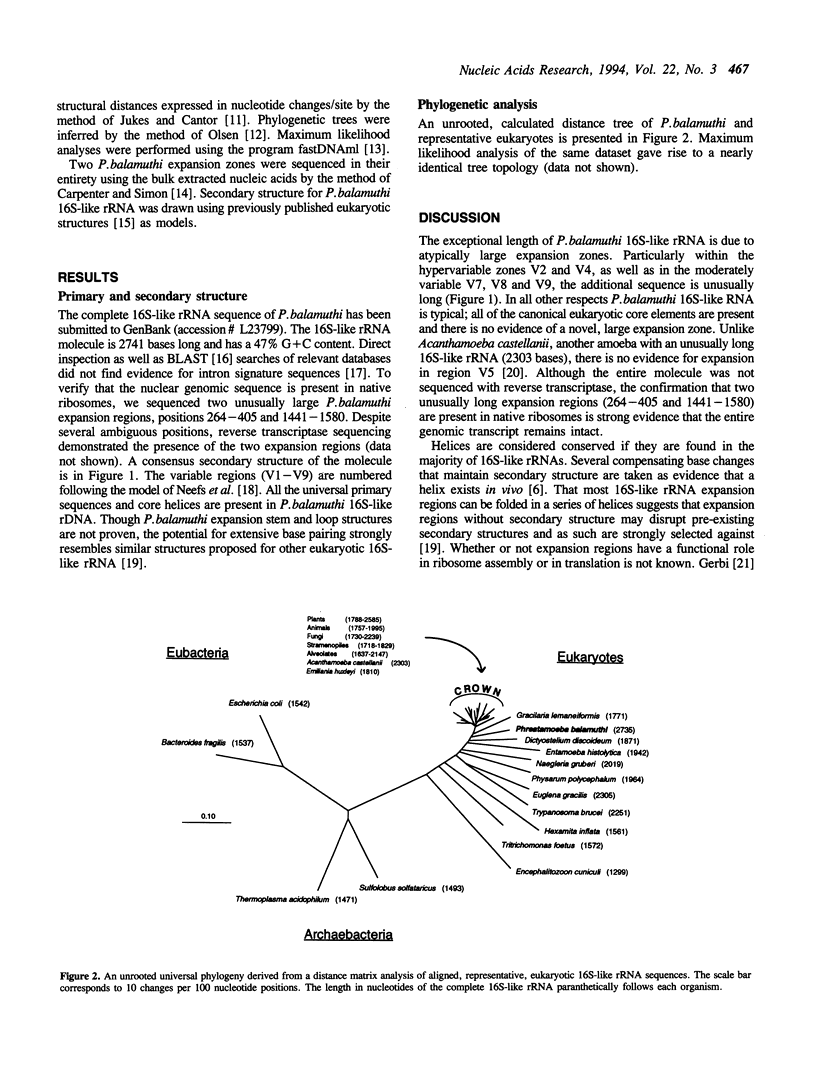


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Simon A. E. Simplified RNA sequencing using dideoxy chain termination. Biotechniques. 1990 Jan;8(1):26–27. [PubMed] [Google Scholar]
- Cech T. R. Conserved sequences and structures of group I introns: building an active site for RNA catalysis--a review. Gene. 1988 Dec 20;73(2):259–271. doi: 10.1016/0378-1119(88)90492-1. [DOI] [PubMed] [Google Scholar]
- Elwood H. J., Olsen G. J., Sogin M. L. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol Biol Evol. 1985 Sep;2(5):399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- Gerbi S. A. The evolution of eukaryotic ribosomal DNA. Biosystems. 1986;19(4):247–258. doi: 10.1016/0303-2647(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Sankoff D., Cedergren R. J. On the evolutionary descent of organisms and organelles: a global phylogeny based on a highly conserved structural core in small subunit ribosomal RNA. Nucleic Acids Res. 1984 Jul 25;12(14):5837–5852. doi: 10.1093/nar/12.14.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L. Length variation in eukaryotic rRNAs: small subunit rRNAs from the protists Acanthamoeba castellanii and Euglena gracilis. Gene. 1986;44(1):63–70. doi: 10.1016/0378-1119(86)90043-0. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L., Wollett G., Hollingdale M., de la Cruz V. F., Waters A. P., McCutchan T. F. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987 Nov 13;238(4829):933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- Gutell R. R. Collection of small subunit (16S- and 16S-like) ribosomal RNA structures. Nucleic Acids Res. 1993 Jul 1;21(13):3051–3054. doi: 10.1093/nar/21.13.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Hancock J. M., Dover G. A. 'Compensatory slippage' in the evolution of ribosomal RNA genes. Nucleic Acids Res. 1990 Oct 25;18(20):5949–5954. doi: 10.1093/nar/18.20.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. M., Dover G. A. Molecular coevolution among cryptically simple expansion segments of eukaryotic 26S/28S rRNAs. Mol Biol Evol. 1988 Jul;5(4):377–391. doi: 10.1093/oxfordjournals.molbev.a040505. [DOI] [PubMed] [Google Scholar]
- Hinkle G., Sogin M. L. The evolution of the Vahlkampfiidae as deduced from 16S-like ribosomal RNA analysis. J Eukaryot Microbiol. 1993 Sep-Oct;40(5):599–603. doi: 10.1111/j.1550-7408.1993.tb06114.x. [DOI] [PubMed] [Google Scholar]
- Knoll A. H. The early evolution of eukaryotes: a geological perspective. Science. 1992 May 1;256(5057):622–627. doi: 10.1126/science.1585174. [DOI] [PubMed] [Google Scholar]
