Abstract
Background:
Intestinal parasitic infections have a worldwide distribution. High prevalence of intestinal parasitic infections in individuals with low socioeconomic status and environmental conditions was found. No study has ever been conducted on the prevalence of these infections in Jiroft. Therefore, in this study prevalence of intestinal parasitic infections was evaluated in Jiroft, Kerman Province, Iran.
Methods:
A total of 1060 individuals from rural and urban areas of Jiroft were sampled accidentally, during 2013–2014. Fresh stool samples were collected from all individuals and examined by formalin ether concentration and agar plate culture. Direct examination was performed on watery samples.
Results:
Out of 1060 individuals, 563 (53.1%) and 497 (46.9%) people were from rural and urban areas, respectively. In general, 297 individuals (28%) were infected with intestinal parasites. The prevalence of infection for protozoa and helminthes infections were 27.4% and 1.8%, respectively. The most prevalent protozoans were Blastocystis hominis (13.7%) and Giardia lamblia (7.8%), and that of helminth was Hymenolepis nana (1.1%).
Conclusion:
Intestinal protozoan parasites were more prevalent than helminth parasites. Source of water supply and personal hygiene were important factors in the distribution of parasites in the study area.
Keywords: Prevalence, Intestinal parasites, Water supply, Iran
Introduction
Intestinal parasitic infections have worldwide distribution. The high prevalence is related to poor personal sanitation, unsafe water supply, and lack of health education (1). Many protozoans live in human gastrointestinal tract. Some of those are pathogenic, like Giardia lamblia, and Entamoeba histolytica, and some are nonpathogenic forms, living in gastrointestinal tract as commensals (1). E. histolytica, causative agent of amoebiasis, is a parasitic infection with considerable worldwide morbidity and mortality (2). Giardia lamblia is another pathogenic Protozoa. G. lamblia is the most common infection in temperate and tropical countries (1). The prevalence of G. lamblia has been estimated that 2– 3% in the developed countries and 20–30% in developing countries (3, 4). Blastocystis hominis is a common intestinal protozoan and its pathogenicity is still controversial (5–7). The prevalence of B. hominis has been reported to be higher in developing countries (30–50%) than developed countries (1.5–10%) (8).
Soil-transmitted helminthes (STH) infections are widely distributed throughout the tropics and subtropics. Climate, moisture and warm temperature are important elements of transmission of these infections (9). The most prevalent these helminthes infections are Ancylostoma duodenale, Necator americanus, Ascaris lumbricoides and Trichuris trichiura that infect approximately one-sixth of people all over the world (10, 11). There are low rates of tapeworm infections in Muslim populations in different regions of Asia and Africa. However, there are some reports from Africa (12–15).
Regarding to climate variation and different social and cultural condition in Iran, epidemiologic survey in different areas is necessary.
Records on intestinal parasitic infections have been reported from some parts of Iran (16–20), however, there are no data available on distribution of intestinal parasitic infection in Jiroft, Kerman Province. In this study we evaluated intestinal parasitic infection rates in Jiroft, Kerman Province.
Material and Methods
Study area
This study was performed in Jiroft, located approximately 248 km 2 southeast of Kerman Province (Fig. 1). It is comprised of four districts, fourteen rural districts, and four towns. Jiroft have a population of 277,748, among them 121,988 people (36%) living in urban areas and the rest in rural areas. It has three different climate zones: cold, warm, and moderate. Humidity stemming from the Indian Ocean causes torrential rains result in floods. Jiroft is considered as the most suitable region in Kerman Province for agriculture (Available at: https://en.wikipedia.org/wiki/Jiroft_County ).
Fig. 1:
Situation of Jiroft in Kerman Province, southeast Iran
Sampling
During 2013–2014, 1060 individuals including 563(53.1%) from rural and 497(46.9%) from urban areas of Jiroft were selected by accidental systematic method. Fresh stool samples were collected from all individuals and examined by formalin ether concentration and agar plate culture. Direct examination was performed on watery samples. Examinations of samples for parasitic infections were performed in the Department of Medical Parasitology, School of Public Health, Tehran University of Medical Sciences.
