Our study shows that the acute impairment of enteric glial metabolism with fluoroacetate (FA) alters specific glial functions that are associated with the modification of neurotransmission in the gut. These include subtle changes to glial agonist-evoked calcium signaling, the subsequent disruption of connexin-43 hemichannels, and changes in protein expression that are consistent with a transition to reactive glia. These changes in glial function offer a mechanistic explanation for the effects of FA on peripheral neuronal networks.
Keywords: autonomic nervous system, enteric glial cells, enteric nervous system, glia, intestine
Abstract
Glia play key roles in the regulation of neurotransmission in the nervous system. Fluoroacetate (FA) is a metabolic poison widely used to study glial functions by disrupting the tricarboxylic acid cycle enzyme aconitase. Despite the widespread use of FA, the effects of FA on essential glial functions such as calcium (Ca2+) signaling and hemichannel function remain unknown. Therefore, our goal was to assess specifically the impact of FA on essential glial cell functions that are involved with neurotransmission in the enteric nervous system. To this end, we generated a new optogenetic mouse model to study specifically the effects of FA on enteric glial Ca2+ signaling by crossing PC::G5-tdTomato mice with Sox10::creERT2 mice. FA did not change the peak glial Ca2+ response when averaged across all glia within a ganglion. However, FA decreased the percent of responding glia by 30% (P < 0.05) and increased the peak Ca2+ response of the glial cells that still exhibited a response by 26% (P < 0.01). Disruption of Ca2+ signaling with FA impaired the activity-dependent uptake of ethidium bromide through connexin-43 (Cx43) hemichannels (P < 0.05) but did not affect baseline Cx43-dependent dye uptake. FA did not cause overt glial or neurodegeneration, but glial cells significantly increased glial fibrillary acid protein by 56% (P < 0.05) following treatment with FA. Together, these data show that the acute impairment of glial metabolism with FA causes key changes in glial functions associated with their roles in neurotransmission and phenotypic changes indicative of reactive gliosis.
NEW & NOTEWORTHY Our study shows that the acute impairment of enteric glial metabolism with fluoroacetate (FA) alters specific glial functions that are associated with the modification of neurotransmission in the gut. These include subtle changes to glial agonist-evoked calcium signaling, the subsequent disruption of connexin-43 hemichannels, and changes in protein expression that are consistent with a transition to reactive glia. These changes in glial function offer a mechanistic explanation for the effects of FA on peripheral neuronal networks.
the partnership between neurons and glia is increasingly recognized as an essential aspect of nervous system function. Glia and, in particular, astrocytes are intimately entwined within neuronal networks and play key roles in the regulation of neuron function and survival [for reviews, see Fellin and Carmignoto (2004); Khakh and Sofroniew (2015); Nimmerjahn (2009); Pekny et al. (2016); Robel and Sontheimer (2016)]. For example, astrocytes have emerged as important regulators of ion homeostasis (Kofuji and Newman 2004; Simard and Nedergaard 2004), neuroprotection (Desagher et al. 1996), and neurotransmission (Gordon et al. 2009; Keyser and Pellmar 1994; Perea and Araque 2005; Schousboe et al. 2004). Many of the key roles of neuroglia in synaptic transmission within the central and peripheral portions of the nervous system are thought to depend on glial activity encoded by fluxes in intracellular calcium concentration ([Ca2+]i) [for examples, see Agulhon et al. (2013); Martín et al. (2015); McClain et al. (2015); Sasaki et al. (2014); Scofield et al. (2015); Wang et al. (2012) and the following reviews: Bazargani and Attwell (2016); Shigetomi et al. (2016)]. It is now clear that glial cells respond to neurotransmitters that are released during synaptic activity through pathways that lead to [Ca2+]i responses. However, the interpretation of the role of glial activity in the regulation of central and peripheral neural networks has been challenging, and results both support (Agulhon et al. 2013; Cao et al. 2013; Martín et al. 2015; McClain et al. 2015; Scofield et al. 2016) and refute (Agulhon et al. 2010; Bonder and McCarthy 2014; Petravicz et al. 2008) major glial contributions.
A portion of the debate surrounding the role of glial cells in neurotransmission has been fueled by a poor understanding of the common model organisms and experimental reagents used to probe glial functions. Although new tools are emerging with high temporal and spatial precision toward glia, many older, more coarse techniques are still widely used. For example, fluoroacetate (FA) and its metabolite fluorocitrate (FC) are important tools that have been used to define the role of astrocytes in brain metabolism (Fonnum et al. 1997; Hassel et al. 1994; Swanson and Graham 1994) and neurotransmission (Clarke 1991; Fonnum et al. 1997; Gordon et al. 2005; Hassel et al. 1994; Keyser and Pellmar 1994; Swanson and Graham 1994). FA and FC are thought to be preferentially taken up by astrocytes and disrupt glial metabolism through effects on the tricarboxylic acid (TCA) cycle enzyme aconitase (Fonnum et al. 1997; Hassel et al. 1992; Paulsen et al. 1987; Swanson and Graham 1994; Willoughby et al. 2003). FA and FC are still widely used to test experimentally the role of glial cells in central (Henneberger et al. 2010; Letellier et al. 2016; Pougnet et al. 2014) and peripheral (MacEachern et al. 2015; Nasser et al. 2006; Wang et al. 2016) neural networks. Despite the widespread use of FA, the actual effects of FA on essential glial functions such as Ca2+ signaling and gliotransmitter release remain questionable.
We addressed this issue by testing the effects of FA on glial Ca2+ signaling, hemichannel function, and reactivity in the enteric nervous system (ENS). The ENS is ideally suited for this type of study because enteric neurons and glia share a similar functional arrangement as neurons and astrocytes in the brain (Gabella 1976), enteric glia and astrocytes both depend on the TCA cycle for metabolic support in adult animals (Krum 1995), and the ENS can be studied in live preparations that maintain network integrity (Broadhead et al. 2012). Importantly, enteric glia respond to neuronal activity with [Ca2+]i responses (Boesmans et al. 2013; Gulbransen and Sharkey 2012) that subsequently enact hemichannel-dependent mechanisms to modify neuronal activity (McClain et al. 2014, 2015). Several studies have used FA and FC to test the roles of enteric glia in peristalsis and gastrointestinal disease (MacEachern et al. 2015; Nasser et al. 2006; Wang et al. 2016), but it is not clear if the acute exposure to FA/FC used in these prior studies has any effect on active glial signaling. We specifically tested this point by monitoring glial activity in samples exposed to FA. To study glial Ca2+ signaling accurately, we generated a novel mouse model where the expression of the optogenetic probe GCaMP5G is specifically targeted to enteric glial cells. Our data show that the acute inhibition of glial metabolism with FA does alter glial cell Ca2+ signaling, hemichannel function and expression of proteins including S100β and glial fibrillary acidic protein (GFAP). However, the effects of FA on glial Ca2+ signaling were relatively minor when viewed at a network level. These findings indicate that great caution is necessary when interpreting the results of studies using FA. Specifically, our results raise the possibility that the main acute effects of FA on intact systems may be due to both an impairment of Ca2+ signaling and a conversion of glia to a reactive state.
MATERIALS AND METHODS
Animals.
All experimental protocols were approved by the Michigan State University Institutional Animal Care and Use Committee. Sox10::creERT2 transgenic mice (C57BL/6 background) (Laranjeira et al. 2011) were a gift from Dr. Vassilis Pachnis (The Francis Crick Institute, London, UK). Sox10::creERT2 mice were bred with PC::G5-tdTomato (PC::G5-tdT) mice (Gee et al. 2014) [B6; 129S6-Polr2atm1(CAG-GCaMP5g,-tdT)Tvrd/J; Stock Number 024477; RRID:IMSR_JAX:024477; The Jackson Laboratory, Bar Harbor, ME] at Michigan State University to generate double-heterozygous mice Sox10::creERT2+/−; PC::G5-tdT+/− (hereafter referred to as Sox10-PC::G5-tdT). Mice of both sexes were used for experiments when they reached 8–12 wk of age, and littermates not expressing Cre (PC::G5-tdT+/− mice) were used as experimental controls (hereafter referred to as “background control”). Mice were genotyped by the Research Technology Support Facility at Michigan State University using standard PCR. Mice were maintained in a temperature-controlled environment (Innocage system with ALPHA-dri bedding; Innovive, San Diego, CA) on a 12-h light:dark cycle with access to acidified water and a minimal phytoestrogen diet (Diet Number 2919; Envigo, Indianapolis, IN) ad libitum. To induce GCaMP5G-tdT expression, mice were fed tamoxifen citrate (400 mg/kg) for 1 wk before experiments or by administration of tamoxifen-free base (1 mg/10 g body wt ip, twice/day for 2 days, in 1:10 ethyl alcohol:sunflower oil mixture), and experiments were performed 5 days after the final injection of tamoxifen.
