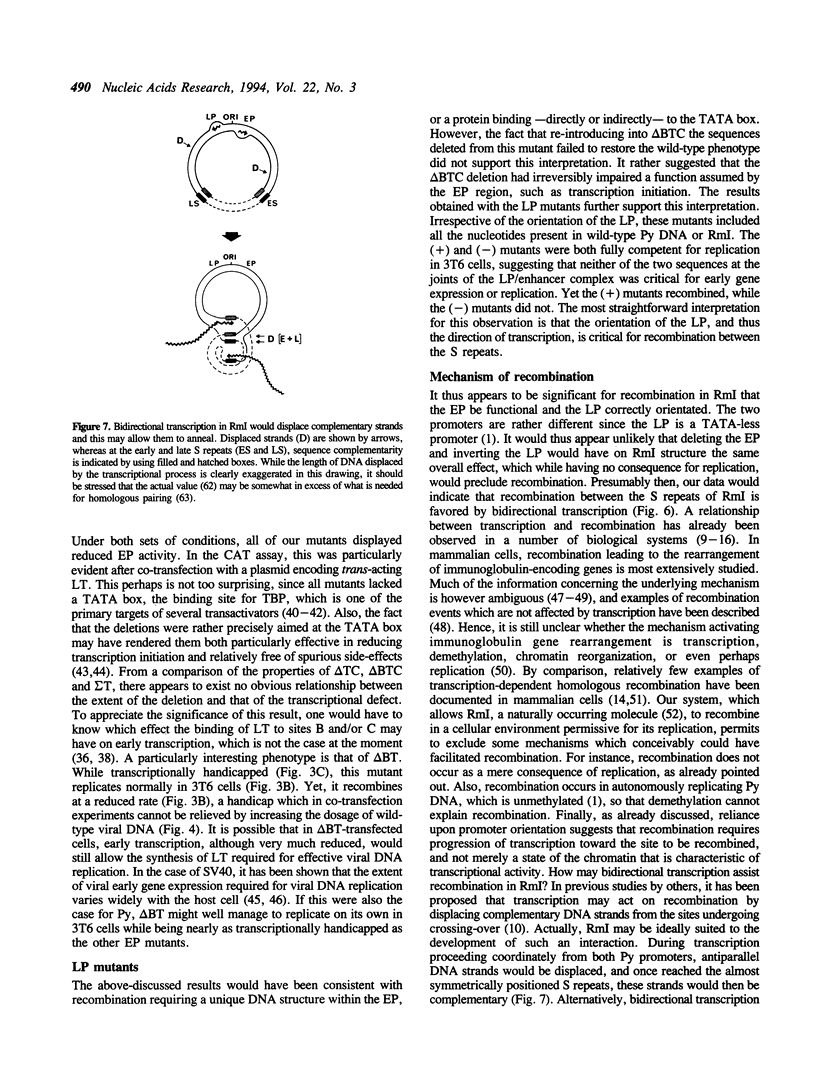
Abstract
We show here that intramolecular homologous recombination in polyomavirus (Py) DNA depends upon discrete sequence elements of the viral regulatory region which are believed to regulate transcription initiation and exert little or no cis-control over replication. Either deleting the viral early promoter (EP) or inverting the viral late promoter (LP) strongly impairs viral DNA recombination under conditions allowing viral DNA replication to proceed undisturbed. These findings suggest that bi-directional transcription proceeding from the intergenic region favors intramolecular recombination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abmayr S. M., Workman J. L., Roeder R. G. The pseudorabies immediate early protein stimulates in vitro transcription by facilitating TFIID: promoter interactions. Genes Dev. 1988 May;2(5):542–553. doi: 10.1101/gad.2.5.542. [DOI] [PubMed] [Google Scholar]
- Alessandrini A., Desiderio S. V. Coordination of immunoglobulin DJH transcription and D-to-JH rearrangement by promoter-enhancer approximation. Mol Cell Biol. 1991 Apr;11(4):2096–2107. doi: 10.1128/mcb.11.4.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendig M. M., Thomas T., Folk W. R. Regulatory mutants of polyoma virus defective in DNA replication and the synthesis of early proteins. Cell. 1980 Jun;20(2):401–409. doi: 10.1016/0092-8674(80)90626-1. [DOI] [PubMed] [Google Scholar]
- Boshart M., Klüppel M., Schmidt A., Schütz G., Luckow B. Reporter constructs with low background activity utilizing the cat gene. Gene. 1992 Jan 2;110(1):129–130. doi: 10.1016/0378-1119(92)90456-y. [DOI] [PubMed] [Google Scholar]
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Delbecchi L., Yu K. K., Herring E., Bourgaux-Ramoisy D. A mouse embryo cell line carrying an inducible, temperature-sensitive, polyoma virus genome. Virology. 1978 Jul 15;88(2):348–360. doi: 10.1016/0042-6822(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Gendron D., Bourgaux-Ramoisy D. Preferred crossover sites on polyomavirus DNA. J Virol. 1990 May;64(5):2327–2336. doi: 10.1128/jvi.64.5.2327-2336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgaux P., Sylla B. S., Chartrand P. Excision of polyoma virus DNA from that of a transformed mouse cell: identification of a hybrid molecule with direct and inverted repeat sequences at the viral-cellular joints. Virology. 1982 Oct 15;122(1):84–97. doi: 10.1016/0042-6822(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Dailey L., Basilico C. Sequences in the polyomavirus DNA regulatory region involved in viral DNA replication and early gene expression. J Virol. 1985 Jun;54(3):739–749. doi: 10.1128/jvi.54.3.739-749.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G. C., Niyogi S. K., Salzman N. P. SV40 promoters and their regulation. Prog Nucleic Acid Res Mol Biol. 1985;32:217–236. doi: 10.1016/s0079-6603(08)60349-9. [DOI] [PubMed] [Google Scholar]
- Della Valle G., Fenton R. G., Basilico C. Polyoma large T antigen regulates the integration of viral DNA sequences into the genome of transformed cells. Cell. 1981 Feb;23(2):347–355. doi: 10.1016/0092-8674(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Engler P., Roth P., Kim J. Y., Storb U. Factors affecting the rearrangement efficiency of an Ig test gene. J Immunol. 1991 Apr 15;146(8):2826–2835. [PubMed] [Google Scholar]
- Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992 Mar;66(3):1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmerie W. G., Folk W. R. Regulation of polyomavirus transcription by large tumor antigen. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6919–6923. doi: 10.1073/pnas.81.22.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Griffin B. E., Lund E., Robberson D. L. Polyoma virus--a study of wild-type, mutant and defective DNAs. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):45–52. doi: 10.1101/sqb.1974.039.01.009. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Hearst J. E. A topological model for transcription based on unwinding angle analysis of E. coli RNA polymerase binary, initiation and ternary complexes. Cell. 1982 May;29(1):81–90. doi: 10.1016/0092-8674(82)90092-7. [DOI] [PubMed] [Google Scholar]
- Gendron D., Delbecchi L., Bourgaux-Ramoisy D., Bourgaux P. A substitution in a nonconserved region of polyomavirus large T antigen which causes a thermosensitive mutation. Virology. 1988 Jul;165(1):165–171. doi: 10.1016/0042-6822(88)90669-1. [DOI] [PubMed] [Google Scholar]
- Gélinas C., Bouchard L., Bastin M. Tumorigenic activity of cloned polyoma virus DNA in newborn rats. Experientia. 1981 Oct 15;37(10):1074–1075. doi: 10.1007/BF02085017. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Fritze C. E., Folk W. R., DePamphilis M. L. The origin of bidirectional DNA replication in polyoma virus. EMBO J. 1987 Jul;6(7):2011–2018. doi: 10.1002/j.1460-2075.1987.tb02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hsieh C. L., McCloskey R. P., Lieber M. R. V(D)J recombination on minichromosomes is not affected by transcription. J Biol Chem. 1992 Aug 5;267(22):15613–15619. [PubMed] [Google Scholar]
- Hsieh P., Camerini-Otero C. S., Camerini-Otero R. D. The synapsis event in the homologous pairing of DNAs: RecA recognizes and pairs less than one helical repeat of DNA. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6492–6496. doi: 10.1073/pnas.89.14.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Matsumoto T. Transcription promotes recA-independent recombination mediated by DNA-dependent RNA polymerase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4571–4575. doi: 10.1073/pnas.76.9.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T. Assembly and disassembly of the Drosophila RNA polymerase II complex during transcription. J Biol Chem. 1990 Feb 15;265(5):2624–2631. [PubMed] [Google Scholar]
- Lania L., Boast S., Fried M. Excision of polyoma virus genomes from chromosomal DNA by homologous recombination. Nature. 1982 Jan 28;295(5847):349–350. doi: 10.1038/295349a0. [DOI] [PubMed] [Google Scholar]
- Larose A., St-Onge L., Bastin M. Mutations in polyomavirus large T affecting immortalization of primary rat embryo fibroblasts. Virology. 1990 May;176(1):98–105. doi: 10.1016/0042-6822(90)90234-i. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Clancy S., Calos M. P. Simian virus 40 replication in adenovirus-transformed human cells antagonizes gene expression. Nature. 1985 Sep 12;317(6033):169–171. doi: 10.1038/317169a0. [DOI] [PubMed] [Google Scholar]
