Abstract
Background:
The aim of this study was to determine the prevalence of the Nosema ceranae and Nosema apis among apiaries using both spore counts and multiplex PCR and the replacement of N. apis by N. ceranae in some regions of Turkey.
Methods:
A hundred honey bee samples were collected from 99 apiaries in 11 different locations in 2011–2012 in Turkey. Nosema infection degree from collected samples was determined using light microscope and molecular detection of Nosema spp. (N. ceranae and N. apis) was performed using specific primers by multiplex PCR.
Results:
N. ceranae was only found spores in sampling areas using molecular diagnosis. N. apis was not detected in whole sampling areas using both techniques. There are no Nosema spores detected in Konya one location using two techniques. The nucleotide sequences from amplification products of the Nosema infested honeybee samples were (98%) identical with the sequence of N. ceranae for many countries deposited in the GenBank database in this study.
Conclusion:
The present study illustrated that N. ceranae is the only spores for sampled areas in 2011–2012. The study could also indicate that N. ceranae has been replaced instead of N. apis in Turkey. In addition, the prevalence of N. ceranae and two microsporodia spores effects on honey bee colonies in Turkey were needed to determine with intensive sampling, periodically.
Keywords: Nosema apis, Nosema ceranae, Honey bees, Molecular diagnosis, Turkey
Introduction
The microsporodia having more than 160 genera and 1300 species has been determined from insects and other species (1–3). The most majority of Nosema species from microsporidia are parasitic for invertabrate (4). In honey bees, N. apis and N.ceranae are called microsporidia and found in adult bees (2). Previously, it was thought that N. apis, caused nosema disease, was specific for Apis mellifera but N. ceranae was found only in Apis ceranae. For the last decade, N. ceranae has been found widespread in Europe, Africa, North America and Australia (5–15).
The life cycles of two Nosema spores are similar but both are distinguished from each other using molecular diagnostic methods. Generally, nosema causes digestive system disorders, shortening the life span, decreasing pollen collection, reducing the colony population size and honey production, increasing the dead bees behind the hive entrance and the losses of colonies (16). The spores spread by using contaminated beekeeping equipments, poor beekeeping practices, temperature fluctuations and the movement of honey bee colonies (17). For the Western honey bee, these two microsporidian species are pathogenic (18, 19). Although, N. apis infection is restricted to the adult bees midgut epithelium (20), N. ceranae also was infect other tissues (21). The type C nosemosis caused by N. ceranae is one of the most prevalent bee pathogens (22, 23). Many factors such as weather conditions, host susceptibility, the age of the bees and beekeeping practices may contribute different effects of N. ceranae on infected colonies (16). Furthermore, the N. apis replacement by N. ceranae was reported by many researchers in the world (13, 22, 23–25).
The molecular diagnosis was performed in order to distinguish N. apis from N. ceranae in honey bees from Turkey in 2010. That was the first time which indicated the presence of N. ceranae in these studies and samples were collected in 2005–06, 07–08 and 09 periods in these studies (8, 26, 27).
In the present study, our goals were to gain a better understanding of the prevalence of the N. ceranae and N. apis among apiaries using both spore counts and multiplex PCR and also the replacement of N. apis by N. ceranae in some regions of Turkey compared with previous studies.
Materials and Methods
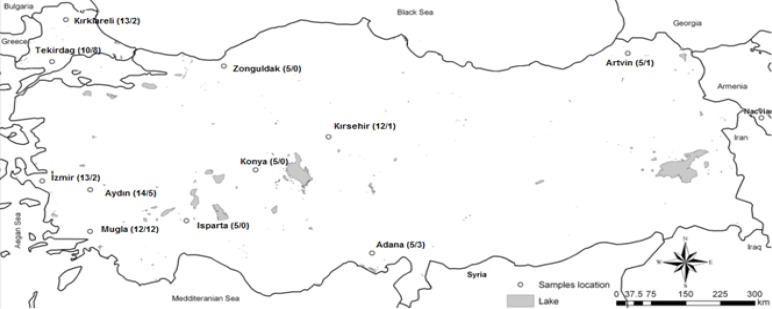
Samples were collected from 99 apiaries in eleven different locations in 2011–2012 in Turkey (Fig. 1). A hundred honey bee samples were collected from each apiary. Nosema infection degree from collected samples was determined using light microscope and molecular detection of Nosema spp. (N. ceranae and N. apis) was performed using specific primers.