Longitudinal muscle myenteric plexus whole-mount preparation.
Colons were carefully removed from euthanized mice and immediately placed in ice-cold DMEM/Ham's F-12 nutrient mixture (Thermo Fisher Scientific, Waltham, MA) containing 3 μM nicardipine and 1 μM scopolamine. Colons were then transferred to a Sylgard-coated petri dish (Dow Corning, Midland, MI) filled with chilled DMEM, secured with insect pins, and opened along the mesenteric border. The full-thickness colon was pinned flat, mucosa facing up, and the mucosa, submucosa, and circular muscle were removed by microdissection [see Fried and Gulbransen (2015) for a detailed description]. The resulting live whole mounts consist of an intact myenteric plexus lying atop longitudinal muscle.
Ca2+ imaging.
Live longitudinal muscle myenteric plexus (LMMP) whole mounts from background control mice not expressing GCaMP5G were loaded with Fluo-4 AM (4 μM in DMEM; Thermo Fisher Scientific) for 45 min at 37°C. Sox10-PC::G5-tdT LMMPs were incubated in DMEM for the same period. Fluorescence imaging used an upright BX51WI fixed-stage microscope (Olympus, Center Valley, PA) fitted with a 40× water-immersion objective (LUMPlan N, 0.8 numerical aperture) and a Lambda DG-4 Plus Xenon light source (Sutter Instrument, Novato, CA). Fluorescence of Fluo-4 and GCaMP5G was excited by light passed through a 485-nm, 20-nm band-pass filter and detected by reflected light passing through a 515-nm long-pass filter. tdT fluorescence was excited by light passed through a 535-nm, 20-nm band-pass filter and detected by reflected light passing through a 610-nm, 75-nm band-pass emission filter. Glial cells were identified by morphology in samples loaded with Fluo-4 as described previously (Gulbransen and Sharkey 2009) and by tdT fluorescence in Sox10-PC::G5-tdT samples. Images of Fluo-4 or GCaMP5G fluorescence were acquired at a rate of 1-0.5 Hz with a Neo sCMOS camera controlled by iQ2 software (Andor, South Windsor, CT). Whole mounts were continually superfused with 37°C Krebs buffer consisting of the following (in mM): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 HEPES, 21.2 NaHCO3, 1 pyruvic acid, 8 glucose (pH adjusted to 7.4 with NaOH), with 3 μM nicardipine and 1 μM scopolamine at a flow rate of 2–3 ml/min. Drugs were diluted in Krebs buffer and bath applied.
Ethidium bromide dye uptake.
Hemichannel activity was measured in enteric glia by ethidium bromide (EtBr) dye uptake as described by Orellana et al. (2010) for astrocytes. Briefly, LMMP whole mounts were prepared from background control mice and incubated with EtBr (5 μM in DMEM) for 10 min at 37°C. Extracellular EtBr was removed by washing the samples with fresh DMEM and EtBr fluorescence was immediately recorded using the Ca2+ imaging microscope described above with the tetramethylrhodamine filter set described for tdT. Basal hemichannel activity was measured as the EtBr fluorescence in nonstimulated glial cells (only exposed to EtBr). Stimulated hemichannel activity was measured as the EtBr fluorescence in glia cotreated with ADP (100 μM) during the 10-min EtBr incubation. The portion of EtBr uptake mediated by connexin-43 (Cx43) hemichannels was measured by preincubating tissue samples in the Cx43 mimetic peptide 43Gap26 (100 μM) (Boitano and Evans 2000; McClain et al. 2014) for 30 min. The effect of Ca2+ on EtBr uptake was determined by cotreatment of EGTA (1 mM) during the 10-min EtBr incubation.
Whole-mount immunohistochemistry.
Whole-mount LMMPs were fixed overnight in Zamboni's fixative at 4°C and processed for immunohistochemistry (IHC) as described previously (Gulbransen et al. 2012). Antibody details are supplied in Table 1. Briefly, LMMPs were rinsed three times (10 min each) in PBS containing 0.1% Triton X-100 followed by a 45-min incubation in blocking solution (containing 4% normal goat serum, 0.4% Triton X-100, and 1% BSA). Primary antibodies were diluted in blocking solution and applied overnight at room temperature. LMMPs were rinsed three times with PBS after removal of primary antibodies the following day, and secondary antibodies (diluted in blocking solution) were applied for 2 h at room temperature. Finally, LMMPs were rinsed in 0.1 M phosphate buffer and mounted on slides with bicarbonate-buffered glycerol consisting of a 1:3 mixture of 142.8 mM sodium bicarbonate and 56.6 mM carbonate to glycerol. Images were acquired through the 40× (0.75 numerical aperture, PlanFluor) objective of an upright epifluorescence microscope (Eclipse Ni; Nikon, Melville, NY) with a Retiga 2000R camera (QImaging, Surrey, BC, Canada) controlled by QCapture Pro 7.0 (QImaging).
Table 1.
Details of primary and secondary antibodies used in this study
| Antibody | Source | Dilution |
|---|---|---|
| Primary antibodies | ||
| Rabbit anti-Cx43 | Sigma-Aldrich, St. Louis, MO | 1:500 |
| Chicken anti-GFAP | Abcam, Cambridge, MA | 1:1,000 |
| Biotin mouse anti-HuC/D | Molecular Probes, Eugene, OR* | 1:200 |
| Rabbit anti-S100β | Abcam, Cambridge, MA | 1:200 |
| Secondary antibodies | ||
| Goat anti-rabbit Alexa Fluor 488 | Invitrogen, Carlsbad, CA* | 1:400 |
| Goat anti-chicken Alexa Fluor 568 | Invitrogen, Carlsbad, CA* | 1:400 |
| Streptavidin Alexa Fluor 594 | Jackson ImmunoResearch Laboratories, West Grove, PA | 1:400 |
Now Thermo Fisher Scientific, Waltham, MA.
Sodium FA treatment.
Glial metabolism was inhibited with the drug sodium FA as previously described by MacEachern et al. (2015) for enteric glia and summarized by Fonnum et al. (1997). Briefly, LMMP whole mounts were incubated with FA (5 mM dissolved in DMEM; 37°C; 5% CO2, 95% air) for 2 h before Ca2+ imaging and dye uptake experiments. For IHC experiments, whole mounts were exposed to FA in Krebs buffer for 2 h, rinsed with Krebs, and then incubated in Krebs buffer for an additional 2 h before being fixed overnight with Zamboni's fixative.
Chemicals and reagents.
ADP sodium salt, EtBr, sodium FA, tamoxifen-free base, and chemicals for Krebs buffer and IHC were purchased from Sigma-Aldrich (St. Louis, MO). Fluo-4 AM, DMEM, and Pluronic F127 were purchased from Thermo Fisher Scientific (Waltham, MA). 43Gap26 was purchased from AnaSpec (Fremont, CA).
Data analysis.
Raw Ca2+ imaging files were analyzed with Andor iQ2 software where regions of interest were drawn around enteric glial cells within a ganglion, and the relative fluorescence intensity was measured. Analysis and generation of traces were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Traces represent the average change in fluorescence (ΔF/F) over time [as described by Takahashi et al. (1999)] for all glial cells within a single ganglion. The half time to peak was calculated as one-half of the time of the peak response from the baseline [time of (Fmax − Fbase)/2]. The full width at half-maximum (FWHM) was calculated by measuring the width of the peak at one-half of the baseline. Half time to peak and FWHM are expressed as time in seconds. The activation profile was calculated by measuring the time to response in seconds of all glial cells after the first glial response within a ganglion.