For every individual a questioner was filled and data about gender, age, place of residency, education, job, gastrointestinal disorder and contact with soil or animals, source of drinking water and use of sanitary toilets were entered.
Analysis
Detection of intestinal parasites was based on morphological characteristic of the parasites. Analysis was performed using SPSS version 18 (Chicago, IL, USA) and Chi-square test was used to analyze statistical relationship between prevalence of parasites and different criteria entered in the questioners.
Results
Out of 1060 individuals, 563 people (53.1%) and 497 people (46.9%) were from rural and urban areas, respectively. Infection rate in rural areas (29.3%) was more than urban areas (26.2%), without significant difference.
In general, 297 individuals (28 %) were infected with intestinal parasites. The prevalence of infection with intestinal protozoa and helminth parasites are summarized in Table 1.The prevalence of infection for protozoa and helminthes infections were 27.4% and 1.8%, respectively. The most prevalent protozoan parasite was B. hominis (13.7%); followed by G. lamblia (7.8%). The most prevalent helminth parasite was Hymenolepis nana (1.1%).
Table 1.
Prevalence of intestinal parasitic infections in Jiroft, Kerman Province, according to gender
| Infection | Gender | Total | ||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Number | Percent (%) | Number | Percent (%) | Number | Percent (%) | |
| Blastocystis hominis | 57 | 11.75 | 88 | 15.3 | 145 | 13.7 |
| Giardia lamblia | 48 | 9.8 | 35 | 6 | 83 | 7.8 |
| Entamoeba coli | 28 | 5.8 | 49 | 8.5 | 77 | 7.2 |
| Entamoeba histolytica/diapar | 4 | 0.8 | 6 | 1 | 10 | 0.9 |
| Iodamoeba butschilli | 10 | 0.2 | 9 | 1.6 | 19 | 1.8 |
| Chilomastix mesnilii | 2 | 0.4 | 6 | 1 | 8 | 0.8 |
| Entamoeba hartmani | 0 | 0 | 2 | 0.3 | 2 | 0.2 |
| Hymenolepis nana | 6 | 1.2 | 6 | 1 | 12 | 1.1 |
| Ascaris lambercoides | 0 | 0 | 2 | 0.3 | 2 | 0.2 |
| Enterobius vermicularis | 2 | 0.4 | 0 | 0 | 2 | 0.2 |
| Trichostrongylus sp | 1 | 0.2 | 0 | 0 | 1 | 0.1 |
| Dicrocoelium dendriticum | 0 | 0 | 1 | 0.2 | 1 | 0.1 |
| Taenia saginata | 1 | 0.2 | 0 | 0 | 1 | 0.1 |
| No parasite | 359 | 74 | 406 | 70.7 | 765 | 72.2 |
| Total | 485 | 45.8 | 574 | 54.2 | 1060 | 100 |
The prevalence rates of infection in females and males were 29% and 27%, respectively. There was no significant correlation between gender and infection rates.
Although the most prevalence rate of infection was found in age group of 30–39 years (34.9%); but, there was no significant correlation between age and prevalence of infection. The highest Giardia infection rate was found in age group 0–9 years old (11.6 %) and there was significant correlation between age and prevalence of Giardia infection (P-value: 0.023).
Respect to the job, the most infected people was pastors (37.5%) and housekeepers (32.2%); but there was no significant correlation between job and infection rates.
