Abstract
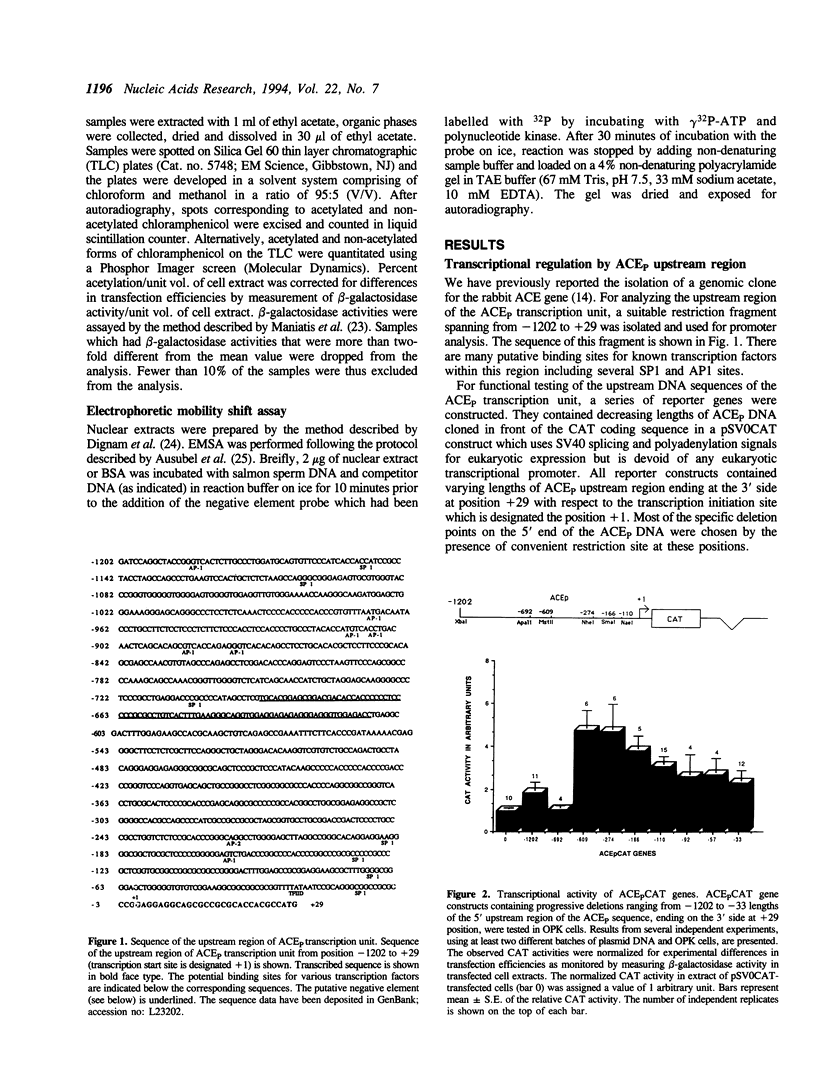
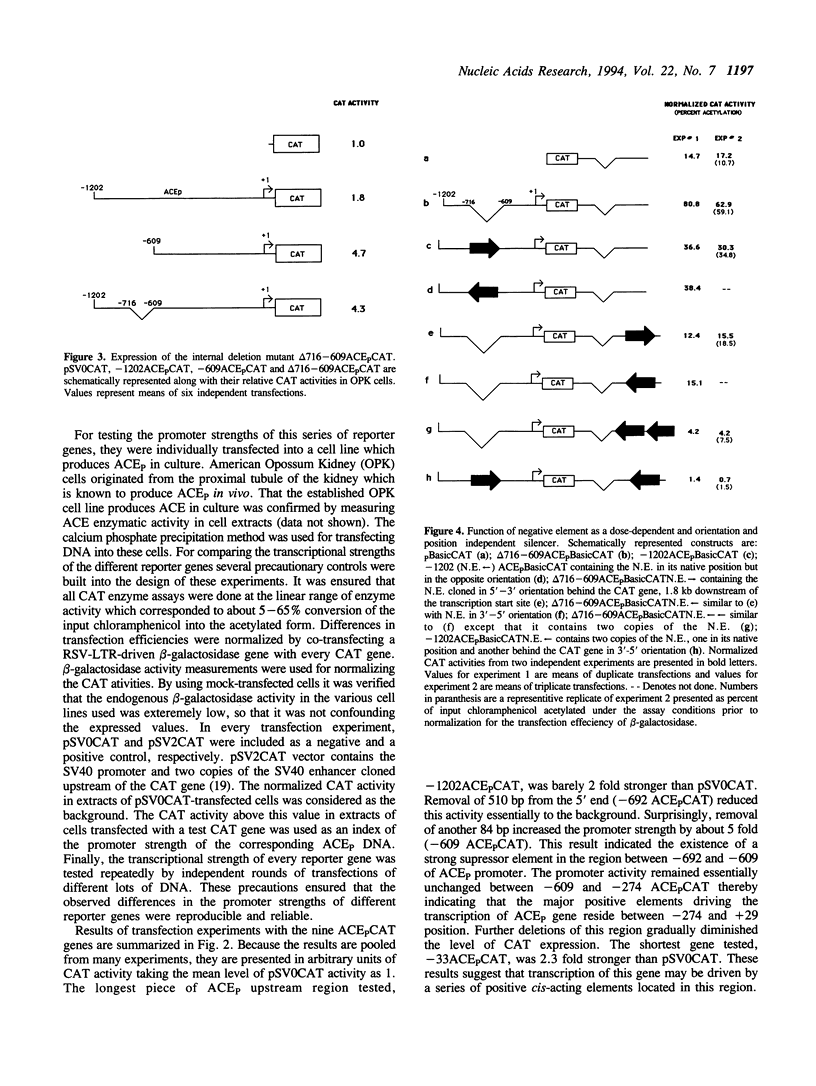
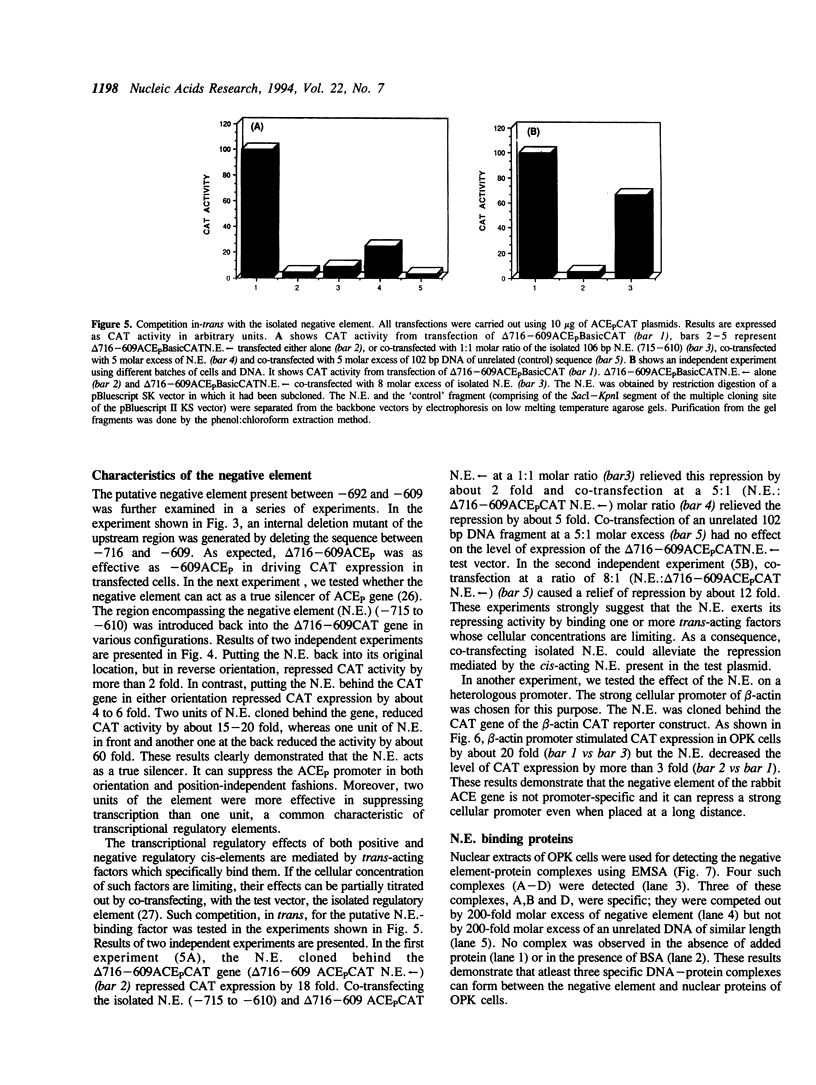
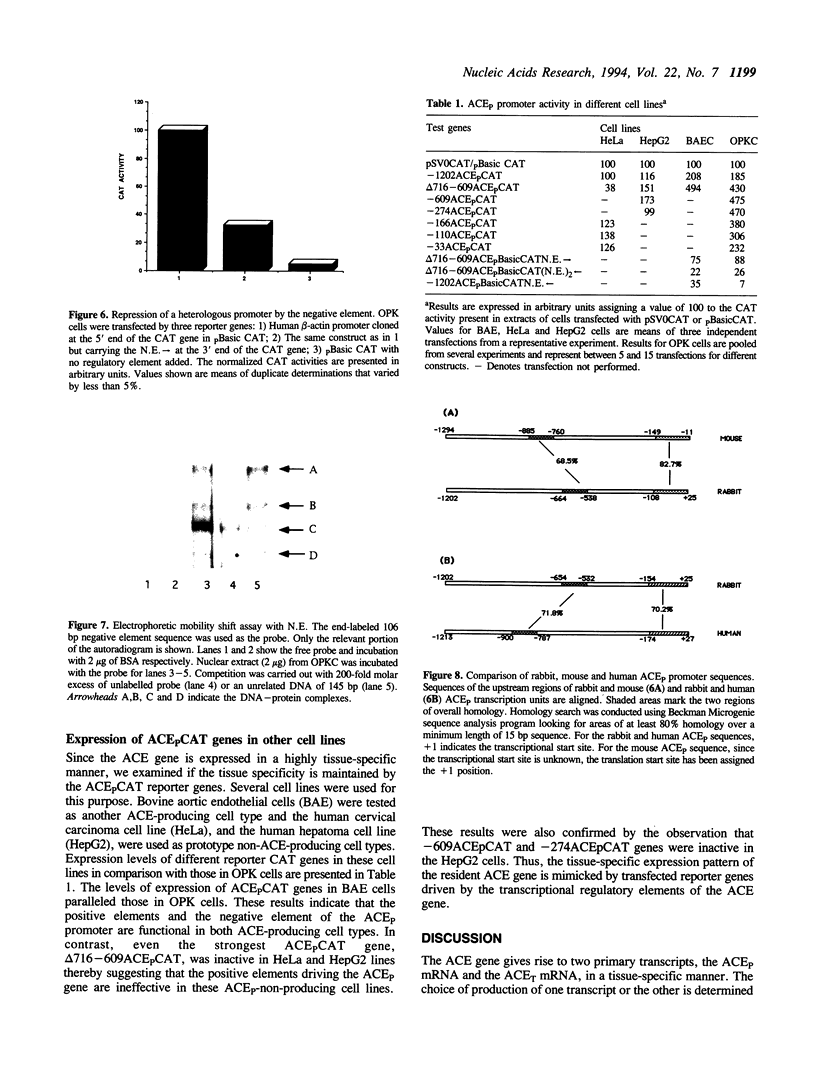
The two tissue-specific mRNAs encoding the isozymes of rabbit angiotensin-converting enzyme (ACE) are generated from the same gene by alternative choice of two transcription initiation sites 5.7 kb apart. In the current study, we have characterized the regulatory sites controlling the transcription of the larger pulmonary isozyme mRNA. For this purpose, reporter genes driven by varying lengths of upstream region of the ACE gene were transfected into ACE-producing cells. Our results demonstrated that the transcription of this gene is primarily driven by positive elements within the first 274 bp DNA upstream of the transcription initiation site. The reporter gene driven by this region was expressed in two ACE-producing cells but not in two ACE-non-producing cells thereby establishing its tissue specificity. Our experiments also revealed the existence of a strong negative element located between -692 and -610 positions. This element suppressed the expression of the reporter gene in a dose-dependent and position and orientation-independent fashion thus suggesting that it is a true silencer element. It could also repress the expression of a reporter gene driven by the heterologous strong promoter of the beta-actin gene. The repressing effects of the negative element could be partially overcome by cotransfecting the isolated negative element along with the reporter gene containing the negative element. This result was possibly due to the functional removal of a limiting trans-acting factor which binds to this element. Electrophoretic mobility shift assays revealed that the negative element can form several complexes with proteins present in the nuclear extract of an ACE-producing cell line. At least part of the negative element is strongly conserved in the upstream regions of the human and mouse ACE genes.
