Abstract
The alcohol dehydrogenase (Adh) gene in the Hawaiian species of fruit fly, Drosophila affinidisjuncta, like the Adh genes from all Drosophila species analyzed, is expressed at high levels in the larval fat body via a larval-specific promoter. To identify the cis-acting elements involved in this highly conserved aspect of Adh gene expression, deleted D. affinidisjuncta genes were introduced into D. melanogaster by somatic transformation. Unlike previously described methods, this transformation system allows analysis of Adh gene expression specifically in the larval fat body. The arrangement of sequences influencing expression of the proximal promoter of this gene in the larval fat body differs markedly from that described for the Adh gene from the distant relative, D. melanogaster. Multiple redundant elements dispersed 5' and 3' to the gene, only some of which map to regions carrying evolutionarily conserved sequences, affect expression in the fat body. D. affinidisjuncta employs a novel mode of Adh gene regulation in which the proximal promoter is influenced by sequences having roles in expression of the distal promoter. This gene is also unique in that far upstream sequences can compensate for loss of sequences within 200 bp of the proximal RNA start site. Furthermore, expression is influenced in an unusual, context-dependent manner by a naturally-occurring 3' duplication of the proximal promoter--a feature found only in Hawaiian species.
Full text
PDF

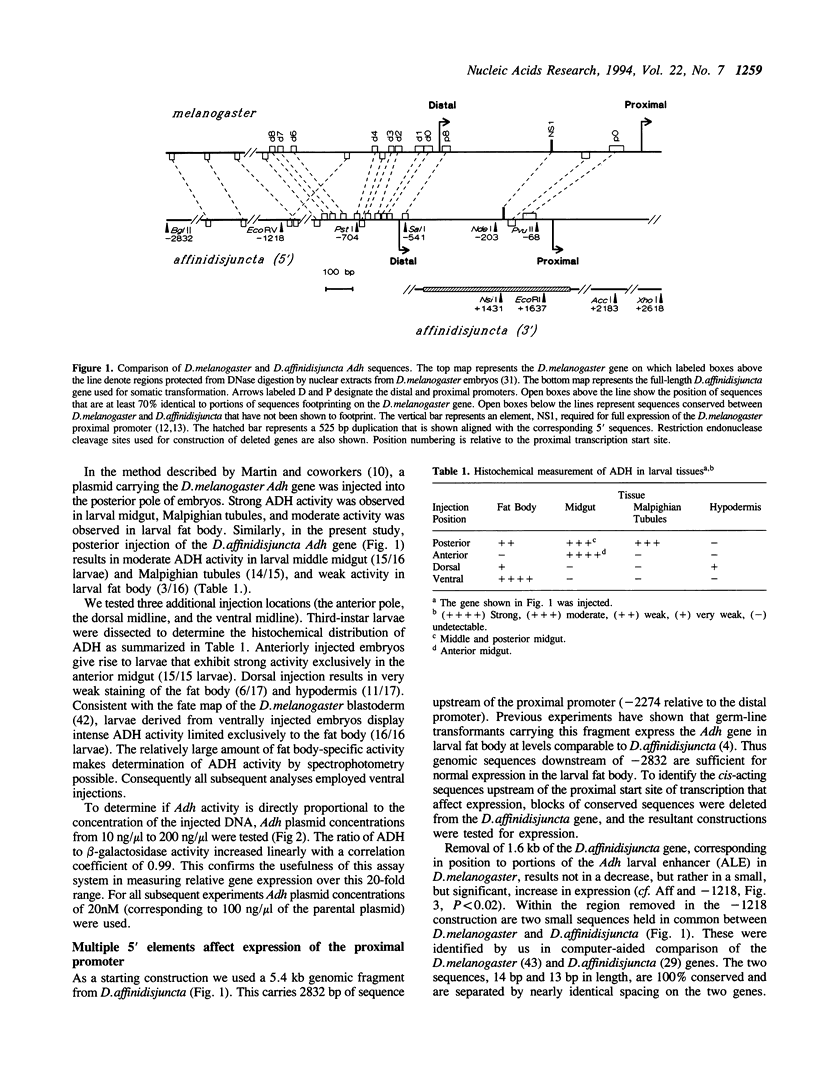
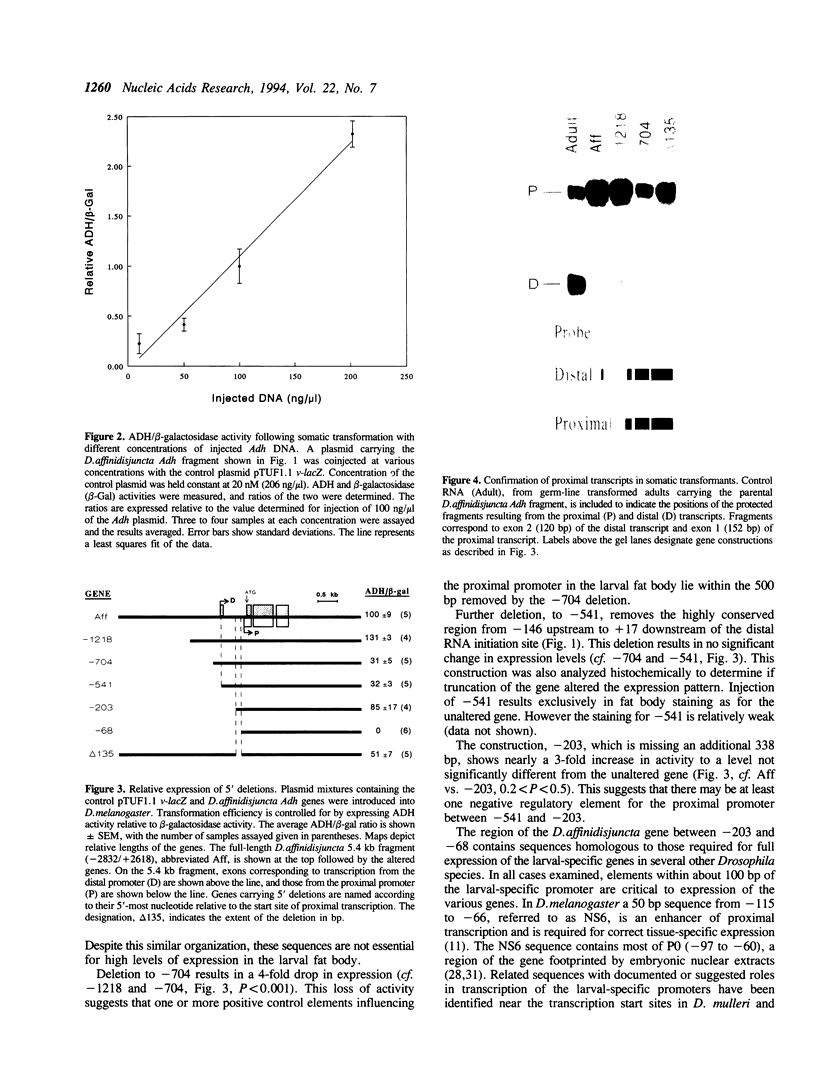

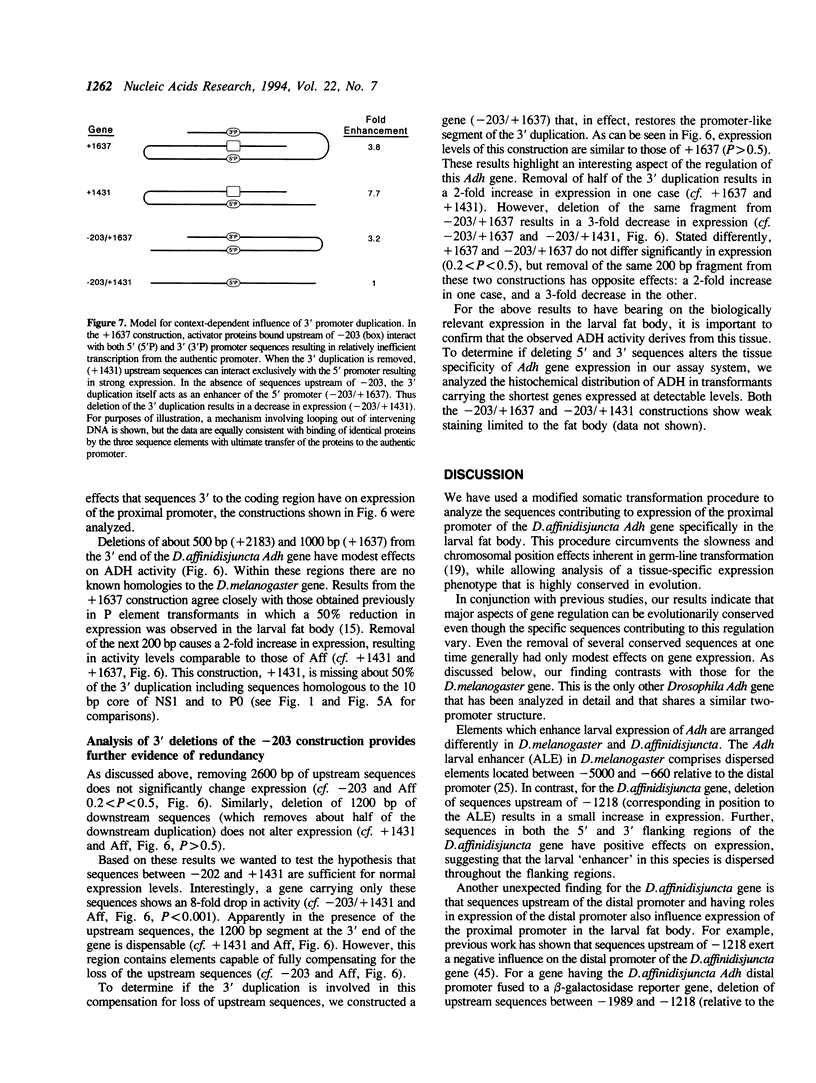


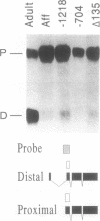
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel T., Bhatt R., Maniatis T. A Drosophila CREB/ATF transcriptional activator binds to both fat body- and liver-specific regulatory elements. Genes Dev. 1992 Mar;6(3):466–480. doi: 10.1101/gad.6.3.466. [DOI] [PubMed] [Google Scholar]