EtBr dye uptake digital images were analyzed offline using ImageJ software (National Institutes of Health, Bethesda, MD). Regions of interest were drawn around enteric glial cells within a ganglion that we identified by morphology [as described by Gulbransen and Sharkey (2009)]. Mean gray values of enteric glial cells were measured to obtain a glial cell population average. EtBr data were performed on a minimum of 10 ganglia/animal from at least 3 mice. The total number of glial cells is represented by n values, and data are expressed as percent buffer control.
Cell counts and ganglionic expression data were analyzed offline using ImageJ software. Cell counts were performed using the cell counter plug-in of ImageJ software. Enteric neuron and glial cell numbers are presented as ganglionic packing density, which was calculated by tracing the ganglionic area and counting the number of HuC/D-immunoreactive neurons or S100β-immunoreactive glia within the defined ganglionic area. The relative ganglionic expression of S100β, GFAP, and Cx43 was measured by recording the mean gray values of S100β, GFAP, and Cx43 fluorescence within a defined ganglionic area. Fluorescence density (integrated density) was calculated as the product of area and mean gray value (McCloy et al. 2014) and is reported as the intensity in arbitrary fluorescence units per squared micrometer of the ganglionic area. Cell counts and ganglionic expression data were performed on a minimum of 10 ganglia per animal and averaged to obtain a value for that animal. The number of animals in each experiment is represented by n values, and data are expressed as percent buffer control.
Statistical analysis.
Data were analyzed using GraphPad Prism 5 and are shown as means ± SE. EtBr dye uptake was analyzed by one-way ANOVA with a Bonferroni post-test. Remaining data were analyzed by Student's t-test, and P < 0.05 was considered significant.
RESULTS
Glial Ca2+ signaling is important in the regulation of neural networks in the periphery (Agulhon et al. 2013; McClain et al. 2015) and central nervous system (Cao et al. 2013; Martín et al. 2015; Scofield et al. 2016). FA is commonly used as an experimental tool to disrupt glial functions, but its impact on glial Ca2+ signaling is not known. Therefore, our first goal was to determine how the acute inhibition of glial metabolism with FA affects glial Ca2+ signaling in the ENS. To this end, we measured glial Ca2+ responses driven by the P2Y1 receptor agonist ADP (Brown et al. 2016; McClain et al. 2014). P2Y1 receptors are enriched in enteric glial cells, and the ability of ADP to drive robust and reproducible Ca2+ responses was ideal for these experiments. We recorded glial Ca2+ responses by selectively expressing the genetically encoded Ca2+ sensor GCaMP5G in enteric glial cells. We chose this model because we reasoned that the genetically encoded Ca2+ sensor would still accurately report Ca2+ levels in the presence of FA, unlike traditional organic Ca2+ indicator dyes that may change fluorescence in the presence of FA due to dye leakage or interactions with the organic dye.
Sox10-PC::G5-tdT mice are a reliable model system to study Ca2+ responses selectively in enteric glia.
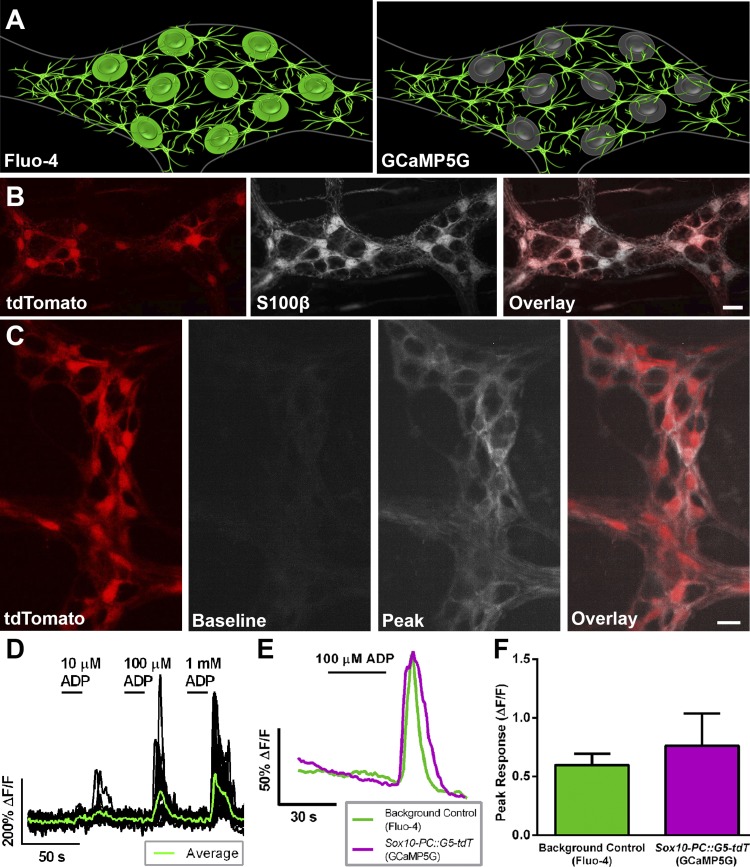
Our cross of Sox10::creERT2 mice with PC::G5-tdT mice yields a novel organism that has not been experimentally validated by prior studies. Therefore, it was necessary to validate Sox10-PC::G5-tdT mice as an appropriate model system to study enteric glial Ca2+ responses before continuing with our experiments (Fig. 1A). The two main parameters that we used to assess Sox10-PC::G5-tdT mice were the expression pattern of the tdT reporter and the change in glial GCaMP5G fluorescence observed upon stimulation with ADP. We observed robust tdT expression in the mouse intestine following the induction of Cre recombinase with tamoxifen. Dual labeling with the glial marker S100β confirmed that all tdT-expressing cells were enteric glial cells (Fig. 1B). Approximately 85% of all S100β-positive enteric glial cells also expressed tdT in the myenteric plexus of the colon 5 days following the induction of Cre. Importantly, tdT expression was absent in background control mice injected with tamoxifen (data not shown). These results show that the induction of Cre recombinase in Sox10-PC::G5-tdT drives the selective expression of GCaMP5G-tdT in the majority of enteric glia.
Fig. 1.
Sox10-PC::G5-tdT transgenic mice are an effective model to study glial [Ca2+]i responses specifically in the enteric nervous system. A: model of our experimental paradigm comparing Fluo-4 and GCaMP5G imaging in the colonic myenteric plexus. Note that Fluo-4 loads both enteric neurons and glia, whereas GCaMP5G expression is isolated to enteric glia in our genetic model. B: representative epifluorescence images showing the specific expression of tdT (red, left) in S100β-immunoreactive enteric glia (grayscale, middle) in the colonic myenteric plexus of Sox10-PC::G5-tdT mice (overlay, right). C: representative still images from a Ca2+ imaging experiment showing GCaMP5G fluorescence (grayscale, middle 2) in enteric glia (identified by tdT fluorescence; red, left) in a myenteric ganglion from a Sox10-PC::G5-tdT mouse. GCaMP5G fluorescence is low at rest (baseline) and increases robustly when glial [Ca2+]i responses are stimulated by ADP (100 μM; peak). Note that [Ca2+]i responses are limited to tdT-positive glial cells (overlay, right). D: representative traces of [Ca2+]i levels in enteric glia (black traces) within a myenteric ganglion (averaged response of all glia within ganglion, overlaid in green) from Sox10-PC::G5-tdT mice exposed to an ADP dose-response curve (10 µM, 100 µM, and 1 mM, subsequently). ΔF/F, change in fluorescence. E: representative traces comparing the mean [Ca2+]i responses of myenteric glia with ADP recorded using GCaMP5G fluorescence of n = 20 glia from a single myenteric ganglion from Sox10-PC::G5-tdT mice (magenta) or traditional Fluo-4 loading of n = 17 glia from a single myenteric ganglion from background control animals (green). F: GCaMP5G fluorescence reports an average peak [Ca2+]i response that is comparable with those reported by Fluo-4. Peak responses are the average of all glia within a myenteric ganglion exposed to ADP of n = 6–7 ganglia from at least 3 mice. Scale bars = 10 μM.
