Abstract
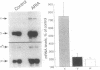
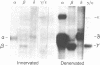
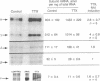
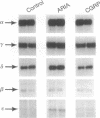
Motor neurons regulate the transcription of acetylcholine receptor subunit genes in postsynaptic muscle fibers both through muscle electrical activity produced by motor neuron acetylcholine release and by mechanisms independent of such transmitter release. Factors secreted by the motor neuron may mediate activity-independent regulation, including the postnatal switch from alpha 2 beta gamma delta (embryonic type) to alpha 2 beta epsilon delta (adult type) receptors. We have investigated the effect of putative trophic factors, agents affecting second-messenger systems, and muscle activity on the levels of acetylcholine receptor subunit mRNAs in primary mouse muscle cultures. We found that ARIA (acetylcholine receptor-inducing activity), a 42-kDa glycoprotein purified on the basis of its ability to increase the synthesis of acetylcholine receptors in chick myotubes, increases epsilon-subunit mRNA levels up to 10-fold. In addition, ARIA stimulated alpha-, gamma-, and delta-subunit mRNA levels 2-fold but had no effect on the expression of the beta-subunit gene. These effects of ARIA were independent of muscle activity, and they were not mimicked by calcitonin gene-related peptide nor by thyroxine, forskolin, phorbol 12-myristate 13-acetate, the calcium ionophore A23187, basic fibroblast growth factor, or transforming growth factor beta. Based on these data, we suggest that ARIA may act at the mammalian neuromuscular junction to induce adult-type acetylcholine receptors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold J., Asmussen M. A., Avise J. C. An epistatic mating system model can produce permanent cytonuclear disequilibria in a hybrid zone. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1893–1896. doi: 10.1073/pnas.85.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Steinbach J. H. The distribution of alpha-bungarotoxin binding sites of mammalian skeletal muscle developing in vivo. J Physiol. 1977 May;267(1):195–213. doi: 10.1113/jphysiol.1977.sp011808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P. Resolving the structural basis for developmental changes in muscle ACh receptor function: it takes nerve. Trends Neurosci. 1989 May;12(5):174–177. doi: 10.1016/0166-2236(89)90064-7. [DOI] [PubMed] [Google Scholar]
- Brenner H. R., Witzemann V., Sakmann B. Imprinting of acetylcholine receptor messenger RNA accumulation in mammalian neuromuscular synapses. Nature. 1990 Apr 5;344(6266):544–547. doi: 10.1038/344544a0. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Merlie J. P. Transcriptional regulation of nicotinic acetylcholine receptor genes during muscle development. J Biol Chem. 1986 Sep 5;261(25):11452–11455. [PubMed] [Google Scholar]
- Buonanno A., Mudd J., Merlie J. P. Isolation and characterization of the beta and epsilon subunit genes of mouse muscle acetylcholine receptor. J Biol Chem. 1989 May 5;264(13):7611–7616. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crowder C. M., Merlie J. P. Stepwise activation of the mouse acetylcholine receptor delta- and gamma-subunit genes in clonal cell lines. Mol Cell Biol. 1988 Dec;8(12):5257–5267. doi: 10.1128/mcb.8.12.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Loftus D. J., Fischbach G. D., Elson E. L. Formation of acetylcholine receptor clusters in chick myotubes: migration or new insertion? J Cell Biol. 1989 Oct;109(4 Pt 1):1733–1743. doi: 10.1083/jcb.109.4.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls D. L., Harris D. A., Johnson F. A., Morgan M. M., Corfas G., Fischbach G. D. Mr 42,000 ARIA: a protein that may regulate the accumulation of acetylcholine receptors at developing chick neuromuscular junctions. Cold Spring Harb Symp Quant Biol. 1990;55:397–406. doi: 10.1101/sqb.1990.055.01.040. [DOI] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Changeux J. P. Calcitonin gene-related peptide and muscle activity regulate acetylcholine receptor alpha-subunit mRNA levels by distinct intracellular pathways. J Cell Biol. 1987 Sep;105(3):1337–1342. doi: 10.1083/jcb.105.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Hökfelt T., Changeux J. P. Calcitonin gene-related peptide, a peptide present in spinal cord motoneurons, increases the number of acetylcholine receptors in primary cultures of chick embryo myotubes. Neurosci Lett. 1986 Oct 30;71(1):59–65. doi: 10.1016/0304-3940(86)90257-0. [DOI] [PubMed] [Google Scholar]
