Abstract
Background:
The aim of the present work was to investigate the prevalence and species of intestinal microsporidiosis among animals in Giza, Egypt.
Methods:
A total of 869 animal fecal samples were collected from domesticated animals (dogs, cats, rabbits, cattle, buffaloes, sheep, goats, donkeys and pigs) living in Giza, Egypt. Spores of microsporidia were concentrated from collected samples by centrifugation and finally stained with modified trichrome (MT) stain to detect microsporidial spores. Microsporidial spores in microscopically-positive samples were molecularly confirmed and identified using species-specific primers.
Results:
Spores of microsporidia were microscopically detected in 17.0% of the examined animal fecal samples. The highest and lowest rates of infection with intestinal microsporidia were recorded in dogs (33.3%) and buffaloes (6.9%), respectively. Molecularly, the obtained microsporidial spores were classified as Enterocytozoon bieneusi and E. intestinalis. Dual infection with both identified species was observed in fecal samples from buffalo, rabbit, goat, cat, pig and dog.
Conclusion:
Domestic animals may play a role in dissemination of intestinal microsporidiosis in the environment. Examined animals were infected with E. bieneusi in a higher percentage than E. intestinalis.
Keywords: Intestinal microsporidia, Modified trichrome, PCR, Domesticated animals, Fecal samples
Introduction
Microsporidia are single-celled microorganisms defined as obligate intracellular eukaryotic parasites capable for infection of protozoa, other invertebrates, and vertebrates (1). Morphologically, spores of microsporidia may be spherical, ovoid, rod-shaped, or crescent-shaped, although most are ovoid (2). These organisms are microscopically defined by the presence of a nucleated sporoplasm, a coiled polar tube and an anchoring disk. They lack several eukaryotic organelles such as mitochondria, Golgi membranes, and eukaryotic ribosomes (3).
Historically, microsporidial spores were first recognized as the causative agent of pébrine (pepper) disease, severely affecting the silk-worm industry in France and Italy during the mid-17th century (4). Thenceforth microsporidiosis have affected honeybee, fish, and mink industries have been compromised by microsporidiosis (5). Since 1985, microsporidia have been incriminated as a causative agent of opportunistic infections associated with persistent diarrhea and weight loss in persons with AIDS (6–8). At first, microsporidia were microscopically considered as primitive protozoa, but in the 1990s, molecular and phylogenetic evidences revealed relationship of these organisms and fungi (9).
More than 1300 species of microsporidia were identified and divided into about 150 genera (10, 11). About 14 microsporidia species infect humans (11). Enterocytozoon bieneusi is considered the most prevalent enteric species infecting humans worldwide, followed by E. intestinalis (12). Vertical transmission of microsporidiosis from mother to offspring has been represented in animals (rodents, rabbits, carnivores, and non-human primates) (13, 14). The presence of microsporidia in the respiratory and intestinal tracts of infected individuals and the excretion of spores in urine and feces illustrate that horizontal transmission is possible through fecal–oral transmission, oral–oral transmission, inhalation of contaminated aerosols, and ingestion of contaminated food and water (8, 15).
Risk factors associated with microsporidiosis that support horizontal transmission include homosexual practices, intravenous drug use, and exposure to water in swimming pools and hot tubs as well as occupational contact with water contaminated with microsporidial spores (16, 17). Those organisms are involved in Drinking Water Contaminant Candidate List of the US Environmental Protection Agency (USEPA) (18). E. bieneusi is found in a variety of mammals including macaques, dogs, cats, cattle, llamas, raccoons, muskrats, beavers, foxes, otters and pigs (19–24).
To our knowledge scarce data is available concerning the occurrence of intestinal microsporidiosis in domestic animals in Egypt. Therefore, the aim of the present work was to investigate the prevalence and species of intestinal microsporidiosis among animals in Giza, Egypt.
Materials and Methods
Animal fecal samples
A total of 869 fecal samples were separately collected from animals in Giza, Egypt in 2012–2013. Fecal samples were collected from 108 dogs “Canis lupus familiaris ”, 104 cats “Felis catus”, 98 cattle “Bos taurus”, 116 buffaloes, “Bubalus bubalis ”, 83 goats “Capra hircus”, 89 sheep “Ovis aries” 96 pigs “Sus scrofa”, 88 donkeys “Equus asinus ” and 87 rabbits “Oryctolagus cuniculus”. Fecal samples were separately collected in clean plastic containers. Animal fecal samples were labeled with species of animal. The collected samples were carried out to the laboratory at the same day of collection.