Next, we tested whether the GCaMP5G expressed by enteric glia is a good indicator of [Ca2+]i transients. GCaMP5G fluorescence was nearly undetectable in quiescent glial cells but dramatically increased in fluorescence upon stimulation of glia with the P2Y1 agonist ADP (100 μM; Fig. 1C and Supplemental Video S1, available in the data supplement online at the Journal of Neurophysiology Web site). Interestingly, GCaMP5G reported Ca2+ transients in glial processes much more effectively than we (Fried and Gulbransen 2015) or others (Broadhead et al. 2012) have observed in past experiments using Fluo-4 (Fig. 1C). The majority of GCaMP5G fluorescence in active glia was present in glial processes, and relatively little activity was observed in the soma. We also frequently observed spontaneous activity in glial cells before stimulation with ADP, suggesting that GCaMP5G is a good indicator of physiological levels of glial activity. Dose-response experiments to assess the sensitivity of the GCaMP5G reporter in Sox10-PC::G5-tdT mice (Fig. 1D) showed that ADP dose-dependently evokes glial Ca2+ responses in Sox10-PC::G5-tdT mice with a calculated EC50 of 29 μM. This agrees well with our prior work using Fluo-4 where we showed that 100 μM elicits robust and consistent increases in [Ca2+]i in enteric glia (Brown et al. 2016). Therefore, we used 100 μM ADP to drive glial [Ca2+]i responses in the remainder of this study.
The usefulness of prior-generation glial GCaMP mice, such as the GFAP-GCaMP3 mice used by Hennig et al. (2015), has been limited due to several drawbacks that include a lack of specific expression in glia and poor temporal resolution and ΔF yield compared with organic dyes such as Fluo-4. The above data show that our model has superior specificity for glia than these previous models, but it was unknown whether the temporal resolution and ΔF yield would also be superior. Therefore, we performed experiments to compare directly the magnitude and temporal kinetics of glial Ca2+ responses reported by GCaMP5G to Fluo-4. Our results from these experiments show that the magnitude and temporal kinetics of glial Ca2+ responses evoked by ADP in tissue from Sox10-PC::G5-tdT mice are comparable with those observed in glia loaded with Fluo-4 in tissue from background control mice (Fig. 1, E and F). The average peak response of all glia within a myenteric ganglion was 0.761 ± 0.274 ΔF/F in Sox10-PC::G5-tdT transgenic mice and 0.597 ± 0.096 ΔF/F in background control mice (P > 0.05; Fig. 1F). Together, these results show that Sox10-PC::G5-tdT mice are comparable with our previous work and a highly effective model system to study enteric glial Ca2+ responses specifically.
Effects of FA on glial Ca2+ responses.
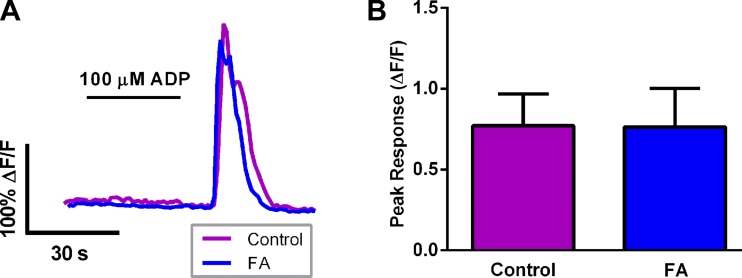
The effectiveness of the Sox10-PC::G5-tdT mice gave us great confidence that we would be able to determine reliably whether FA has a significant effect on glial Ca2+ signaling. To this end, we studied the effects of an acute exposure to FA (2 h) on glial Ca2+ responses evoked by ADP in tissue from Sox10-PC::G5-tdT mice (Fig. 2). Surprisingly, FA had no effect on the glial response evoked by ADP when we expressed these data as an average response of all glia within a ganglion (Fig. 2A). The mean peak response of all glia within a myenteric ganglion was 0.772 ± 0.195 ΔF/F in control tissue and 0.763 ± 0.240 ΔF/F in FA-pretreated tissue (P > 0.05; Fig. 2B).
Fig. 2.
The effect of fluoroacetate (FA) on averaged ganglionic glial [Ca2+]i responses triggered by ADP in Sox10-PC::G5-tdT mice. A: representative traces showing the average [Ca2+]i response of all glia within a myenteric ganglion driven by ADP (100 μM) in control samples (magenta) or samples exposed to FA (5 mM, blue). The control trace is the averaged response of n = 20 glia within a single myenteric ganglion, and the FA trace is the averaged response of n = 26 glia within a single myenteric ganglion. B: quantification of the effect of FA on peak glial [Ca2+]i responses driven by ADP. Data are expressed as an averaged response of all glia within a myenteric ganglion (averaged ganglionic response) of n = 9 ganglia from at least 5 mice.
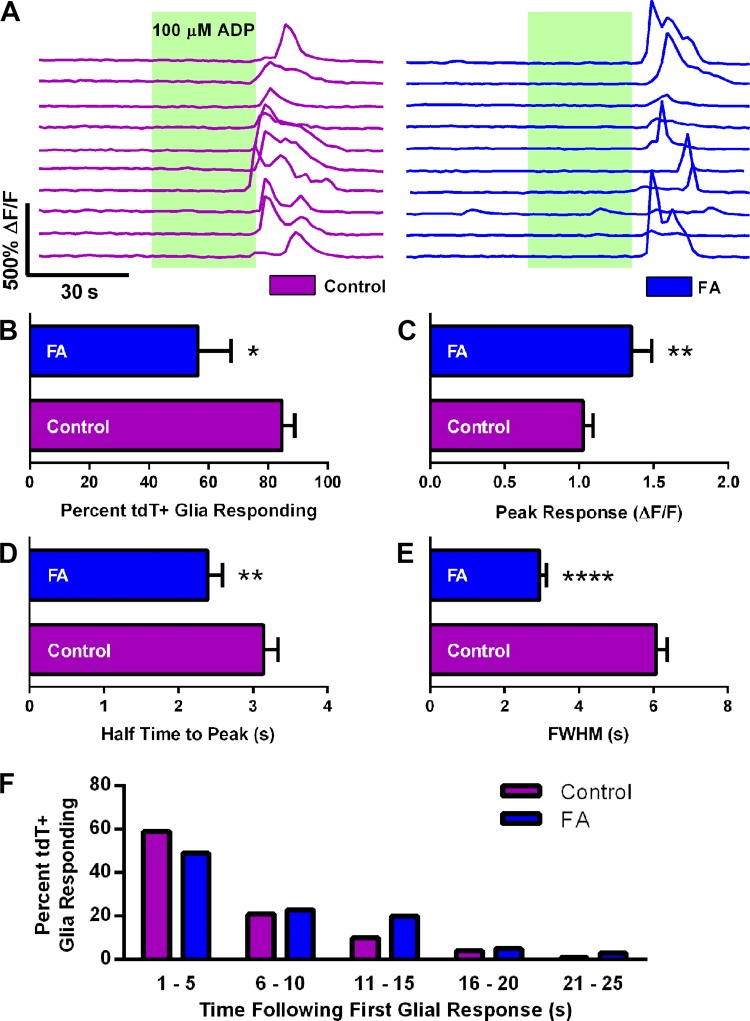
The above data were puzzling because they suggested that FA has little to no effect on glial Ca2+ waves despite a number of studies showing a significant effect of FA on functions mediated by glia (Fonnum et al. 1997; Gordon et al. 2005; Hassel et al. 1994; Keyser and Pellmar 1994; MacEachern et al. 2015; Nasser et al. 2006; Swanson and Graham 1994). One explanation for the lack of effect is that the compression of the data to an averaged ganglionic glial Ca2+ response masks important, subtle changes in individual cells. We tested this hypothesis by closely analyzing specific parameters of the glial Ca2+ response on a cell-by-cell basis and found that differences did begin to emerge when we took this more detailed look at the data (Fig. 3). Specifically, fewer glial cells per ganglion responded to ADP in tissue treated with FA (84.67 ± 4.20% control vs. 56.44 ± 10.96% FA, P < 0.05; Fig. 3, A and B), but the peak response of glia still exhibiting a response to ADP in FA-treated tissue was larger than control (average peak response of responding glial cells: 1.00 ± 0.07 ΔF/F control vs. 1.26 ± 0.13 ΔF/F FA, P < 0.01; Fig. 3C). FA also significantly altered the kinetics of the Ca2+ responses observed in responding cells. Specifically, the assessment of the quickness of the response by measuring the half time to peak and the length of the response by measuring the FWHM revealed that glia elevated Ca2+ more rapidly after treatment with FA (half time to peak: 3.14 ± 0.19 s control vs. 2.39 ± 0.20 s FA, P < 0.01; Fig. 3D) and that these glial responses were more brief (FWHM: 6.07 ± 0.29 s control vs. 2.94 ± 0.19 s FA, P < 0.0001; Fig. 3E). Despite these alterations, the activation profile of responding glia exposed to FA was comparable with control glia (Fig. 3F). These data show that FA has significant effects on glial cell Ca2+ transients that are masked by averaging the activity of all cells within an individual ganglion.