- Leong K., Brunet L., Berk A. J. Factors responsible for the higher transcriptional activity of extracts of adenovirus-infected cells fractionate with the TATA box transcription factor. Mol Cell Biol. 1988 Apr;8(4):1765–1774. doi: 10.1128/mcb.8.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H., Maizels N. Transcriptional regulatory elements stimulate recombination in extrachromosomal substrates carrying immunoglobulin switch-region sequences. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4154–4158. doi: 10.1073/pnas.89.9.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. D., Manley J. L. Repression of simian virus 40 early transcription by viral DNA replication in human 293 cells. Nature. 1985 Sep 12;317(6033):172–175. doi: 10.1038/317172a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Miller J., Bullock P., Botchan M. Simian virus 40 T antigen is required for viral excision from chromosomes. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7534–7538. doi: 10.1073/pnas.81.23.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff J. A., Reynolds R. J. Transcription stimulates homologous recombination in mammalian cells. Mol Cell Biol. 1990 Sep;10(9):4837–4845. doi: 10.1128/mcb.10.9.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff J. A. Transcription enhances intrachromosomal homologous recombination in mammalian cells. Mol Cell Biol. 1992 Dec;12(12):5311–5318. doi: 10.1128/mcb.12.12.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piché A., Bourgaux P. Resolution of a polyomavirus-mouse hybrid replicon: release of genomic viral DNA. J Virol. 1987 Mar;61(3):840–844. doi: 10.1128/jvi.61.3.840-844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piché A., Bourgaux P. Resolution of a polyomavirus-mouse hybrid replicon: viral function required for recombination. J Virol. 1987 Mar;61(3):845–850. doi: 10.1128/jvi.61.3.845-850.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautmann G., Glaichenhaus N., Nahgashfar Z., Breathnach R., Rassoulzadegan M. Complementation of a tsa mutant and replication of a recombinant DNA carrying the viral ori region in mouse cells transformed by polyoma virus. Virology. 1982 Oct 30;122(2):306–317. doi: 10.1016/0042-6822(82)90230-6. [DOI] [PubMed] [Google Scholar]
- Rothwell V. M., Folk W. R. Comparison of the DNA sequence of the Crawford small-plaque variant of polyomavirus with those of polyomaviruses A2 and strain 3. J Virol. 1983 Nov;48(2):472–480. doi: 10.1128/jvi.48.2.472-480.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Sentenac A. RNA polymerase B (II) and general transcription factors. Annu Rev Biochem. 1990;59:711–754. doi: 10.1146/annurev.bi.59.070190.003431. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Corcoran L. M., Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J Exp Med. 1991 Mar 1;173(3):711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M., Voronova A., Baltimore D. Helix-loop-helix transcription factor E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev. 1991 Aug;5(8):1367–1376. doi: 10.1101/gad.5.8.1367. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart S. E., Roeder G. S. Transcription by RNA polymerase I stimulates mitotic recombination in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Aug;9(8):3464–3472. doi: 10.1128/mcb.9.8.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Berg P. Homologous and nonhomologous recombination in monkey cells. Mol Cell Biol. 1983 Jun;3(6):1040–1052. doi: 10.1128/mcb.3.6.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla B. S., Huberdeau D., Bourgaux-Ramoisy D., Bourgaux P. Site-specific excision of integrated polyoma DNA. Cell. 1984 Jun;37(2):661–667. doi: 10.1016/0092-8674(84)90398-2. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Matthes H., Wintzerith M., Chambon P. Transcription from the SV40 early-early and late-early overlapping promoters in the absence of DNA replication. EMBO J. 1983;2(9):1605–1611. doi: 10.1002/j.1460-2075.1983.tb01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]