Fig. 1:
Map of sample collection sites in Turkey. The total numbers of apiaries / infected apiaries for Nosema spores using microscopic diagnose were given in parentheses
Determination of Nosema infection level
Abdomens of twenty adult bees from a single colony were crushed in 10 ml. of distilled water. The suspended samples were filtered through two layers of muslin and centrifuged at 1000 g for 5 min. and the supernatants were removed. Pellets were resuspended in 10 ml of distilled water. Each homogenate was microscopically examined for the presence of Nosema spp. spores at 400X magnification and the spores were counted on the haemocytometer (28).
Molecular detection of Nosema spp.
Each homagenate was kept at 4 °C. Total DNA was extracted from samples using DNA isolation kit (Fermentas K512) and isolated DNA was analyzed in order to confirm the Nosema species of the spores by PCR as previously described using 218MITOC FOR/218MITOC REV and 321APIS FOR/32-1APIS REV primers specific for N. ceranae or N. apis, respectively (29). Multiplex PCR reaction mix contains 10 ng DNA, 0.5 U Taq DNA polymerase (Fermentas), 10XPCR buffer (Fermentas), 0.3 mM of each dNTP (Fermentas) 2.5 mM MgCl 2 (Fermentas), 0.4 μM primers and high pure H 2 O as required in order to make up to 20 μl total volume. The PCR protocol was 2 min at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at 55 °C, and 1 min at 72 °C and a final extension step at 72 °C for 5 min. PCR amplification was detected agarose gel (1.5%) electrophoresis and was visualized by ultraviolet (UV) after ethidium bromide staining (29). The positive PCR products were compared with positive for N. ceranae and N. apis provided from Etlik Veterinary Control Central Research Institute. Three positive PCR products for N. ceranae were sequenced and the sequence similarity analyses were performed using BLAST database search.
Results
The microscopic examination results of Nosema spores in samples were illustrated in Table 1 and Fig. 1. The highest percentages (100%) of Nosema positive samples were found in Muğla located in the southwestern part of Anatolia having typical Mediterranean climatic condition. There were no Nosema spores observed in three locations (Isparta, Konya and Zonguldak) in microscopic diagnosis. The lowest percentage (8.3%) of Nosema spores was detected in Kırşehir located in the central part of Anatolia. After microscopic diagnosis, the homogenates from all different 99 apiaries were used for DNA isolation and N. apis and N. ceranae were investigated by multiplex PCR.
Table 1.
Results of light microscopy examination and PCR results for Nosema spp. spores in common samples originating from each location in Turkey
| Locations | # of Apiaries | Negative | Positive | ||||
|---|---|---|---|---|---|---|---|
| (N) | (n−) | (%) | (n+) | (%) | N. ceranae | N. Apis | |
| İzmir | 13 | 11 | 84.6 | 2 | 15.4 | Yes | No |
| Aydın | 14 | 9 | 64.3 | 5 | 35.7 | Yes | No |
| Muğla | 12 | 0 | 0.0 | 12 | 100.0 | Yes | No |
| Tekirdag | 10 | 2 | 20.0 | 8 | 80.0 | Yes | No |
| Kırklareli | 13 | 11 | 84.6 | 2 | 15.4 | Yes | No |
| Zonguldak | 5 | 5 | 100.0 | 0 | 0.0 | Yes | No |
| Artvin | 5 | 4 | 80.0 | 1 | 20.0 | Yes | No |
| Isparta | 5 | 5 | 100.0 | 0 | 0.0 | Yes | No |
| Adana | 5 | 4 | 80.0 | 3 | 60.0 | Yes | No |
| Konya | 5 | 5 | 100.0 | 0 | 0.0 | No | No |
| Kırşehir | 12 | 11 | 91.7 | 1 | 8.3 | Yes | No |
(N=Total number of Apiaries; (n−)= Negative number of infected colonies; (n+)= Positive number of infected colonies)
Molecular diagnosis of the samples illustrated that N. ceranae spore was the only Nosema species found to honeybees from in Turkey (Fig. 2).