Concentration of microsporidial spores
Fecal samples were separately homogenized with clean spatula and then divided into two equal parts. One part of each fecal specimen was preserved in 10% formalin solution (Merck) and used for microscopic detection of microsporidial spores. The second part of each fecal sample was kept at −20 °C until used for DNA extraction. Ethyl acetate concentration method was used to concentrate the spores of microsporidia in fecal samples (25).
Staining and light microscopy
The obtained concentrated pellet was fixed on a clean glass slide using absolute methyl alcohol (Merck) and stained with modified trichrome (MT) stain (26).
Stained smears were microscopically examined with oil immersion lens for the presence of microsporidia spores. Stained microsporidia spores appeared reddish with clearly defined edges and a vacuole against a green background.
DNA extraction
The preserved part of each fecal sample was washed with phosphate buffer saline (PBS) and centrifuged at 2500 g for 5 min. The supernatant was decanted and the remaining pellet was washed again two times as mentioned before. The final washed pellet was re-suspended in 1 ml of PBS. Two hundred microliters of fecal suspension were extracted using the Qiagen QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA) with a modified protocol. The temperature of the initial lysis step was increased to 95 °C for 5 min, followed by incubation with Proteinase K for 4 h in a water bath at 55 °C, and then 100 μl of AE buffer was used for elution. The elution step was repeated to increase the amount of DNA yield by reapplying the original 100 μl to the spin column. DNA eluate was stored at −20 °C until PCR analysis.
PCR amplification and electrophoresis
PCR was performed using three different diagnostic primer pairs: i) generic microsporidia primer pair (PMP1 and PMP2) to confirm the presence of microsporidia (27); ii) species specific primer pair (EBIEF1/EBIER1) for amplification of microsporidial small subunit rRNA (SSU-rRNA) coding regions of E. bieneusi (28); and iii) species specific primer pair (SINTF/SINTR) for E. intestinalis (29) (Table 1).
Table 1:
Primers of microsporidia, Enterocytozoon bieneusi and Encephalitozoon intestinalis
| Organism | Primer name | Sequence | Fragment length (bp) | Reference |
|---|---|---|---|---|
| Microsporidia | PMP1 | CACCAGGTTGATTCTGCCTGAC | 250 :E. bieneusi | 27 |
| PMP2 | CCTCTCCGGAACCAAACCCTG | 268 : E. cuniculi | ||
| 270 : E. intestinalis | ||||
| 279 : E. hellem. | ||||
| Enterocytozoon bieneusi | EBIEF1 | GAAACTTGTCCACTCCTTACG | 607 | 28 |
| EBIER1 | CCATGCACCACTCCTGCCATT | |||
| Encephalitozoon intestinalis | SINTF | TATGAGAAGTGAGTTTTTTTC | 545 | 29 |
| SINTR | CCGTCCTCGTTCTCCTGCCCG |
Amplification of DNA was performed using Maxima Hot Start Green PCR master mix (Thermo Scientific). A hot-start procedure for microsporidia and E. bieneusi was used with an initial denaturation at 95 °C for 4 min followed by 35 cycles of denaturation at 94 °C for 30s, primer annealing at 60 °C for 30 s and extension at 72 °C for 30s. A final extension step was performed at 72 °C for 10 min (27, 28). The optimal PCR conditions for the SINTF/SINTR primers began with an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30s, annealing at 55 °C for 30s, and extension at 72 °C for 90 s. A final extension step was performed at 72 °C for 10 min (29). Agarose gel electrophoresis was consequently used for the detection of PCR products.
Results
Microscopic examination of 869 fecal samples from different animals revealed the presence of intestinal microsporidia in 17.0% of them by using modified trichrome stain. The highest rate of infection with intestinal microsporidia was recorded in dogs (33.3%), followed by 23.1, 20.5, 18.8, 14.9, 14.3, 11.2, 9.1 and 6.9% in cats, goats, pigs, rabbits, cattle, sheep, donkeys and buffaloes, respectively (Table 2 and Fig. 1).