- Ayer S., Benyajati C. Conserved enhancer and silencer elements responsible for differential Adh transcription in Drosophila cell lines. Mol Cell Biol. 1990 Jul;10(7):3512–3523. doi: 10.1128/mcb.10.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer S., Benyajati C. The binding site of a steroid hormone receptor-like protein within the Drosophila Adh adult enhancer is required for high levels of tissue-specific alcohol dehydrogenase expression. Mol Cell Biol. 1992 Feb;12(2):661–673. doi: 10.1128/mcb.12.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer C. A., Curtiss S. W., Weaver J. A., Sullivan D. T. Delineation of cis-acting sequences required for expression of Drosophila mojavensis Adh-1. Genetics. 1992 May;131(1):143–153. doi: 10.1093/genetics/131.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Ewel A., McKeon J., Chovav M., Juan E. Characterization and purification of Adh distal promoter factor 2, Adf-2, a cell-specific and promoter-specific repressor in Drosophila. Nucleic Acids Res. 1992 Sep 11;20(17):4481–4489. doi: 10.1093/nar/20.17.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Place A. R., Wang N., Pentz E., Sofer W. Deletions at intervening sequence splice sites in the alcohol dehydrogenase gene of Drosophila. Nucleic Acids Res. 1982 Nov 25;10(22):7261–7272. doi: 10.1093/nar/10.22.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Wilson A. C. Ancient origin for Hawaiian Drosophilinae inferred from protein comparisons. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4753–4757. doi: 10.1073/pnas.82.14.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Wilson A. C. Molecular evolution of Drosophila and higher Diptera. I. Micro-complement fixation studies of a larval hemolymph protein. J Mol Evol. 1982;18(4):251–264. doi: 10.1007/BF01734103. [DOI] [PubMed] [Google Scholar]
- Brennan M. D., Dickinson W. J. Complex developmental regulation of the Drosophila affinidisjuncta alcohol dehydrogenase gene in Drosophila melanogaster. Dev Biol. 1988 Jan;125(1):64–74. doi: 10.1016/0012-1606(88)90059-0. [DOI] [PubMed] [Google Scholar]