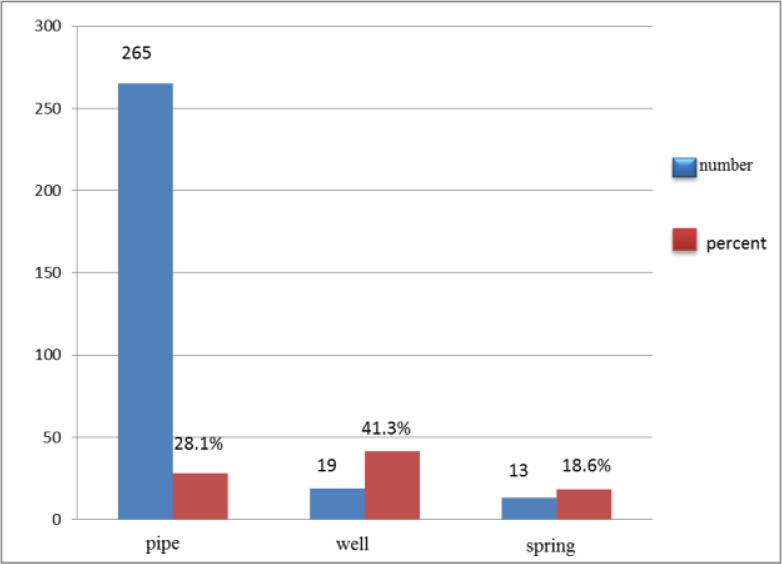
The prevalence rates of infection in people who consumed drinking water from different sources including wells, pipe and spring were 41.3%, 28.1% and 18.6%, respectively. There was statistical correlation between water supply and infection rates (P-value: 0.001) (Fig. 2).
Fig. 2:
Prevalence of intestinal parasites in Jiroft, Kerman according to the source of drinking water
There was significant correlation between use of unsanitary toilet and rate of infection (P: 0.031). There was no statistical correlation between gastrointestinal disorders, and contact with soil or animal and infectivity with intestinal parasites. In general, 29 individuals (28%) were infected with intestinal parasites; among those, 254 (85.5%), 35(11.8%), 7 (2.4%), and 1(0.3%) individuals had one, two, three and four parasites, respectively.
Discussion
Consumption of unsafe drinking water, Lack of sanitation and hygiene are strong determinants for infection with intestinal protozoa (21–23). In the current study, 28% of people were infected with intestinal parasites. In a survey (24), parasitic infection rate in Kerman was 47%. Prevalence of parasitic infection in part of Khuzestan Province, southwest Iran was 25.2% in 2007 (20).
The prevalence of infection for protozoan and helminthic infection in the present study were 27.4% and 1.8%, respectively. The result of this study showed the highest prevalence of intestinal protozoa was related to B. hominis (13.7%), followed by G. lamblia (7.8%). Prevalence of E. coli, I. butschilli and E. histolytica/dispar were 7.2% and 1.8% and 0.9, respectively. B. hominis and G. lamblia were the most common parasites in some of studies (1, 17–20). B.hominis (31.7%) and G. lamblia (29.6%) were the most prevalent protozoa in the Tonekabon (18). Shojaei, et al. reported the prevalence of 10.7% for intestinal parasites in Tehran during 2004–2005 and B. hominis and G. lamblia were the most frequent intestinal parasites (25).
In a survey on 196 disable people in Golestan Province in 2009, protozoan and helminth prevalence rates were 11.2% and 0.5%, respectively. Enterobius vermicularis was the only helminth was found. The most common protozoa was B.hominis (4.2%) and prevalence of G. lamblia, E. coli and E. histolytica/dispar were (3.1%), (2.5%) and (1.5%), respectively (26).
The prevalence rates of infection in this study were 29% and 27% in female and male, respectively. There was no significant correlation between gender and infection rates. Similarly, the results of some other studies showed that gender is not a factor subscribe to the differences in possibility of intestinal parasitic infections (17, 18, 27, 28).
In the present study, the highest infection rate (34.9%) was found in 30–39 yr old age group, although there was no significant correlation between age and parasitic infection rates. The highest Giardia infection (11.6%) was found in 0–9 yr old age group, as it is expected, There was significant correlation between age and Giardia infection rates (P= 0.023).
In the present study, the most prevalent helminth infection was Hymenolepis nana (1.1%), followed by Ascaris lumbricoides 0.2%) and Enterobius vermicularis (0.2%).