Table 2:
Prevalence of intestinal microsporidia in animal fecal samples by modified trichrome stain
| Source of fecal samples | Fecal samples | ||
|---|---|---|---|
| Total no. examined | Microsporidia +Positive by MT stain | ||
| No. | % | ||
| Cattle | 98 | 14 | 14.3 |
| Buffalo | 116 | 8 | 6.9 |
| Sheep | 89 | 10 | 11.2 |
| Goat | 83 | 17 | 20.5 |
| Rabbit | 87 | 13 | 14.9 |
| Cat | 104 | 24 | 23.1 |
| Dog | 108 | 36 | 33.3 |
| Donkey | 88 | 8 | 9.1 |
| Pig | 96 | 18 | 18.8 |
| Total | 869 | 148 | 17.0 |
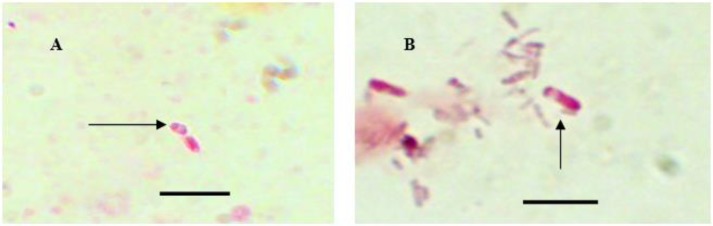
Fig. 1:
Original picture of different microsporidial spores stained with MT stain. Bar = 5μm
Molecularly, intestinal microsporidial spores were recorded in 95 (64.2%) out of the 148 microscopically positive samples. 76.8% and 42.1 % of the PCR positive animal fecal samples had E. bieneusi and E. intestinalis, respectively. The highest rate of infection with E. bieneusi reached 100% in cattle, buffaloes, sheep and goats. No infection with E. bieneusi was recorded in donkey fecal samples. The highest rate of infection with E. intestinalis was recorded in donkeys (100%), followed by 82.6, 36.4, 31.3, 26.7, 25.0 and 20.0%, in dogs, goats, cats, pigs, buffaloes and rabbits, respectively. There was no infection with E. intestinalis in cattle and sheep. Dual infection with both E. bieneusi and E. intestinalis was recorded in examined fecal samples from buffalo, rabbit, goat, cat, dog and pig (Table 3 and Fig 2–4).
Table 3:
Species identification of intestinal microsporidia in examined animals by PCR
| Sources of fecal samples | PCR positive fecal samples for intestinal microsporidia | ||||
|---|---|---|---|---|---|
| Total + positive samples by PCR | E. bieneusi positive samples | E. intestinals positive samples | |||
| NO. | % | NO. | % | ||
| Cattle | 9 | 9 | 100 | 0 | 0.0 |
| Buffaloes | 4 | 4 | 100 | 1 | 25.0 |
| Rabbits | 5 | 4 | 80 | 1 | 20.0 |
| Sheep | 6 | 6 | 100 | 0 | 0.0 |
| Goat | 11 | 11 | 100 | 4 | 36.4 |
| Cats | 16 | 13 | 81.3 | 5 | 31.3 |
| Dogs | 23 | 14 | 60.9 | 19 | 82.6 |
| Donkeys | 6 | 0 | 0.0 | 6 | 100 |
| Pigs | 15 | 12 | 80 | 4 | 26.7 |
| Total | 95 | 73 | 76.8 | 40 | 42.1 |
Fig. 2:
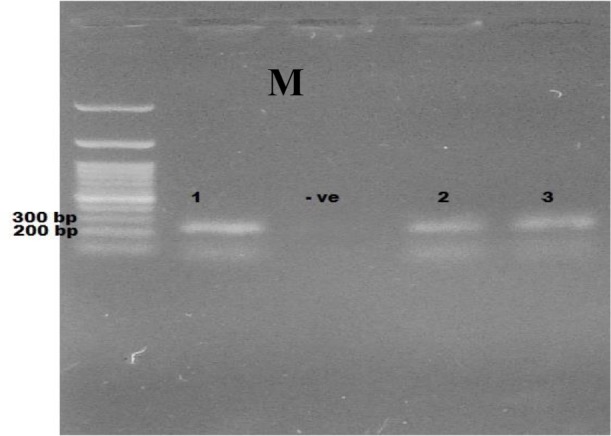
Ethidium bromide stained 2% agrose showing PCR products of microsporidia. M: Marker (100 plus bp), −ve: negative control, samples 1,2,3: positive samples at 250–279bp.