Fig. 2:
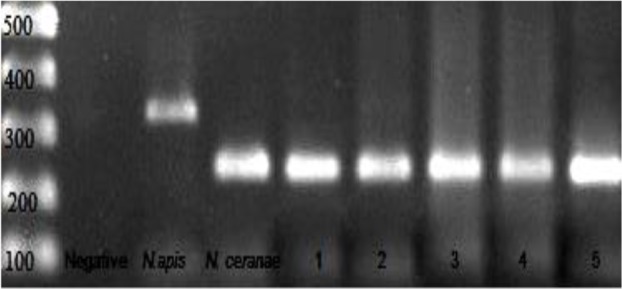
PCR products of negative, positive controls and infected colonies: Line 1: 100bp DNA ladder, line 2: negative control, line 3: N. apis, line 4: N. ceranae, line 5–9: nosema positive samples
The main point is that all samples were negative for N. apis despite of the presence of N. apis illustrating in previous studies. The positive samples were from beekeepers located in Aegean, Mediterranean, Thrace, Black Sea and Central Anatolia regions in Turkey. There is no Nosema spore detected in Konya (in Central Anatolia) using both microscopic and molecular diagnosis. Other important point is that Nosema spores were not observed in Isparta and Zonguldak honey bee samples in microscopic diagnoses; however, N. ceranae was detected in samples using multiplex PCR. The nucleotide sequences of amplification products from the Nosema infested samples were (98%) identical with the sequence of N. ceranae from many countries deposited in the Gen-Bank database in this study. A nucleotide blast illustrated that N. ceranae samples from Turkey was highly identical (98%) from N. ceranae sequences from many European countries (Lithuania, Poland, France, Italy, Germany and Austria), Morrocco, Lebonan, Iran, Mexico, Argentina, Australia and Thailand.
Discussion
The presence of N. ceranae and N. apis in Turkey were determined using molecular techniques in previous studies (8, 26, 27). The sampling period of these studies included 2005 to 2009. The detection of the two Nosema spores was important suspicious for colony losses in Turkey in those periods. Because N. ceranae were detected in Artvin, Muğla and Hatay provinces (8) and high rates of colony losses were detected by Giray et al. (30). At that point, Whitaker et al. (8) mentioned that “Though the cause of the colony losses cannot be conclusively attributed to N. ceranae infection, there were a potentially significant relationship between its presence and the occurrence of the losses”.
Besides, N. ceranae is more pathogen than N. apis and it has been spread all over the world (8, 13, 22, 24). In 2005 and 2006, N. ceranae was detected from three samples from the Hatay, Muğla and Artvin provinces (8). N. apis was detected in samples from the Sivas, Izmir, Gaziantep and Bitlis provinces (8). In the samples from Black Sea (Samsun and Giresun), N. ceranae were detected in 2007 and 2008 (26). The other study informed the presence of N. cerana and N. apis in Hatay and southeastern Marmara region between 2007 and 2009 (27).
The present study results illustrated that N. ceranae is the only Nosema species detected into infect honey bee in Turkey and N. apis were not detected using both techniques for collected samples between 2011–2012. But, the presence of N. apis was informed in previous studies (8, 26, 27). The other study illustrated the presence of N. ceranae in the samples of migratory beekeepers but not within the samples of local beekeepers (31). They also concluded that the infectivity of N. ceranae expands with migratory beekeeping activities (31). However, the samples of the present study were collected mostly in local beekeepers in Zonguldak, Isparta, Izmir, Kırşehir and others except Muğla and N. ceranae spores found nearly all locations except Konya samples, which were also collected from local beekeepers. The present study does not support that N. ceranae expands with migratory beekeeping activities or found in migratory beekepers. The recent studies indicated the replacement of N. apis by N. ceranae in many regions in Turkey (31, 32).
The present results illustrated that N. ceranae is the only spore in sampled areas which is found instead of N. apis in Turkey. The sequence results for present study also showed high-level identity of N. ceranae sequences from many European countries Morrocco, Lebanon, Iran, Mexico, Argentina, Australia and Thailand. This result can be explained with the properties of pathogen, which has high level of pathogenicity and transmission rate. The other reason of N. ceranae, which is the only spores in sampled areas, could be explained the lack of symptoms of N. ceranae led to insufficient attention or neglect nosemosis of beekeepers (12, 17).