Intestinal helminth infection in Isfahan in 1987 (19) was reported 80.7% and the most common helminthes were Trichuris trichiura (76.7%) and A. lumbricoides (46.7%). Parasitic infection rate in a study was 69.2% in 1990 (17). The most common helminthes infections in that study were T. trichiura (26.8%) and A. lumbricoides (17.8%). Prevalence of parasitic infection in Tonekabon in 1992 (18) was 74.6% and high rates of helminthes infections were related to T. trichiura (22.5%) and A.lumbricoides (6.3%). Low prevalence of intestinal helminth infections in Jiroft is in concordance with the result of recent studies in other parts of Iran (16, 19, 20), indicating decrease of infection compared to last decades.
In the current study, coinfection was found from two to four parasites. The most common co-infection was observed between B. hominis and E. coli in13 cases (4.3%).
Considering other criteria, there was no significant correlation between place of residency (rural or urban), education, job, gastrointestinal disorder, and contact with soil or animals and parasitic infection rates. However, source of water supply was important criteria for infectivity with intestinal parasites. Unsafe water supply led to higher prevalence of infection. So that, prevalence of infection in people who consumed well water (41.3%) and untreated pipe water (28.1%) were more than that of spring water (18.6%); and the difference between water supply and infection rate was statistically significant (P: 0.001). In addition, in this study, there was significant correlation between use of unsanitary toilet and infection rate. Therefore, unsafe water supply and unsanitary toilets are important causative agents for intestinal parasitic infection in the study area.
Conclusion
In spite of decreasing trend of parasitic infection in Iran, compared to past decades, nowadays-intestinal parasitic infections are still one of the public health problems wherever safe water and hygiene measures are lacking.
Acknowledgement
This research was financially supported by Tehran University of Medical Sciences, Tehran, Iran. The authors declare that there is no conflict of interest.
References
- 1. Noor Azian M, San Y, Gan C, Yusri M, Nurulsyamzawaty Y, Zuhaizam A, et al. Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Trop Biomed. 2007; 24 (1): 55– 62. [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380 (9859): 2095– 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Escobedo AA, Cimerman S. Giardiasis: a pharmacotherapy review. Expert Opin Pharmacother. 2007; 8 (12): 1885– 1902. [DOI] [PubMed] [Google Scholar]
- 4. Speich B, Marti H, Ame SM, Ali SM, Bogoch II, Utzinger J, et al. Prevalence of intestinal protozoa infection among school-aged children on Pemba Island, Tanzania, and effect of single-dose albendazole, nitazoxanide and albendazole-nitazoxanide. Parasit Vectors. 2013; 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stensvold C, Lewis H, Hammerum AM, Porsbo LJ, Nielsen S, Olsen K, et al. Blastocystis: unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol Infect. 2009; 137 (11): 1655– 63. [DOI] [PubMed] [Google Scholar]
- 6. Leder K, Hellard ME, Sinclair MI, Fairley CK, Wolfe R. No correlation between clinical symptoms and Blastocystis hominis in immunocompetent individuals. J Gastroenterol Hepatol. 2005; 20 (9): 1390– 1394. [DOI] [PubMed] [Google Scholar]
- 7. Sheehan DJ, Raucher B, Mckitrick JC. Association of Blastocystis hominis with signs and symptoms of human disease. J Clin Microbiol. 1986; 24 (4): 548– 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Windsor J, Macfarlane L, Whiteside T, Chalmers R, Thomas A, Joynson D. Blastocystis hominis: a common yet neglected human parasite. Brit J Biomed Sci. 2001; 58 (2): 129. [PubMed] [Google Scholar]