Fig. 4:
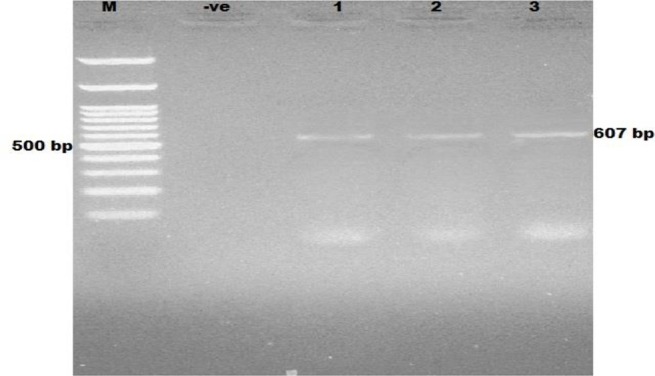
Ethidium bromide stained 2% agrose showing PCR products of Enterocytozoon bieneusi. M: Marker (100 plus bp); −ve: negative control; lanes 1–3: positive samples
Fig. 3:
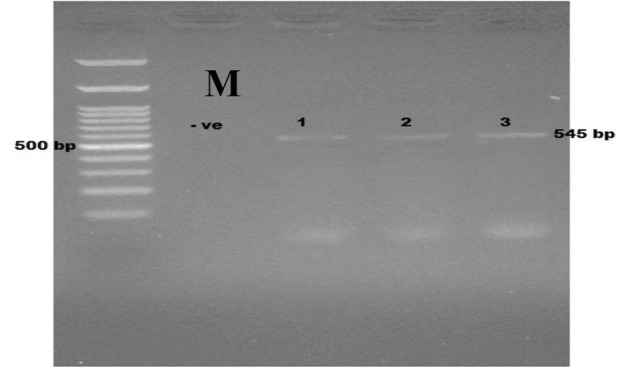
Ethidium bromide stained 2% agrose showing PCR products of Encephalitozoon intestinalis. M: Marker (100 plus bp); −ve: negative control; lanes 1–3: positive samples
Discussion
Overall, 148 out of 869 examined animal fecal samples were positive for intestinal microsporidia by MT stain. Only 95 out of 148 microscopically positive fecal samples were molecularly positive for intestinal microsporidia. This result agreed with another study (30) where the detection of microsporidia by PCR showed lower correlation with their detection by light microscopy. Furthermore, in Portugal the inhibitors could interfere with PCR results and therefore PCR was postulated to have a limited value for identification of the species of microsporidia present in fecal samples (31).
In the present study, a total of 14 (14.3%) cattle fecal samples were positive for intestinal microsporidia by modified trichrome stain. Other workers in Spain found no infection with intestinal microsporidia in fecal samples from three caws by Weber’s chromotrope stain and this might be attributed to the small number of samples (32). In Mexico, E. intestinalis was detected in one caw fecal sample stained with quick-hot Gram chromotrope stain (33).
The present result showed that nine out of 14 microscopically-positive cattle fecal samples were molecularly-positive and identified as E. bieneusi. In other molecular studies, E. bieneusi was reported in cattle feces in Germany (11.7%) (34), USA (9.5%) (35), Korea (14.9%) (36) and Argentina (14.3%) (37).
In our study, a total of 8/88 (9.1%) donkeys had intestinal microsporidia by MT stain. Our result was greatly lower than that of Lores et al. (32) where one of two examined fecal samples of donkeys had intestinal microsporidia and Bornaylinares et al. (33) who examined only two donkeys and found that one of them had intestinal microsporidia. The greater difference in the number of examined samples could not be dependent due the very low number of examined animals.