- Brennan M. D., Rowan R. G., Rabinow L., Dickinson W. J. Isolation and initial characterization of the alcohol dehydrogenase gene from Drosophila affinidisjuncta. J Mol Appl Genet. 1984;2(5):436–446. [PubMed] [Google Scholar]
- Brennan M. D., Wu C. Y., Berry A. J. Tissue-specific regulatory differences for the alcohol dehydrogenase genes of Hawaiian Drosophila are conserved in Drosophila melanogaster transformants. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6866–6869. doi: 10.1073/pnas.85.18.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. Identification of cis-regulatory elements required for larval expression of the Drosophila melanogaster alcohol dehydrogenase gene. Genetics. 1990 Mar;124(3):637–646. doi: 10.1093/genetics/124.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. Role of transcriptional interference in the Drosophila melanogaster Adh promoter switch. Nature. 1989 Jan 19;337(6204):279–282. doi: 10.1038/337279a0. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. The role of specific enhancer-promoter interactions in the Drosophila Adh promoter switch. Genes Dev. 1989 Dec;3(12B):2191–2120. doi: 10.1101/gad.3.12b.2191. [DOI] [PubMed] [Google Scholar]
- Dickinson W. J. Evolution of patterns of gene expression in hawaiian picture-winged Drosophila. J Mol Evol. 1980 Dec;16(2):73–94. doi: 10.1007/BF01731579. [DOI] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falb D., Maniatis T. A conserved regulatory unit implicated in tissue-specific gene expression in Drosophila and man. Genes Dev. 1992 Mar;6(3):454–465. doi: 10.1101/gad.6.3.454. [DOI] [PubMed] [Google Scholar]