- Fontaine B., Sassoon D., Buckingham M., Changeux J. P. Detection of the nicotinic acetylcholine receptor alpha-subunit mRNA by in situ hybridization at neuromuscular junctions of 15-day-old chick striated muscles. EMBO J. 1988 Mar;7(3):603–609. doi: 10.1002/j.1460-2075.1988.tb02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frail D. E., Musil L. S., Buonanno A., Merlie J. P. Expression of RAPsyn (43K protein) and nicotinic acetylcholine receptor genes is not coordinately regulated in mouse muscle. Neuron. 1989 Jan;2(1):1077–1086. doi: 10.1016/0896-6273(89)90232-8. [DOI] [PubMed] [Google Scholar]
- Goldman D., Brenner H. R., Heinemann S. Acetylcholine receptor alpha-, beta-, gamma-, and delta-subunit mRNA levels are regulated by muscle activity. Neuron. 1988 Jun;1(4):329–333. doi: 10.1016/0896-6273(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Goldman D., Staple J. Spatial and temporal expression of acetylcholine receptor RNAs in innervated and denervated rat soleus muscle. Neuron. 1989 Aug;3(2):219–228. doi: 10.1016/0896-6273(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Falls D. L., Fischbach G. D. Differential activation of myotube nuclei following exposure to an acetylcholine receptor-inducing factor. Nature. 1989 Jan 12;337(6203):173–176. doi: 10.1038/337173a0. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A., Changeux J. P. Activity regulates the levels of acetylcholine receptor alpha-subunit mRNA in cultured chicken myotubes. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4558–4562. doi: 10.1073/pnas.82.13.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou J. C., Merlie J. P. Nerve-dependent modulation of acetylcholine receptor epsilon-subunit gene expression. J Neurosci. 1991 May;11(5):1291–1299. doi: 10.1523/JNEUROSCI.11-05-01291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Isenberg K. E., Russell S. D., Sanes J. R. Denervation supersensitivity in skeletal muscle: analysis with a cloned cDNA probe. J Cell Biol. 1984 Jul;99(1 Pt 1):332–335. doi: 10.1083/jcb.99.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Sanes J. R. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature. 1985 Sep 5;317(6032):66–68. doi: 10.1038/317066a0. [DOI] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Moss S. J., Beeson D. M., Jackson J. F., Darlison M. G., Barnard E. A. Differential expression of nicotinic acetylcholine receptor genes in innervated and denervated chicken muscle. EMBO J. 1987 Dec 20;6(13):3917–3921. doi: 10.1002/j.1460-2075.1987.tb02732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New H. V., Mudge A. W. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. 1986 Oct 30-Nov 5Nature. 323(6091):809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- Osterlund M., Fontaine B., Devillers-Thiery A., Geoffroy B., Changeux J. P. Acetylcholine receptor expression in primary cultures of embryonic chick myotubes--I. Discoordinate regulation of alpha-, gamma- and delta-subunit gene expression by calcitonin gene-related peptide and by muscle electrical activity. Neuroscience. 1989;32(2):279–287. doi: 10.1016/0306-4522(89)90078-x. [DOI] [PubMed] [Google Scholar]
- Role L. W., Matossian V. R., O'Brien R. J., Fischbach G. D. On the mechanism of acetylcholine receptor accumulation at newly formed synapses on chick myotubes. J Neurosci. 1985 Aug;5(8):2197–2204. doi: 10.1523/JNEUROSCI.05-08-02197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S. M., Role L. W. Developmental regulation of nicotinic acetylcholine receptors. Annu Rev Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M. The acetylcholine channel open time in chick muscle is not decreased following innervation. J Physiol. 1980 Jun;303:111–124. doi: 10.1113/jphysiol.1980.sp013274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh B. H., Ballivet M., Schmidt J. Acetylcholine receptor synthesis rate and levels of receptor subunit messenger RNAs in chick muscle. Neuroscience. 1988 Jan;24(1):175–187. doi: 10.1016/0306-4522(88)90321-1. [DOI] [PubMed] [Google Scholar]
- Usdin T. B., Fischbach G. D. Purification and characterization of a polypeptide from chick brain that promotes the accumulation of acetylcholine receptors in chick myotubes. J Cell Biol. 1986 Aug;103(2):493–507. doi: 10.1083/jcb.103.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzemann V., Barg B., Criado M., Stein E., Sakmann B. Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett. 1989 Jan 2;242(2):419–424. doi: 10.1016/0014-5793(89)80514-9. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Barg B., Nishikawa Y., Sakmann B., Numa S. Differential regulation of muscle acetylcholine receptor gamma- and epsilon-subunit mRNAs. FEBS Lett. 1987 Oct 19;223(1):104–112. doi: 10.1016/0014-5793(87)80518-5. [DOI] [PubMed] [Google Scholar]