Full text
PDF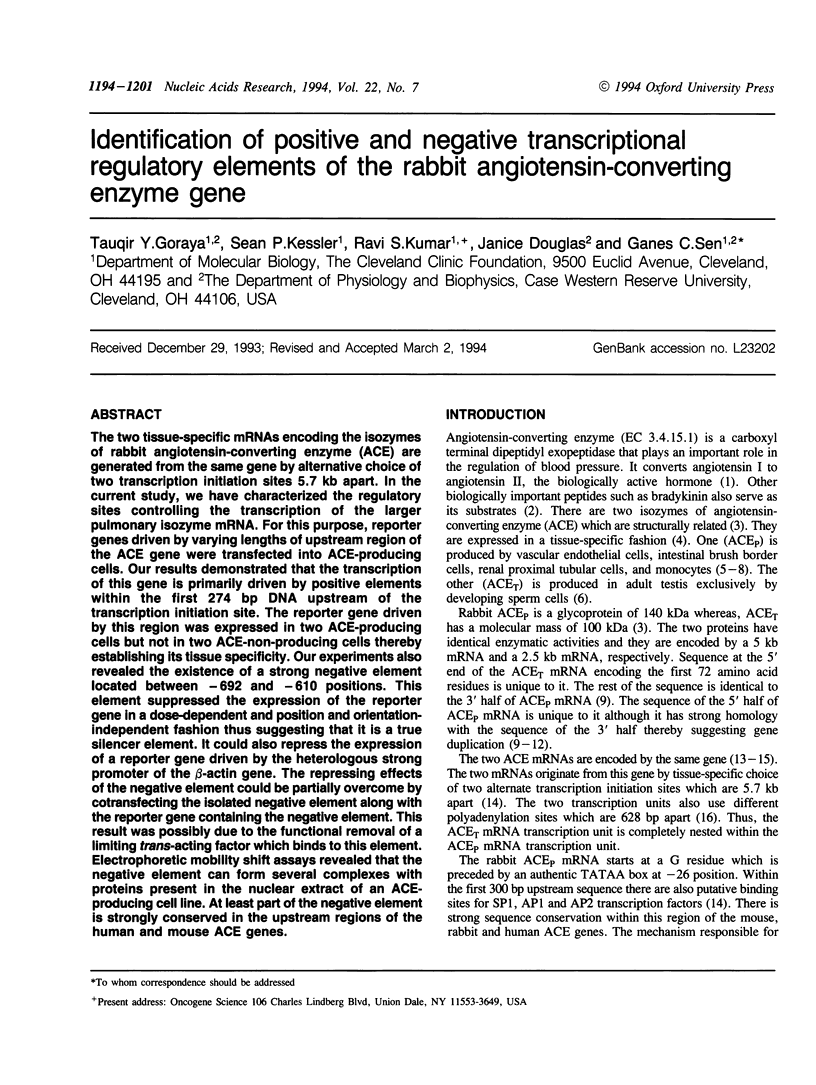



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg T., Sulner J., Lai C. Y., Soffer R. L. Immunohistochemical localization of two angiotensin I-converting isoenzymes in the reproductive tract of the male rabbit. J Histochem Cytochem. 1986 Jun;34(6):753–760. doi: 10.1177/34.6.3009604. [DOI] [PubMed] [Google Scholar]
- Bernstein K. E., Martin B. M., Edwards A. S., Bernstein E. A. Mouse angiotensin-converting enzyme is a protein composed of two homologous domains. J Biol Chem. 1989 Jul 15;264(20):11945–11951. [PubMed] [Google Scholar]
- Braun T., Tannich E., Buschhausen-Denker G., Arnold H. H. Promoter upstream elements of the chicken cardiac myosin light-chain 2-A gene interact with trans-acting regulatory factors for muscle-specific transcription. Mol Cell Biol. 1989 Jun;9(6):2513–2525. doi: 10.1128/mcb.9.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. R., Fox E. A., Strydom D. J., Riordan J. F. Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dorry H. A., Bull H. G., Iwata K., Thornberry N. A., Cordes E. H., Soffer R. L. Molecular and catalytic properties of rabbit testicular dipeptidyl carboxypeptidase. J Biol Chem. 1982 Dec 10;257(23):14128–14133. [PubMed] [Google Scholar]
- El-Dorry H. A., Pickett C. B., MacGregor J. S., Soffer R. L. Tissue-specific expression of mRNAs for dipeptidyl carboxypeptidase isoenzymes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4295–4297. doi: 10.1073/pnas.79.14.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J., Setton C., Silverstein E. Induction of angiotensin converting enzyme in human monocytes in culture. Biochem Biophys Res Commun. 1978 Aug 14;83(3):843–849. doi: 10.1016/0006-291x(78)91471-7. [DOI] [PubMed] [Google Scholar]
- Goodbourn S. Negative regulation of transcriptional initiation in eukaryotes. Biochim Biophys Acta. 1990 Jun 1;1032(1):53–77. doi: 10.1016/0304-419x(90)90012-p. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Tamura T., Mikoshiba K., Masamune Y., Nakanishi Y. Transcription inhibition of the somatic-type phosphoglycerate kinase 1 gene in vitro by a testis-specific factor that recognizes a sequence similar to the binding site for Ets oncoproteins. Nucleic Acids Res. 1991 Jul 25;19(14):3959–3963. doi: 10.1093/nar/19.14.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T. E., Shai S. Y., Langford K. G., Martin B. M., Bernstein K. E. Transcription of testicular angiotensin-converting enzyme (ACE) is initiated within the 12th intron of the somatic ACE gene. Mol Cell Biol. 1990 Aug;10(8):4294–4302. doi: 10.1128/mcb.10.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert C., Houot A. M., Corvol P., Soubrier F. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem. 1991 Aug 15;266(23):15377–15383. [PubMed] [Google Scholar]