Fig. 3.
The effect of fluoroacetate (FA) on [Ca2+]i responses in individual enteric glial cells triggered by ADP in Sox10-PC::G5-tdT mice. A: representative traces showing [Ca2+]i responses in enteric glial cells within a myenteric ganglion that were evoked by exposure to ADP (green-shaded areas). Magenta traces show the responses of glial cells in a control ganglion (left); blue traces show the responses of glial cells in a ganglion exposed to 5 mM FA (right). Each trace represents the responses of 1 glial cell. B–F: quantification of the effects of FA on the percentage of glia [tdT-positive cells (tdT+)] responding to ADP (B), the peak [Ca2+]i response of cells still exhibiting a response (C), the half time to peak of glial [Ca2+]i responses (D), the full width at half max (FWHM; E), and the activation profile of glial [Ca2+]i responses (F). Data are representative of recordings in n = 90 and 160 glial cells (FA and control, respectively) from at least 5 mice. *P < 0.05, **P < 0.01, ****P < 0.0001, Student's t-test.
Effects of FA on glial hemichannel function.
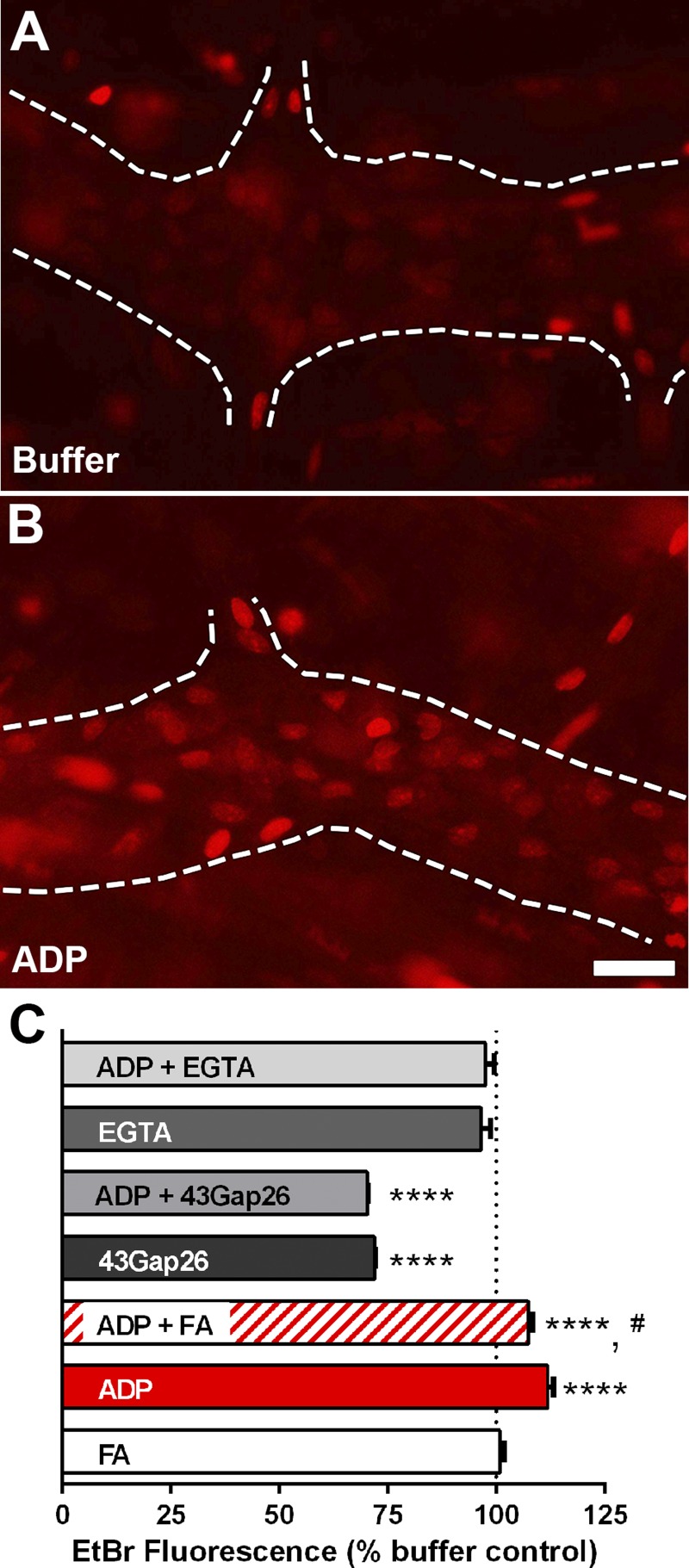
Given that our data suggest that FA alters glial Ca2+ responses, FA should also disrupt downstream mechanisms that are regulated by Ca2+ signaling. We have previously shown that the activation of P2Y1 receptors in enteric glia drives Ca2+ responses that subsequently enact pathways leading to the opening of hemichannels composed of Cx43 and that this is a key mechanism of intercellular communication used by enteric glia (McClain et al. 2014). Likewise, the regulation of Cx43 hemichannels by pathways downstream of Ca2+ responses is well described in other types of glia (De Vuyst et al. 2009; Kang et al. 2008; Wang et al. 2013). Hemichannels composed of Cx43 are permeable to EtBr (Contreras et al. 2002), and EtBr uptake is an efficient method to test their activity (Orellana et al. 2011; Retamal et al. 2006, 2007). Therefore, we used this technique to determine whether FA affects Cx43 hemichannel activity in enteric glia at rest or following stimulation of intracellular Ca2+ signaling with the P2Y1 receptor agonist ADP. EtBr uptake by astrocytes under normal conditions is low due to the low open probability of Cx43 hemichannels at rest (Retamal et al. 2007; Sáez et al. 2005). In agreement, our results show that enteric glial cells display a very small amount of baseline uptake of EtBr in control (nonstimulated) tissue (Fig. 4). This baseline uptake was at least partly mediated by Cx43 hemichannels because preincubation with the Cx43 mimetic peptide 43Gap26 (100 μM) reduced baseline uptake by 28% (Fig. 4C). Incubation with FA had no effect on baseline uptake of EtBr (Fig. 4C). Stimulation of glial Ca2+ signaling with ADP increased EtBr uptake by 12%, and ADP-driven EtBr uptake was completely abolished by 43Gap26 (Fig. 4C), indicating that the ADP-driven increase was entirely Cx43 dependent. Interestingly, we found that FA inhibited the maximal ADP-dependent increase in glial EtBr uptake (Fig. 4C). Ca2+ chelation with EGTA did not affect baseline EtBr uptake but completely abolished the ADP-driven uptake of EtBr (Fig. 4C). These data show that dysregulation of glial Ca2+ signaling by FA impacts downstream mechanisms such as Cx43 hemichannel opening.
Fig. 4.
The effect of fluoroacetate (FA) on hemichannel-dependent dye uptake by myenteric glia in the mouse colon. A and B: representative epifluorescence images showing ethidium bromide (EtBr) fluorescence in whole-mount preparations of the myenteric plexus from the mouse colon exposed to buffer (A) or ADP (B). Glial cells within myenteric ganglia (outlined by dashed lines) normally display a low amount of dye uptake (A) that increases robustly when stimulated with ADP (B). C: quantification of the effects of FA, the connexin-43 mimetic peptide 43Gap26, the Ca2+ chelator EGTA, and ADP on mean glial cell EtBr fluorescence in whole-mount preparations of the mouse myenteric plexus. Scale bar (B) = 10 μm and applies to A and B. Measurements are representative of n = 225 glia to 373 glial cells from at least 3 mice. ****P < 0.0001 compared with buffer, #P < 0.05 compared with ADP, ANOVA.