Conclusion
N. ceranae is the only spores for sampled areas in 2011–2012. The study could also indicate that N. ceranae has been replaced instead of N. apis in Turkey according to sampling areas. Also the prevalence of N. ceranea and two microsporidia spores effects on honey bee colonies in Turkey were needed to determine with intensive sampling, periodically.
Acknowledgments
The authors declare that there is no conflict of interest.
References
- 1. Canning EU. Microsporidia. In: Kreier JP, Baker JR, editors. Parasitic protozoa. New York : : Academic Press ; ; 1993. . p. 299 –385. [Google Scholar]
- 2. Chen YP, Evans JD, Murphy C, Gutell R, Zuker M, Gundensen-Rindal D, Pettis JS. Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera. J Eukaryot Microbiol. 2009. ; 56 : (2 ): 142 –7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh T, Bhat MM, Khan MA. Microsporidiosis in the Silkworm, Bombyx mori L. (Lepidoptera: Bombycidae). Pertanika J Trop Agric Sci. 2012; 35 (3): 387– 406. [Google Scholar]
- 4. Canning EU, Curry A, Cheney SA, Lafranchi-Tristem NJ, Kawakami Y, Hatakeyama Y, Iwano H, Ishihara R. Nosema tyriae and Nosema sp., microsporidian parasites of cinnabar moth Tyria jacobaeae. J Invertebr Pathol. 1999; 74 (1): 29– 38. [DOI] [PubMed] [Google Scholar]
- 5. Higes M, Martin R, Meana A. Nosema ceranae, a new microsporidian parasite in honey bees in Europe. J Invertebr Pathol. 2006; 92 (2): 93– 5. [DOI] [PubMed] [Google Scholar]
- 6. Higes M, Martin-Hernandez R, Garrido-Bailon E, Gonzalez-Porto AV, Garcia-Palencia P, Meana A, Del Nozal MJ, Mayo R, Bernal JL. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Env Microbiol Rep. 2009; 1: 110– 3. [DOI] [PubMed] [Google Scholar]
- 7. Huang WF, Jiang JH, Chen YW, Wang CH. A Nosema ceranae isolate from the honeybees Apis mellifera. Apidologie 2007; 38: 30– 7. [Google Scholar]
- 8. Whitaker J, Szalanski AL, Kence M. Molecular detection of Nosema ceranae and N. apis from Turkish honey bees. Apidologie 2011; 42: 174– 80. [Google Scholar]
- 9. Chauzat MP, Carpentier P, Martel AC, Bougeard S, Cougoule N, Porta P, Lachaıze J, Madec F, Aubert M, Faucon JP. Influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environ Entomol. 2009; 38 ( 3): 514– 23. [DOI] [PubMed] [Google Scholar]
- 10. Williams GR, Shafer ABA, Rogers REL, Shutler D, Stewart DT. First detection of Nosema ceranae, a microsporidian parasite of European honeybees (Apis mellifera) in Canada and central USA. J Invertebr Pathol. 2008; 97 ( 2): 189– 92. [DOI] [PubMed] [Google Scholar]
- 11. Klee J, Besana A, Genersch E, Gisder S, Nanetti A, Tam DQ, Chinh TX, Puerta F, Ruz JM, Kryger P, Message D, Hatjına F, Korpela S, Fries I, Paxton RJ. Widespread dispersal of the microsporidium Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J Invertebr Pathol. 2007; 96 ( 1): 1– 10. [DOI] [PubMed] [Google Scholar]
- 12. Martin-Hernandez R, Meana A, Prieto L, Martinez-Salvador A, Garrido-Bailon E, Higes M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl Environ Microbiol. 2007; 73: 6331– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paxton RJ, Klee J, Korpela S, Fries I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007; 38: 558– 65. [Google Scholar]
- 14. Tapaszti Z, Forgach P, Kovago C, Bekesi L, Bakonyi T, Rusvai M. First detection and dominance of Nosema ceranae in Hungarian honeybee colonies. Acta Vet Hung. 2009; 57: 383– 8. [DOI] [PubMed] [Google Scholar]
