Abstract
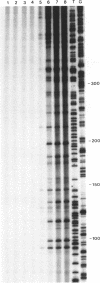
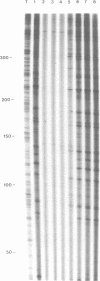
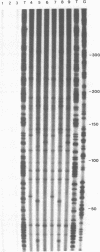
IodoHoechst 33258 sensitizes DNA to cleavage by near ultraviolet light (UV-A). Following an earlier study which showed that the UV-induced cleavage is at discrete locations corresponding to the ligand binding sites, this paper reports a more extensive analysis of the sequence specificity of cleavage. The experiments involved use of double-stranded DNA synthesised on primed M13 templates. Analysis of cleavage in a 280bp sequence in M13mp18 and a 310bp sequence in three M13mp9 clones ('alpha-32', 'alpha-82' and 'alpha-22') derived from human alpha-DNA, showed that for all of the twenty-nine strong and very strong damage sites, cleavage was at the 3' end of a run of three or more consecutive AT base pairs. The extent of cleavage was higher for sites with consecutive Ts than for consecutive As, and greatest for the sequence cTTTTca. Comparison of three closely-related alpha-DNA clones enabled assessment of single bp changes and essentially confirmed the results of detailed analysis of binding cleavage sites in mp18 and alpha-32. Decreasing the input ratio of iodoHoechst/per bp DNA from 0.13 to 0.013 altered the sequence specificity, and sites possessing only three consecutive AT bps were generally not cleaved. The contributions of both the strength of ligand binding and the efficiency of photolytic cleavage to the overall extent of cleavage by UV/iodoHoechst are discussed, in view of the potential utility of the ligand as a probe of DNA conformation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrondo M. A., Coll M., Aymami J., Wang A. H., van der Marel G. A., van Boom J. H., Rich A. Binding of a Hoechst dye to d(CGCGATATCGCG) and its influence on the conformation of the DNA fragment. Biochemistry. 1989 Sep 19;28(19):7849–7859. doi: 10.1021/bi00445a047. [DOI] [PubMed] [Google Scholar]
- Charnas R. L., Goldberg I. H. Neocarzinostatin abstracts a hydrogen during formation of nucleotide 5'-aldehyde on DNA. Biochem Biophys Res Commun. 1984 Jul 31;122(2):642–648. doi: 10.1016/s0006-291x(84)80081-9. [DOI] [PubMed] [Google Scholar]
- Chin D. H., Kappen L. S., Goldberg I. H. 3'-Formyl phosphate-ended DNA: high-energy intermediate in antibiotic-induced DNA sugar damage. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7070–7074. doi: 10.1073/pnas.84.20.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fede A., Labhardt A., Bannwarth W., Leupin W. Dynamics and binding mode of Hoechst 33258 to d(GTGGAATTCCAC)2 in the 1:1 solution complex as determined by two-dimensional 1H NMR. Biochemistry. 1991 Dec 3;30(48):11377–11388. doi: 10.1021/bi00112a004. [DOI] [PubMed] [Google Scholar]
- Harshman K. D., Dervan P. B. Molecular recognition of B-DNA by Hoechst 33258. Nucleic Acids Res. 1985 Jul 11;13(13):4825–4835. doi: 10.1093/nar/13.13.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A. L., Bostock C. J., Bak A. L. Chromosome-specific subfamilies within human alphoid repetitive DNA. J Mol Biol. 1986 Jan 20;187(2):185–196. doi: 10.1016/0022-2836(86)90227-5. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Deoxyribonucleic acid damage by neocarzinostatin chromophore: strand breaks generated by selective oxidation of C-5' of deoxyribose. Biochemistry. 1983 Oct 11;22(21):4872–4878. doi: 10.1021/bi00290a002. [DOI] [PubMed] [Google Scholar]
- Martin R. F., Holmes N. Use of an 125I-labelled DNA ligand to probe DNA structure. 1983 Mar 31-Apr 6Nature. 302(5907):452–454. doi: 10.1038/302452a0. [DOI] [PubMed] [Google Scholar]
- Martin R. F., Murray V., D'Cunha G., Pardee M., Kampouris E., Haigh A., Kelly D. P., Hodgson G. S. Radiation sensitization by an iodine-labelled DNA ligand. Int J Radiat Biol. 1990 May;57(5):939–946. doi: 10.1080/09553009014551061. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989 Nov 11;17(21):8889–8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray V., Martin R. F. Nucleotide sequences of human alpha-DNA repeats. Gene. 1987;57(2-3):255–259. doi: 10.1016/0378-1119(87)90129-6. [DOI] [PubMed] [Google Scholar]
- Murray V., Martin R. F. Sequence specificity of 125I-labelled Hoechst 33258 damage in six closely related DNA sequences. J Mol Biol. 1988 Sep 5;203(1):63–73. doi: 10.1016/0022-2836(88)90091-5. [DOI] [PubMed] [Google Scholar]
- Murray V., Martin R. F. Sequence specificity of 125I-labelled Hoechst 33258 in intact human cells. J Mol Biol. 1988 May 20;201(2):437–442. doi: 10.1016/0022-2836(88)90150-7. [DOI] [PubMed] [Google Scholar]
- Murray V., Martin R. F. The degree of ultraviolet light damage to DNA containing iododeoxyuridine or bromodeoxyuridine is dependent on the DNA sequence. Nucleic Acids Res. 1989 Apr 11;17(7):2675–2691. doi: 10.1093/nar/17.7.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Parkinson J. A., Barber J., Douglas K. T., Rosamond J., Sharples D. Minor-groove recognition of the self-complementary duplex d(CGCGAATTCGCG)2 by Hoechst 33258: a high-field NMR study. Biochemistry. 1990 Nov 6;29(44):10181–10190. doi: 10.1021/bi00496a005. [DOI] [PubMed] [Google Scholar]
- Pjura P. E., Grzeskowiak K., Dickerson R. E. Binding of Hoechst 33258 to the minor groove of B-DNA. J Mol Biol. 1987 Sep 20;197(2):257–271. doi: 10.1016/0022-2836(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Portugal J., Waring M. J. Assignment of DNA binding sites for 4',6-diamidine-2-phenylindole and bisbenzimide (Hoechst 33258). A comparative footprinting study. Biochim Biophys Acta. 1988 Feb 28;949(2):158–168. doi: 10.1016/0167-4781(88)90079-6. [DOI] [PubMed] [Google Scholar]
- Quintana J. R., Lipanov A. A., Dickerson R. E. Low-temperature crystallographic analyses of the binding of Hoechst 33258 to the double-helical DNA dodecamer C-G-C-G-A-A-T-T-C-G-C-G. Biochemistry. 1991 Oct 22;30(42):10294–10306. doi: 10.1021/bi00106a030. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Teng M. K., Usman N., Frederick C. A., Wang A. H. The molecular structure of the complex of Hoechst 33258 and the DNA dodecamer d(CGCGAATTCGCG). Nucleic Acids Res. 1988 Mar 25;16(6):2671–2690. doi: 10.1093/nar/16.6.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngquist R. S., Dervan P. B. Sequence-specific recognition of B-DNA by oligo(N-methylpyrrolecarboxamide)s. Proc Natl Acad Sci U S A. 1985 May;82(9):2565–2569. doi: 10.1073/pnas.82.9.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein N., Sinha A. M., McGahren W. J., Ellestad G. A. Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science. 1988 May 27;240(4856):1198–1201. doi: 10.1126/science.3240341. [DOI] [PubMed] [Google Scholar]