Effects of FA on neuron and glial survival and glial expression of key proteins.
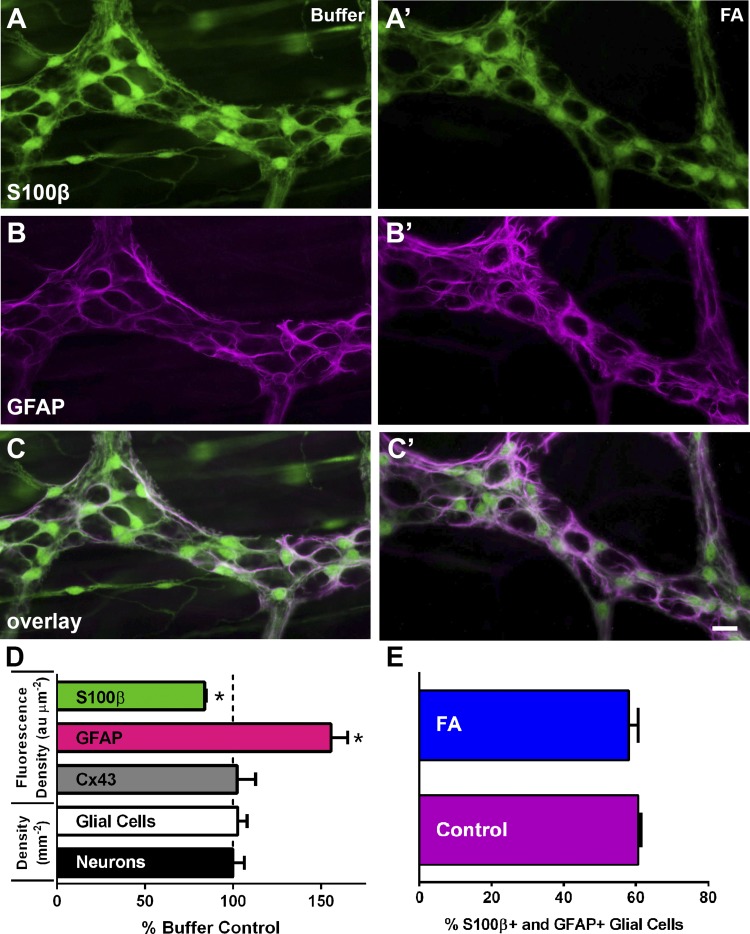
The effects of FA on glial cell function may be due, in part, to changes in glial protein expression or neuron and/or glial cell survival when glial metabolic pathways are impaired. We tested this possibility by quantifying the expression of the key glial cell proteins Cx43, S100β, and GFAP after incubation in FA (Fig. 5). We also assessed whether FA altered neuron and glial survival by quantifying neuronal density with HuC/D labeling and glial density with S100β. We were able to rule out the possibility that FA causes neuron or glial degeneration because we did not observe any change in neuron or glial packing density following FA treatment (Fig. 5D). We also did not observe any change in Cx43 expression (102.4 ± 10.45% of control, P > 0.05). In contrast, we observed a 17% decrease in the intensity of S100β labeling (P < 0.05; Fig. 5, A and A′) and a 56% increase in the intensity of GFAP immunoreactivity (P < 0.05; Fig. 5, B and B′). We did not observe a difference in glial cell coexpression of S100β and GFAP following FA treatment (P < 0.05; Fig. 5, C, C′, and E). These results suggest that altered glial phenotype, rather than survival, may contribute to altered glial cell function following acute exposure to FA.
Fig. 5.
The effects of fluoroacetate (FA) on ganglionic cell density and glial expression of key proteins. A–C and A′–C′: representative epifluorescence images of S100β immunoreactivity (green, A and A′), glial fibrillary acidic protein (GFAP) immunoreactivity (magenta, B and B′), and overlay (C and C′) in myenteric ganglia from control whole mounts (A, B, and C) and whole mounts exposed to FA (A′, B′, and C′). Note that S100β immunoreactivity decreases in glia exposed to FA (green, A′) and that GFAP immunoreactivity increases in glia exposed to FA (magenta, B′). D: quantification of ganglionic S100β, GFAP, and connexin-43 (Cx43) immunoreactivity, expressed as the fluorescence density [arbitrary fluorescence units per square micrometer (au μm−2)], and the packing density of myenteric glial and neurons [number of S100β+ glia and HuC/D+ neurons per square millimeter (mm−2)] expressed as the percentage of buffer control levels. Scale bar (C′) = 10 μm and applies to A–C and A′–C′. E: quantification of glial cells coexpressing S100β and GFAP. Measurements were obtained by sampling ganglia from n = 4 animals. *P < 0.05, Student's t-test.
DISCUSSION
The main results of our study show that the acute inhibition of glial metabolism with FA affects essential glial functions including [Ca2+]i signaling, hemichannel function, and expression of key glial markers such as S100β and GFAP. FA had relatively minor effects on glial Ca2+ responses when viewed at the population level. However, FA significantly altered the kinetics of the Ca2+ responses in individual cells and decreased the total number of cells responding. Our data suggest that the latter of these two effects is caused by a disruption in hemichannel opening downstream of Ca2+ responses. Interestingly, enteric glia responded to acute metabolic inhibition with changes in protein expression consistent with a conversion to a reactive state.
Our study provides direct evidence that FA affects glial cell [Ca2+]i signaling. Yet the effects of FA were surprisingly minor given the body of published work showing effects of FA/FC on functions that are presumably mediated by glia (Henneberger et al. 2010; Letellier et al. 2016; MacEachern et al. 2015; Nasser et al. 2006; Pougnet et al. 2014; Wang et al. 2016). This may indicate that the predominant effects of FA are on glial functions that are not mediated by Ca2+-dependent signaling pathways. However, an alternate interpretation is that even the minor disruption of a key regulatory signal, such as [Ca2+]i in glia, can have broad effects on neuronal networks. Glial [Ca2+]i signaling is a “master regulator” of multiple signal transduction pathways that control important glial mechanisms such as the release of mediators through hemichannels composed of Cx43 (De Vuyst et al. 2009). The opening of Cx43 hemichannels is an important mechanism of intercellular communication used by enteric glia (McClain et al. 2014; Zhang et al. 2003) and astrocytes (Chever et al. 2016; Stout et al. 2002; Theis and Giaume 2012), and the selective impairment of glial Cx43 function in genetic models has major effects on neural networks (Chever et al. 2014, 2016; Frisch et al. 2003; McClain et al. 2014). In our study, we found that the minor impairment of glial [Ca2+]i signaling caused by FA produced large effects on the activity-dependent opening of Cx43 hemichannels but not on Cx43 protein expression or basal function of Cx43 hemichannels. Therefore, our data support the conclusion that FA significantly disrupts activity-dependent functions of glia that are associated with synaptic signaling. This relatively minor impairment of glial [Ca2+]i signals presumably has disproportionately large effects on neuronal networks by broadly influencing multiple key signaling pathways such as we showed here for Cx43 hemichannel opening.
Our data show that the impairment of the glial TCA cycle with FA had no effect on baseline Cx43 dye uptake but impaired activity-dependent dye uptake through Cx43. In support of our findings, the impairment of the glial TCA cycle with FC also decreases dipeptide uptake into enteric glia (Nasser et al. 2006). However, other reports suggest that the impairment of glial metabolism increases the uptake of dyes through Cx43. For example, Contreras et al. (2002) used an in vitro model of cortical ischemia to show that the inhibition of oxidative and glycolytic metabolism in cultured astrocytes induces the opening of Cx43 hemichannels. In this study, astrocyte metabolism was impaired with a combination of antimycin A and iodoacetic acid. These drugs are presumed to impair glycolysis and oxidative phosphorylation through specific effects on GAPDH (iodoacetic acid) and cytochrome c reductase (antimycin A), respectively (Dairaku et al. 2004; Rego et al. 1999). However, both of these compounds have significant effects on mechanisms that control Cx43 hemichannel function that are independent of their effects on metabolic inhibition. For example, iodoacetic acid is an alkylating agent that reacts with cysteine residues, and cysteines are key regulatory elements of connexin hemichannels (Retamal et al. 2006, 2009). Likewise, the inhibition of cytochrome c reductase with antimycin A generates the free radical superoxide (Dairaku et al. 2004), and oxidative stress induces Cx43 hemichannel permeation (Retamal et al. 2006). These confounding factors make the interpretation of data from this study difficult and whether the effects on glial Cx43 hemichannel function observed by Contreras et al. (2002) were due to metabolic inhibition or the unanticipated side effects of the drugs used remains unclear.