- 15. Giersch T, Berg T, Galea F, Hornitzky M. Nosema ceranae infects honey bees (Apis mellifera) and contaminates honey in Australia. Apidologie 2009; 40: 117– 23. [Google Scholar]
- 16. Higes M, Meana A, Bartolome C, Botias C, Martin-Hernandez R. Nosema ceranae (Micro-sporida), a controversial 21st century honey bee pathogen. Environ Microbiol Rep. 2013; 5: 17– 29. [DOI] [PubMed] [Google Scholar]
- 17. Gajger TV, Vugrek O, Grilec OD, Petrinec Z. Prevalence and distribution of Nosema ceranae in Croatian honeybee colonies. Vet Med. 2010; 55 (9): 457– 62. [Google Scholar]
- 18. Fries I, Feng F, Da Silva A, Slemenda SB, Pieniazek NJ. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis ceranae (Hymenoptera, Apidae). Eur J Protistol. 1996; 32: 356– 65. [Google Scholar]
- 19. Fries I, Martin R, Meana A, Garcia-Palencia P, Higes M. Natural infections of Nosema ceranae in European honey bees. J Apic Res. 2006; 45: 230– 3. [Google Scholar]
- 20. Fries I. The intracellular development of Nosema apis Z. Apidologie 1989; 20: 502– 3. [Google Scholar]
- 21. Gisder S, Hedtke K, Möckel N, Frielitz MC, Linde A, Genersch E. Five-year cohort study of Nosema spp. in Germany: does climate shape virulence and assertiveness of Nosema ceranae? Appl Environ Microbiol. 2010; 76 (9): 3032– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higes M, Martin-Hernandez R, Meana A. Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 2010; 41: 375– 92. [Google Scholar]
- 23. Fries I. Nosema ceranae in European honey bees (Apis mellifera). J Invertebr Pathol. 2010. ; 103 : Suppl 1 : S73 –S79. [DOI] [PubMed] [Google Scholar]
- 24. Paxton RJ. Does infection by Nosema ceranae cause “colony collapse disorder” in honey bees (Apis mellifera)? J Apic Res. 2010. ; 49 : 80 –4. [Google Scholar]
- 25. Martin-Hernandez R, Meana A, Garcia-Palencia P, Marin P, Botias C, Garrido-Bailon E, Barrios L, Higes M. Effect of temperature on the biotic potential of honeybee microsporidia. Appl Environ Microbiol. 2009; 75: 2554– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Utuk AE, Piskin FC, Kurt M. First molecular detection of Nosema ceranae in Turkey. Vet J Ankara Univ. 2010; 57: 275– 8. [Google Scholar]
- 27. Muz MN, Girisgin AO, Muz D, Aydin L. Molecular detection of Nosema ceranae and Nosema apis infections in Turkish apiaries with collapsed colonies. J Apic Res. 2010; 49 (4): 342. [Google Scholar]
- 28. Office International des Epizooties (OIE) (2008. ) Manual of Standards for Diagnostic Test and Vaccines. http://www.oie.int/doc/ged/D7710.PDF . [DOI] [PubMed]
- 29. Fries I, Chauzat MP, Chen YP, Doublet V, Genersch E, Gisder S, Higes M, Mcmahon DP, Martinhernandez R, Natsopoulou M, Paxton RJ, Tanner G, Webster TC, Williams GR. Standard methods for Nosema research. J Apic Res. 2013; 52 (1): 1– 28. [Google Scholar]
- 30. Giray T, Kence M, Oskay D, Doke MA, Kence A. Scientific note: colony losses survey in Turkey and causes of bee deaths. Apidologie. 2010; 41: 451– 3. [Google Scholar]
- 31. Tozkar CO, Kence M, Evans J, Kence A. Identification of Nosema spp among honey bees from different regions of Turkey. WG2 Workshop: Nosema, from knowledge to experimental setup, 2012, Istanbul, Turkey 3rd–4th of March p. 25 . [Google Scholar]
- 32. Yalçinkaya A, Martin-Hernandez R, Higes M, Ozkirim A. Epidemiology of Nosema spp. in Turkey. WG2 Workshop: Nosema, from knowledge to experimental setup 2012– Istanbul, Turkey 3rd–4th of March p. 27. [Google Scholar]