The present results showed that 6 out of 8 donkey fecal samples (positive for microsporidia by MT stain) had intestinal microsporidia by PCR. Molecularly, the identified species in these 6 donkeys were all E. intestinalis. In USA, molecular examination of only 2 donkeys revealed that one of them had intestinal microsporidia identified as E. intestinalis (33), while in Spain no microsporidia was detected by PCR in donkey fecal samples that were microscopically positive for microsporidia (32).
Microscopic examination of pig fecal samples stained with MT stain in the current investigation revealed the presence of microporidial spores in 18.8% of them. A higher occurrence (82%) of intestinal microsporidia in pigs was recorded in Peru using calcoflour M2R stain (38). This variation between our result and that of Sak et al. (38) might be attributed to difference in diagnostic tools. In Spain, no intestinal microsporidia was observed in 4 pigs by using Weber’s chromotrope stain and this might be attributed to the low number of the examined fecal samples (32).
The current work revealed that 15 out of 18 pig fecal samples (that were microscopically positive for microsporidia) were positive by PCR. The identified species of microsporidia in these samples were: 12 pigs had E. bieneusi, four pigs had E. intestinalis. Other workers recorded higher incidences (94% and 92.6%) of E. bieneusi by PCR in the examined pig fecal samples from Czech Republic and Slovakia (38, 39), while lower incidences were reported in Switzerland, Massachusetts (USA) and Japan (40–43).
To our knowledge there were no available data concerning the detection of intestinal microsporidia in sheep by using staining techniques. Using PCR technique in the present work, it was found that 60% of microscopically positive sheep fecal samples for intestinal microsporidia were PCR positive and all samples had only a single infection with E. bieneusi. Other workers agreed with our result in that E. bieneusi was the only species identified in feces of sheep (43).
In the present work, 20.5% of the examined goat fecal samples (n=83) by MT stain were microscopically positive for intestinal microsporidia. Other workers in Spain observed clusters of microsporidia-like spores within a vacuole inside epithelial cells in fecal smears of one goat using Weber’s chromotrope-based stain (32).
In the present study, 64.7% of microscopically positive goat fecal samples (n=17) were positive by PCR. In addition, 41.2% of PCR positive goat fecal samples had a single infection with E. bieneusi. Other workers in Spain (32) and Peru (43) found that 14.2% and 2% of the examined goat fecal samples had E. bieneusi only, while Bornay-linares et al. (33) in USA examined one goat fecal sample by PCR and identified E. intestinalis in it.
14.9% of the examined rabbit fecal samples had intestinal microsporidia by MT stain which was higher than the result of Lores et al. (32) in Spain who found that 2/22 (9.1%) of rabbits had intestinal microsporidia by Weber’s chromotrope stain.
Molecularly, single as well as mixed infections with E. bieneusi and E. intestinalis were observed in pellets of rabbits in the present work. E. bieneusi was microscopically identified and by PCR for the first time in rabbit fecal samples in Spain, a single infection of rabbits with either E. bieneusi (44) or E. cuniculi (32) was recorded, while no infection with intestinal microsporidia was reported in Germany (34).
Intestinal microsporidia were microscopically detected in cat feces in our study in Egypt (23.1%) and in Portugal (29.4%) (31), but not in Spain (32) might be attributed to difference in geographic criteria of these different countries.
The present study showed that both E. bieneusi and E. intestinalis were present in feces of cats. In Germany (34), Japan (42) and USA (45), E. bieneusi was the only identified species in 5, 17 and 14.3% of cat fecal samples by PCR, respectively. In Portugal, both E. bieneusi and E. cuniculi were detected in feces of cats (31), while no intestinal microsporidia were found in ten examined cat fecal samples in Spain (32).
In the present study, 36/108 (33.3%) of dogs had intestinal microsoporidia by MT stain. Other workers in Spain found a lower incidence (5.9%) of intestinal microsporidia in dogs by Weber’s chromotrope stain (32). In Portugal, a lower incidence [13.8% (5/36)] of intestinal microsporidia was detected in dogs by MT stain (31). The variation between our result and the previous results (31, 32) might be attributed to difference in the number of the examined samples.
The present work declared that dogs could have E. bieneusi, E. intestinalis or both in their feces. Other workers in Colombia (46), Spain ((32), Portugal (31) and Japan (42) found molecularly that 15, 11.8, 60 and 2.5% of examined dogs had intestinal microsporidia belonging to only E. bieneusi, while in no microsporidial spores were found in 60 dog fecal samples examined by PCR in Germany (34).