- 9. Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006; 367 (9521): 1521– 1532. [DOI] [PubMed] [Google Scholar]
- 10. Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009; 373 (9674): 1570– 1575. [DOI] [PubMed] [Google Scholar]
- 11. Boa M, Mukaratirwa S, Willingham AL, Johansen MV. Regional action plan for combating Taenia solium cysticercosis/taeniosis in Eastern and Southern Africa. Acta Trop. 2003; 87 (1): 183– 6. [DOI] [PubMed] [Google Scholar]
- 12. Carabin H, Krecek R, Cowan L, Michael L, Foyaca-Sibat H, Nash T, et al. Estimation of the cost of Taenia solium cysticercosis in Eastern Cape Province, South Africa. Trop Med Int Health 2006; 11 (6): 906– 916. [DOI] [PubMed] [Google Scholar]
- 13. Zoli AP, Shey-Njila O, Nforninwe DN, Speybroeck N, Ito A, Sato MO, et al. Neurocysticercosis and epilepsy in Cameroon. Trans R Soc Trop Med Hyg. 2003; 97 (6): 683– 6. [DOI] [PubMed] [Google Scholar]
- 14. Abunna F, Tilahun G, Megersa B, Regassa A. Taeniasis and its socioeconomic implication in Awassa town and its surroundings, Southern Ethiopia. East Afr J Public Health. 2007; 4 (2): 73– 9. [PubMed] [Google Scholar]
- 15. Elmahdi I, Ali Q, Magzoub M, Ibrahim A, Saad M, Romig T. Cystic echinococcosis of livestock and humans in central Sudan. Ann Trop Med Parasitol. 2004; 98 (5): 473– 9. [DOI] [PubMed] [Google Scholar]
- 16. Sheiban J, Rezaian M. Study on intestinal protozoa in seven villages of Bandarabass. Iran J Public Health. 1981; 10 (1–4): 45– 54. [Google Scholar]
- 17. Rezaeian M, Saraei M, A survey of the prevalence of human parasites in rural areas of Lahijan . Iran J Public Health. 1992 . ; 4 ( 1 ): 29 –35. [Google Scholar]
- 18. Rezaiian M, Hooshyar H. The prevalence of intestinal parasitic infection in rural areas of Tonekabon, Iran. Iran J Public Health. 1996; 25 (3–4): 47– 58. [Google Scholar]
- 19. Jalayer T, Farid H, Katiraei A. Prevalence of intestinal parasitic infection in Dorchepiaz, Isfahan. Iran J Public Health. 1977; 6 (1): 9– 15. [Google Scholar]
- 20. Daryani EN. Prevalence of intestinal parasitic infection in Khuzestan province. J Gasteros. 2007; 12 (4): 218– 19. [Google Scholar]
- 21. Yoder JS, Beach MJ, Centers for Disease Control and Prevention (CDC) Giardiasis surveillance—United States, 2003–2005. MMWR Surveill Summ. 2007; 56 (7): 11– 8. [PubMed] [Google Scholar]
- 22. Hellard ME, Sinclair MI, Hogg GG, Fairley CK. Prevalence of enteric pathogens among community based asymptomatic individuals. J Gastroenterol Hepatol. 2000; 15 (3): 290– 293. [DOI] [PubMed] [Google Scholar]
- 23. Taylor DN, Houston R, Shlim DR, Bhaibulaya M, Ungar BL, Echeverria P. Etiology of diarrhea among travelers and foreign residents in Nepal. JAMA. 1988; 260 (9): 1245– 8. [PubMed] [Google Scholar]
- 24. Zia-Al N, Masood J. A survey of the prevalence of intestinal parasities in the city of Kerman. J Kerman Univ Med Sci. 1996; 3 (3): 129– 34. [Google Scholar]
- 25. Arani AS, Alaghehbandan R, Akhlaghi L, Shahi M, Lari AR. Prevalence of intestinal parasites in a population in south of Tehran, Iran. Rev Inst Med Trop São Paulo. 2008; 50 (3): 145– 9. [DOI] [PubMed] [Google Scholar]
- 26. Soosaraie M, Pagheh A, Gholami S. Prevalence of Intestinal Parasitic Infections in Rehabilitation Centers in Golestan Province, Iran. Med Lab J. 2014; 8 (1): 42– 7. [Google Scholar]
- 27. Aza N, Ashley S, Albert J. Parasitic infections in human communities living on the fringes of the Crocker Range Park Sabah, Malaysia. ASEAN Review of Biodiversity and Environmental Conservation (ARBEC). 2003 . Jan ; 1 –4. [Google Scholar]
- 28. Okyay P, Ertug S, Gultekin B, Onen O, Beser E. Intestinal parasites prevalence and related factors in school children, a western city sample-Turkey. BMC Public Health. 2004; 4: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]