- Kalvakolanu D. V., Bandyopadhyay S. K., Tiwari R. K., Sen G. C. Enhancement of expression of exogenous genes by 2-aminopurine. Regulation at the post-transcriptional level. J Biol Chem. 1991 Jan 15;266(2):873–879. [PubMed] [Google Scholar]
- Kumar R. S., Kusari J., Roy S. N., Soffer R. L., Sen G. C. Structure of testicular angiotensin-converting enzyme. A segmental mosaic isozyme. J Biol Chem. 1989 Oct 5;264(28):16754–16758. [PubMed] [Google Scholar]
- Kumar R. S., Thekkumkara T. J., Sen G. C. The mRNAs encoding the two angiotensin-converting isozymes are transcribed from the same gene by a tissue-specific choice of alternative transcription initiation sites. J Biol Chem. 1991 Feb 25;266(6):3854–3862. [PubMed] [Google Scholar]
- Ng S. Y., Gunning P., Eddy R., Ponte P., Leavitt J., Shows T., Kedes L. Evolution of the functional human beta-actin gene and its multi-pseudogene family: conservation of noncoding regions and chromosomal dispersion of pseudogenes. Mol Cell Biol. 1985 Oct;5(10):2720–2732. doi: 10.1128/mcb.5.10.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchett A. A., Cordes E. H. The design and properties of N-carboxyalkyldipeptide inhibitors of angiotensin-converting enzyme. Adv Enzymol Relat Areas Mol Biol. 1985;57:1–84. doi: 10.1002/9780470123034.ch1. [DOI] [PubMed] [Google Scholar]
- Roy S. N., Kusari J., Soffer R. L., Lai C. Y., Sen G. C. Isolation of cDNA clones of rabbit angiotensin converting enzyme: identification of two distinct mRNAs for the pulmonary and the testicular isozymes. Biochem Biophys Res Commun. 1988 Sep 15;155(2):678–684. doi: 10.1016/s0006-291x(88)80548-5. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Webster K. A., Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990 Oct;4(10):1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- Shai S. Y., Langford K. G., Martin B. M., Bernstein K. E. Genomic DNA 5' to the mouse and human angiotensin-converting enzyme genes contains two distinct regions of conserved sequence. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1128–1133. doi: 10.1016/0006-291x(90)90640-9. [DOI] [PubMed] [Google Scholar]
- Soubrier F., Alhenc-Gelas F., Hubert C., Allegrini J., John M., Tregear G., Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner C., Muller M., Baniahmad A., Renkawitz R. Lysozyme gene activity in chicken macrophages is controlled by positive and negative regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4163–4178. doi: 10.1093/nar/15.10.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thekkumkara T. J., Livingston W., 3rd, Kumar R. S., Sen G. C. Use of alternative polyadenylation sites for tissue-specific transcription of two angiotensin-converting enzyme mRNAs. Nucleic Acids Res. 1992 Feb 25;20(4):683–687. doi: 10.1093/nar/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]