Conclusion
E. bieneusi was the most prevalent intestinal microsporidia in domesticated animals that may play a role in dissemination of intestinal microsporidiosis in the environment.
Acknowledgments
The authors thank Gamal Yamamah for technical services in support of this study. The authors declare that there is no conflict of interest.
References
- 1. Cuomo CA, Desjardins CA, Bakowski MA, Goldberg J, Ma AT, Becnel JJ, Didier ES, Fan L, Heiman DI, Levin JZ, Young S, Zeng Q, Troemel ER. Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Res. 2012; 22 12: 2478– 2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vavra J, Larsson JIR. Structure of the microsporidia. In: Wittner M. (editor). The microsporidia and microsporidiosis. Washington DC: ASM Press, 1999; pp. 7– 84. [Google Scholar]
- 3. Mathis A, Weber R, Deplazes P. Zoonotic potential of the microsporidia. Clin Microbiol Rev. 2005; 1 8: 423– 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wittner M. Historic perspective on the microsporidia: expanding horizons. In: Wittner M, Weiss L, (editors.). The microsporidia and microsporidiosis. Washington DC: ASM Press, 1999; pp. 1– 6. [Google Scholar]
- 5. Canning EU, Lom J. The Microsporidia of vertebrates. New York: Academic press, 1986; pp. 289. [Google Scholar]
- 6. Kotler DP, Orenstein JM. Clinical syndromes associated with microsporidiosis. Adv Parasitol. 1998; 40: 321– 349. [DOI] [PubMed] [Google Scholar]
- 7. Kotler D, Orenstein JM. Clinical syndromes associated with microsporidiosis. In: Wittner M., Weiss L. (editors.). The microsporidia and microsporidiosis. Washington DC: ASM press, 1999; pp. 258– 292. [Google Scholar]
- 8. Weber R, Deplazes P, Schwartz D. Diagnosis and clinical aspects of human microsporidiosis. Contrib Microbiol. 2000; 6: 166– 192. [DOI] [PubMed] [Google Scholar]
- 9. Thomarat F, Vivares CP, Gouy M. Phylogenetic analysis of the complete genome sequence of Encephalitozoon cuniculi supports the fungal origin of microsporidia and reveals a high frequency of fast-evolving genes. J Mol Evol. 2004; 59 6: 780– 791. [DOI] [PubMed] [Google Scholar]
- 10. Keeling PJ, Fast NM. Microsporidia: biology and evolution of highly reduced intracellular parasites. Annu Rev Microbiol. 2002; 56: 93– 116. [DOI] [PubMed] [Google Scholar]
- 11. Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006; 19 5: 485– 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Didier ES, Stovall ME, Green LC, Brindley PJ, Sestak K, Didier PJ. Epidemiology of microsporidiosis: sources and modes of transmission. Vet Parasitol. 2004; 126: 145– 166. [DOI] [PubMed] [Google Scholar]
- 13. Snowden KF, Didier ES, Orenstein JM, Shadduck JA. Animal models of human microsporidial infections. Lab Anim Sci. 1998; 48 6: 589– 592. [PubMed] [Google Scholar]
- 14. Snowden KF, Shadduck JA. Microsporidia of higher vertebrates. In: Wittner M., Weiss L. (editors.). The microsporidia and microsporidiosis. Washington, DC; ASM press; 1999; pp. 393– 419. [Google Scholar]
- 15. Deplazes P, Mathis A, Weber R. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib Microbiol. 2000; 6: 236– 260. [DOI] [PubMed] [Google Scholar]
- 16. Hutin YJ, Sombardier MN, Liguory O, Sarfati C, Derouin F, Modai J, Molina JM. Risk factors for intestinal microsporidiosis in patients with human immunodeficiency virus infection: a case-control study. J Infect Dis. 1998; 178: 904– 907. [DOI] [PubMed] [Google Scholar]
- 17. Dascomb K, Frazer T, Clark RA, Kissinger P, Didier E. Microsporidiosis and HIV. J Acquir Immune Defic Syndr. 2000; 24 3: 290– 292. [DOI] [PubMed] [Google Scholar]
- 18. USEPA Contaminant Candidate List – CCL1. 1998. http://water.epa.gov/scitech/drinkingwater/dws/ccl/ccl1.cfm#list .