We believe that the most reasonable mechanistic explanation that links impaired glial metabolism with altered [Ca2+]i signals is the energy dependence of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pump. The release of Ca2+ from intracellular stores is the main source of glial Ca2+ transients (Finkbeiner 1993), and the spatiotemporal regulation of Ca2+ within glial cells is regulated by the activity of the SERCA pump (Simpson and Russell 1997). This is a highly energy-dependent process (Chinet et al. 1992; Smith et al. 2013) that would be among the first impacted by metabolic disruption. Although we did not directly test the activity of glial SERCA pumps in this study, the specific characteristics of the [Ca2+]i responses after exposure to FA in our study such as reduced response length, a shorter time to peak, and a larger peak all imply altered SERCA activity. However, many mechanisms could conceivably contribute to the altered kinetics of glial [Ca2+]i responses during metabolic inhibition.
The changes that we observed in protein expression indicate that enteric glia react to metabolic impairment/starvation by becoming “reactive.” For example, we observed a significant increase in GFAP immunoreactivity following FA treatment, and the upregulation of GFAP is a classic characteristic of reactive gliosis (Bradley et al. 1997; Gabella 1984; Thacker et al. 2011; von Boyen et al. 2004). The fact that other groups have observed similar changes in GFAP expression in an ischemia-reperfusion model of ENS metabolic injury (Thacker et al. 2011) strongly suggests that the general response of enteric glia to metabolic stress is to become reactive. If this is true, then extreme caution will be needed when conducting or interpreting any study that uses FA/FC because subsequent changes in neuronal function may be due to a loss of glial influence and/or a transition to reactive gliosis. Interestingly, Nasser et al. (2006) did not report altered GFAP expression in the myenteric plexus of mice treated with FC for 1 wk in vivo. This difference may indicate that the reactive response of glia to a metabolic challenge is transient or that glia compensate for long-term inhibition of the TCA cycle by transitioning to alternate metabolic pathways. However, Nasser et al. (2006) did not actually quantify the expression of GFAP in the myenteric plexus so it is difficult to determine whether the lack of change reported is accurate. Interestingly, Nasser et al. (2006) did observe a significant upregulation of phosphorylated ERK1/2 in enteric glia in tissue samples acutely exposed to FC in vitro. These findings support our observations, because phosphorylated ERK1/2 is linked to an increase in reactive gliosis and GFAP expression in astrocytes (Heffron and Mandell 2005).
Reactive gliosis is typically considered a defensive response of glia to protect neural networks from damage during disease or injury (Burda and Sofroniew 2014). Importantly, new data show that reactive gliosis is also required for repair processes after injury such as axon regeneration (Anderson et al. 2016). The role of reactive gliosis in the gut is currently unclear, but available data do support a similar protective role. For example, reactive gliosis observed by Thacker et al. (2011) during ischemia-reperfusion is most likely a protective response to preserve neuron integrity during transient metabolic stress. Reactive gliosis is a broad spectrum of glial responses to insults that should not be confused with physiological glial activation or glial activation during acute inflammation. For example, we recently showed that glial activation by neuron danger cues released during acute inflammation is responsible for driving enteric neuron death (Brown et al. 2016). Likewise, we showed that glial activity encoded by [Ca2+]i responses plays an important role in the physiological regulation of gut motility (McClain et al. 2014, 2015). Importantly, the influence of glial activation on neural networks during inflammation and during normal physiology requires that the activity-dependent opening of glial Cx43 hemichannels that we showed here depends on glial [Ca2+]i responses. Our results in this study show that the conversion to reactive gliosis initiated by FA has the opposite effect on glial activity and decreases Cx43 hemichannel opening. Given that glial Cx43 opening is critical for neurodegeneration during inflammation (Brown et al. 2016), the decrease of its activity during metabolic stress likely functions to protect against inadvertently killing neurons.
The direct effects of FA on the loading or function of organic Ca2+ indicator dyes such as Fluo-4 are potential confounding factors in any study that aims to examine the effects of FA on glial Ca2+ signaling. We avoided this issue in the current study by generating a novel optogenetic mouse model to study enteric glial Ca2+ responses specifically. Our results show that the Sox10 promoter is highly effective at specifically driving the expression of GCaMP5G-tdT in enteric glial cells within the gut. The temporal kinetics and fluorescence yield of GCaMP5G were also impressive compared with prior-generation GCaMP mice used to study enteric glia (Hennig et al. 2015). The large, dynamic range of GCaMP5G (Akerboom et al. 2012) combined with the specificity of the Sox10 promoter will be a significant benefit to future work that aims to study glial Ca2+ responses in the gut. In particular, the excellent responses that we observed in glial processes suggest that this model will be particularly useful to study physiological signaling events that occur in the fine processes of glial cells.
In conclusion, our results provide important insight into how the metabolic impairment of glia with FA affects specific glial functions. This is a classic technique that has been used for many years to study the role of glia in the nervous system. However, our understanding of how it affects specific glial functions involved in neurotransmission such as Ca2+ signaling and hemichannel function is limited. Our work provides a basic understanding of how FA affects key functions of enteric glial cells that are involved with neurotransmission in the gut. We feel that this information will be broadly beneficial to the field in terms of planning future studies and in interpreting prior work that used metabolic toxins.
GRANTS
Support for this project was provided by grants to B. D. Gulbransen from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK103723) and the Crohn's and Colitis Foundation of America (CCFA; Senior Research Award).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.D.G. conceived and designed research; J.L.M. performed experiments; J.L.M. analyzed data; J.L.M. and B.D.G. interpreted results of experiments; J.L.M. and B.D.G. prepared figures; J.L.M. and B.D.G. drafted manuscript; J.L.M. and B.D.G. edited and revised manuscript; J.L.M. and B.D.G. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Vassilis Pachnis (MRC National Institute for Medical Research) for his generous donation of Sox10::creERT2 transgenic mice.