- Kwon O. Y., Ogino K., Ishikawa H. The longest 18S ribosomal RNA ever known. Nucleotide sequence and presumed secondary structure of the 18S rRNA of the pea aphid, Acyrthosiphon pisum. Eur J Biochem. 1991 Dec 18;202(3):827–833. doi: 10.1111/j.1432-1033.1991.tb16439.x. [DOI] [PubMed] [Google Scholar]
- Larsen N., Olsen G. J., Maidak B. L., McCaughey M. J., Overbeek R., Macke T. J., Marsh T. L., Woese C. R. The ribosomal database project. Nucleic Acids Res. 1993 Jul 1;21(13):3021–3023. doi: 10.1093/nar/21.13.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlin L., Elwood H. J., Stickel S., Sogin M. L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988 Nov 30;71(2):491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., De Rijk P., Chapelle S., De Wachter R. Compilation of small ribosomal subunit RNA structures. Nucleic Acids Res. 1993 Jul 1;21(13):3025–3049. doi: 10.1093/nar/21.13.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G. J. Phylogenetic analysis using ribosomal RNA. Methods Enzymol. 1988;164:793–812. doi: 10.1016/s0076-6879(88)64084-5. [DOI] [PubMed] [Google Scholar]
- Sogin M. L. Early evolution and the origin of eukaryotes. Curr Opin Genet Dev. 1991 Dec;1(4):457–463. doi: 10.1016/s0959-437x(05)80192-3. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Edman J. C. A self-splicing intron in the small subunit rRNA gene of Pneumocystis carinii. Nucleic Acids Res. 1989 Jul 11;17(13):5349–5359. doi: 10.1093/nar/17.13.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H. Structural diversity of eukaryotic small subunit ribosomal RNAs. Evolutionary implications. Ann N Y Acad Sci. 1987;503:125–139. doi: 10.1111/j.1749-6632.1987.tb40603.x. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Maddox J. V., Friedman S., Debrunner-Vossbrinck B. A., Woese C. R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. 1987 Mar 26-Apr 1Nature. 326(6111):411–414. doi: 10.1038/326411a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Winker S., Gutell R. R. Architecture of ribosomal RNA: constraints on the sequence of "tetra-loops". Proc Natl Acad Sci U S A. 1990 Nov;87(21):8467–8471. doi: 10.1073/pnas.87.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Keulen H., Gutell R. R., Gates M. A., Campbell S. R., Erlandsen S. L., Jarroll E. L., Kulda J., Meyer E. A. Unique phylogenetic position of Diplomonadida based on the complete small subunit ribosomal RNA sequence of Giardia ardeae, G. muris, G. duodenalis and Hexamita sp. FASEB J. 1993 Jan;7(1):223–231. doi: 10.1096/fasebj.7.1.8422968. [DOI] [PubMed] [Google Scholar]