- 19. Galván-Díaz AL, Magnet A, Fenoy S, Henriques-Gil N, Haro M, Gordo FP, Miró G, del Águila C, Izquierdo F. Microsporidia detection and genotyping study of human pathogenic E. bieneusi in animals from Spain. PLoS One. 2014; 9: e92289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lobo ML, Xiao L, Cama V, Magalhaes N, Antunes F, Matos O. Identification of potentially human-pathogenic Enterocytozoon bieneusi genotypes in various birds. Appl Environ Microbiol. 2006; 72: 7380– 7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mori H, Mahittikorn A, Thammasonthijarern N, Chaisiri K, Rojekittikhun W, Sukthana Y. Presence of zoonotic Enterocytozoon bieneusi in cats in a temple in central Thailand. Vet Parasitol. 2013; 197: 696– 701. [DOI] [PubMed] [Google Scholar]
- 22. Sak B, Petrzelkova KJ, Kvetonova D, Mynarova A, Shutt KA, Pomajbikova K, Kalousova B, Modry D, Benavides J, Todd A, Kvac M. Long-term monitoring of microsporidia, Cryptosporidium and Giardia infections in western Lowland Gorillas (Gorilla gorilla gorilla) at different stages of habituation in Dzanga Sangha Protected Areas, Central African Republic. PLoS One. 2013; 8: e71840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ye J, Xiao L, Li J, Huang W, Amer SE, Guo Y, Roellig D, Feng Y. Occurrence of human-pathogenic Enterocytozoon bieneusi, Giardia duodenalis and Cryptosporidium genotypes in laboratory macaques in Guangxi, China. Parasitol Int. 2014; 63: 132– 137. [DOI] [PubMed] [Google Scholar]
- 24. Li W, Diao R, Yang J, Xiao L, Lu Y, Li Y, Song M. High diversity of human-pathogenic Enterocytozoon bieneusi genotypes in Swine in northeast China. Parasitol Res. 2014; 113: 1147– 1153. [DOI] [PubMed] [Google Scholar]
- 25. Bern C, Hernandez B, Lopez MB, Arrowood MJ, de Mejia MA, de Merida AM, Hightower AW, Venczel L, Herwaldt BL, Klein RE. Epidemiologic study of Cyclospora cayetanensis in Guatemala. Emerg Infect Dis. 1999; 5: 766– 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med. 1992; 326: 161– 166. [DOI] [PubMed] [Google Scholar]
- 27. Fedorko DP, Nelson NA, Cartwright CP. Identification of microsporidia in stool specimens by using PCR and restriction endonucleases. J Clin Microbiol. 1995; 33: 1739– 1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Da Silva AJ, Schwartz DA, Visvesvara GS, Moura H, Slemenda SB, Pieniazek NJ. Sensitive PCR diagnosis of infections by Enterocytozoon bieneusi (Microsporidia) using primers based on the region coding for small-subunit rRNA. J Clin Microbiol. 1996; 34: 986– 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Da Silva AJ, Slemenda SB, Visvesvara GS, Schwartz DA, Wilcox CM, Wallace S, Pieniazek NJ. Detection of Septata intestinalis (Microsporidia), Cali et al. 1993, using polymerase chain reaction primers targeting the small subunit ribosomal RNA coding region. Mol Diagn. 1997; 2: 47– 52. [DOI] [PubMed] [Google Scholar]
- 30. Katzwinkel-Wladarsch S, Deplazes P, Weber R, Löscher T, Rinder H. Comparison of polymerase chain reaction with light microscopy for detection of microsporidia in clinical specimens. Eur J Clin Microbiol Infect Dis. 1997; 16: 7– 10. [DOI] [PubMed] [Google Scholar]
- 31. Lobo ML, Teles A, da Cunha MB, Henriques J, Lourenço AM, Antunes F, Matos O. Microsporidia detection in stools from pets and animals from the zoo in Portugal: a preliminary study. J Eukaryot Microbiol. 2003; 50: 581– 582. [DOI] [PubMed] [Google Scholar]