REFERENCES
- Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J Physiol 591: 5599–5609, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327: 1250–1254, 2010. [DOI] [PubMed] [Google Scholar]
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci 32: 13819–13840, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532: 195–200, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci 19: 182–189, 2016. [DOI] [PubMed] [Google Scholar]
- Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J, Vanden Berghe P. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25: e151–e160, 2013. [DOI] [PubMed] [Google Scholar]
- Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca(2+) signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol 279: L623–L630, 2000. [DOI] [PubMed] [Google Scholar]
- Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci 34: 13139–13150, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JS, Parr EJ, Sharkey KA. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res 289: 455–461, 1997. [DOI] [PubMed] [Google Scholar]
- Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ, Smith TK. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J Physiol 590: 335–350, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81: 229–248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, Yan HC, Gao YB, Liu JH, Li XW, Sun LR, Zeng YN, Zhu XH, Gao TM. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med 19: 773–777, 2013. [DOI] [PubMed] [Google Scholar]
- Chever O, Dossi E, Pannasch U, Derangeon M, Rouach N. Astroglial networks promote neuronal coordination. Sci Signal 9: ra6, 2016. [DOI] [PubMed] [Google Scholar]
- Chever O, Lee CY, Rouach N. Astroglial connexin43 hemichannels tune basal excitatory synaptic transmission. J Neurosci 34: 11228–11232, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinet A, Decrouy A, Even PC. Ca(2+)-dependent heat production under basal and near-basal conditions in the mouse soleus muscle. J Physiol 455: 663–678, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DD. Fluoroacetate and fluorocitrate: mechanism of action. Neurochem Res 16: 1055–1058, 1991. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA 99: 495–500, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairaku N, Kato K, Honda K, Koike T, Iijima K, Imatani A, Sekine H, Ohara S, Matsui H, Shimosegawa T. Oligomycin and antimycin A prevent nitric oxide-induced apoptosis by blocking cytochrome C leakage. J Lab Clin Med 143: 143–151, 2004. [DOI] [PubMed] [Google Scholar]
- De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 46: 176–187, 2009. [DOI] [PubMed] [Google Scholar]
- Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci 16: 2553–2562, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol 559: 3–15, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner SM. Glial calcium. Glia 9: 83–104, 1993. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia 21: 106–113, 1997. [PubMed] [Google Scholar]
- Fried DE, Gulbransen BD. In situ Ca2+ imaging of the enteric nervous system. J Vis Exp e52506, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch C, Theis M, De Souza Silva MA, Dere E, Söhl G, Teubner B, Namestkova K, Willecke K, Huston JP. Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur J Neurosci 18: 2313–2318, 2003. [DOI] [PubMed] [Google Scholar]
- Gabella G. Intramural ganglia. In: Structure of the Autonomic Nervous System. New York: Chapman and Hall, 1976, p. 117–132. [Google Scholar]
- Gabella G. Size of neurons and glial cells in the intramural ganglia of the hypertrophic intestine of the guinea-pig. J Neurocytol 13: 73–84, 1984. [DOI] [PubMed] [Google Scholar]
- Gee JM, Smith NA, Fernandez FR, Economo MN, Brunert D, Rothermel M, Morris SC, Talbot A, Palumbos S, Ichida JM, Shepherd JD, West PJ, Wachowiak M, Capecchi MR, Wilcox KS, White JA, Tvrdik P. Imaging activity in neurons and glia with a Polr2a-based and cre-dependent GCaMP5G-IRES-tdTomato reporter mouse. Neuron 83: 1058–1072, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 8: 1078–1086, 2005. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 64: 391–403, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18: 600–604, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9: 625–632, 2012. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136: 1349–1358, 2009. [DOI] [PubMed] [Google Scholar]
- Hassel B, Paulsen RE, Johnsen A, Fonnum F. Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res 576: 120–124, 1992. [DOI] [PubMed] [Google Scholar]
- Hassel B, Sonnewald U, Unsgård G, Fonnum F. NMR spectroscopy of cultured astrocytes: effects of glutamine and the gliotoxin fluorocitrate. J Neurochem 62: 2187–2194, 1994. [DOI] [PubMed] [Google Scholar]
- Heffron DS, Mandell JW. Opposing roles of ERK and p38 MAP kinases in FGF2-induced astroglial process extension. Mol Cell Neurosci 28: 779–790, 2005. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463: 232–236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Gould TW, Koh SD, Corrigan RD, Heredia DJ, Shonnard MC, Smith TK. Use of genetically encoded calcium indicators (GECIs) combined with advanced motion tracking techniques to examine the behavior of neurons and glia in the enteric nervous system of the intact murine colon. Front Cell Neurosci 9: 436, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci 28: 4702–4711, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser DO, Pellmar TC. Synaptic transmission in the hippocampus: critical role for glial cells. Glia 10: 237–243, 1994. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18: 942–952, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience 129: 1045–1056, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum JM. Age-dependent susceptibility of CNS glial populations in situ to the antimetabolite 6-aminonicotinamide. Mol Chem Neuropathol 26: 79–94, 1995. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, Pachnis V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 121: 3412–3424, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letellier M, Park YK, Chater TE, Chipman PH, Gautam SG, Oshima-Takago T, Goda Y. Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc Natl Acad Sci USA 113: E2685–E2694, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEachern SJ, Patel BA, Keenan CM, Dicay M, Chapman K, McCafferty DM, Savidge TC, Beck PL, MacNaughton WK, Sharkey KA. Inhibiting inducible nitric oxide synthase in enteric glia restores electrogenic ion transport in mice with colitis. Gastroenterology 149: 445–455.e3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R, Bajo-Grañeras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349: 730–734, 2015. [DOI] [PubMed] [Google Scholar]
- McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca(2+) signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1: 631–645, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JL, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146: 497–507.e1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13: 1400–1412, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser Y, Fernandez E, Keenan CM, Ho W, Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Rühl A, Sharkey KA. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol 291: G912–G927, 2006. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A. Astrocytes going live: advances and challenges. J Physiol 587: 1639–1647, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Hernández DE, Ezan P, Velarde V, Bennett MV, Giaume C, Sáez JC. Hypoxia in high glucose followed by reoxygenation in normal glucose reduces the viability of cortical astrocytes through increased permeability of connexin 43 hemichannels. Glia 58: 329–343, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Sáez PJ, Jiang JX, Naus CC, Sáez JC, Giaume C. Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J Neurosci 31: 4962–4977, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem 48: 1377–1385, 1987. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. Astrocytes: a central element in neurological diseases. Acta Neuropathol 131: 323–345, 2016. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Glial calcium signaling and neuron-glia communication. Cell Calcium 38: 375–382, 2005. [DOI] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 28: 4967–4973, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pougnet JT, Toulme E, Martinez A, Choquet D, Hosy E, Boué-Grabot E. ATP P2X receptors downregulate AMPA receptor trafficking and postsynaptic efficacy in hippocampal neurons. Neuron 83: 417–430, 2014. [DOI] [PubMed] [Google Scholar]
- Rego AC, Areias FM, Santos MS, Oliveira CR. Distinct glycolysis inhibitors determine retinal cell sensitivity to glutamate-mediated injury. Neurochem Res 24: 351–358, 1999. [DOI] [PubMed] [Google Scholar]
- Retamal MA, Cortés CJ, Reuss L, Bennett MV, Sáez JC. S-Nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103: 4475–4480, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27: 13781–13792, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Yin S, Altenberg GA, Reuss L. Modulation of Cx46 hemichannels by nitric oxide. Am J Physiol Cell Physiol 296: C1356–C1363, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Sontheimer H. Glia as drivers of abnormal neuronal activity. Nat Neurosci 19: 28–33, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 1711: 215–224, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Ishikawa T, Abe R, Nakayama R, Asada A, Matsuki N, Ikegaya Y. Astrocyte calcium signalling orchestrates neuronal synchronization in organotypic hippocampal slices. J Physiol 592: 2771–2783, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem Int 45: 521–527, 2004. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking. Biol Psychiatry 78: 441–451, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, Boger HA, Kalivas PW, Reissner KJ. Cocaine self-administration and extinction leads to reduced glial fibrillary acidic protein expression and morphometric features of astrocytes in the nucleus accumbens core. Biol Psychiatry 80: 207–215, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Patel S, Khakh BS. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol 26: 300–312, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129: 877–896, 2004. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Russell JT. Role of sarcoplasmic/endoplasmic-reticulum Ca2+-ATPases in mediating Ca2+ waves and local Ca2+-release microdomains in cultured glia. Biochem J 325: 239–247, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IC, Bombardier E, Vigna C, Tupling AR. ATP consumption by sarcoplasmic reticulum Ca2+pumps accounts for 40–50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PLoS One 8: e68924, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277: 10482–10488, 2002. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Graham SH. Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res 664: 94–100, 1994. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev 79: 1089–1125, 1999. [DOI] [PubMed] [Google Scholar]
- Thacker M, Rivera LR, Cho HJ, Furness JB. The relationship between glial distortion and neuronal changes following intestinal ischemia and reperfusion. Neurogastroenterol Motil 23: e500–e509, 2011. [DOI] [PubMed] [Google Scholar]
- Theis M, Giaume C. Connexin-based intercellular communication and astrocyte heterogeneity. Brain Res 1487: 88–98, 2012. [DOI] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53: 222–228, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci Signal 5: ra26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta 1828: 35–50, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Du C, Chen FX, Li CQ, Yu YB, Han T, Akhtar S, Zuo XL, Tan XD, Li YQ. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep 6: 20320, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby JO, Mackenzie L, Broberg M, Thoren AE, Medvedev A, Sims NR, Nilsson M. Fluorocitrate-mediated astroglial dysfunction causes seizures. J Neurosci Res 74: 160–166, 2003. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40: 971–982, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.