- 32. Lores B, del Aguila C, Arias C. Enterocytozoon bieneusi (microsporidia) in fecal samples from domestic animals from Galicia, Spain. Mem Inst Oswaldo Cruz. 2002; 97: 941– 945. [DOI] [PubMed] [Google Scholar]
- 33. Bornay-Linares FJ, da Silva AJ, Moura H, Schwartz DA, Visvesvara GS, Pieniazek NJ, Cruz-Lopez A, Hernandez-Jauregui P, Guerrero J, Enriquez FJ. Immunologic, microscopic, and molecular evidence of Encephalitozoon intestinalis (Septata intestinalis) infection in mammals other than humans. J Infect Dis. 1998; 178: 820– 826. [DOI] [PubMed] [Google Scholar]
- 34. Dengjel B, Zahler M, Hermanns W, Heinritzi K, Spillmann T, Thomschke A, Löscher T, Gothe R, Rinder H. Zoonotic potential of Enterocytozoon bieneusi. J Clin Microbiol. 2001; 39: 4495– 4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sulaiman IM, Fayer R, Yang C, Santin M, Matos O, Xiao L. Molecular characterization of Enterocytozoon bieneusi in cattle indicates that only some isolates have zoonotic potential. Parasitol Res. 2004; 92: 328– 334. [DOI] [PubMed] [Google Scholar]
- 36. Lee JH. Molecular detection of Enterocytozoon bieneusi and identification of a potentially human-pathogenic genotype in milk. Appl Environ Microbiol. 2008; 74: 1664– 1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Del Coco VF, Córdoba MA, Bilbao G, de Almeida Castro P, Basualdo JA, Santín M. First report of Enterocytozoon bieneusi from dairy cattle in Argentina. Vet Parasitol. 2014; 199: 112– 115. [DOI] [PubMed] [Google Scholar]
- 38. Sak B, Kváč M, Hanzlíková D, Vitaliano C. First report of Enterocytozoon bieneusi infection on a pig farm in the Czech Republic. Vet Parasitol. 2008; 153: 220– 224. [DOI] [PubMed] [Google Scholar]
- 39. Valencáková A, Balent P, Húska M, Novotný F, Luptáková L. First report on Encephalitozoon intestinalis infection of swine in Europe. Acta Vet Hung. 2006; 54: 407– 411. [DOI] [PubMed] [Google Scholar]
- 40. Breitenmoser AC, Mathis A, Bürgi E, Weber R, Deplazes P. High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology. 1999; 118: 447– 453. [DOI] [PubMed] [Google Scholar]
- 41. Buckholt MA, Lee JH, Tzipori S. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughter house in Massachusetts. Appl Environ Microbiol. 2002; 68: 2595– 2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abe N, Kimata I, Iseki M. Molecular evidence of Enterocytozoon bieneusi in Japan. J Vet Med Sci. 2009; 71: 217– 219. [DOI] [PubMed] [Google Scholar]
- 43. Cama V, Cabrera L, Lopera C, Vargas M, Taquiri C, Smit H, Xiao L. Molecular characterization of Cryptosporidium and microsporidia from goats and sheep in Peru, abstr. 10th International workshops on opportunistic protists, Boston, MA 2008; PL3. [Google Scholar]
- 44. Del Aguila C, Izquierdo F, Navajas R, Pieniazek NJ, Miró G, Alonso AI, Da Silva AJ, Fenoy S. Enterocytozoon bieneusi in animals: rabbits and dogs as new hosts. J Eukaryot Microbiol. 1999; 46: 8S– 9S. [PubMed] [Google Scholar]
- 45. Santín M, Trout JM, Vecino JA, Dubey JP, Fayer R. Cryptosporidium, Giardia and Enterocytozoon bieneusi in cats from Bogota (Colombia) and genotyping of isolates. Vet Parasitol. 2006; 141: 334– 339. [DOI] [PubMed] [Google Scholar]
- 46. Santín M, Cortés Vecino JA, Fayer R. Enterocytozoon bieneusi genotypes in dogs in Bogota, Colombia. Am J Trop Med Hyg. 2008; 79: 215– 217. [PubMed] [Google Scholar]