- Falb D., Maniatis T. Drosophila transcriptional repressor protein that binds specifically to negative control elements in fat body enhancers. Mol Cell Biol. 1992 Sep;12(9):4093–4103. doi: 10.1128/mcb.12.9.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X. M., Brennan M. D. Multiple cis-acting sequences contribute to evolved regulatory variation for Drosophila Adh genes. Genetics. 1992 Jun;131(2):333–343. doi: 10.1093/genetics/131.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X. M., Wu C. Y., Brennan M. D. Complexity in evolved regulatory variation for alcohol dehydrogenase genes in Hawaiian Drosophila. J Mol Evol. 1991 Mar;32(3):220–226. doi: 10.1007/BF02342744. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Drosophila Adh: a promoter element expands the tissue specificity of an enhancer. Cell. 1988 May 6;53(3):451–461. doi: 10.1016/0092-8674(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Regulatory elements involved in Drosophila Adh gene expression are conserved in divergent species and separate elements mediate expression in different tissues. EMBO J. 1986 Jun;5(6):1275–1289. doi: 10.1002/j.1460-2075.1986.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell Y. W., Searles L. L. In vivo transcriptional analysis of the TATA-less promoter of the Drosophila melanogaster vermilion gene. Mol Cell Biol. 1992 Oct;12(10):4571–4577. doi: 10.1128/mcb.12.10.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. A., Posakony J. W., Maniatis T. Correct developmental expression of a cloned alcohol dehydrogenase gene transduced into the Drosophila germ line. Cell. 1983 Aug;34(1):59–73. doi: 10.1016/0092-8674(83)90136-8. [DOI] [PubMed] [Google Scholar]
- Heberlein U., England B., Tjian R. Characterization of Drosophila transcription factors that activate the tandem promoters of the alcohol dehydrogenase gene. Cell. 1985 Jul;41(3):965–977. doi: 10.1016/s0092-8674(85)80077-5. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Livant D. L., Cutting A. E., Britten R. J., Davidson E. H. An in vivo titration of regulatory factors required for expression of a fusion gene in transgenic sea urchin embryos. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7607–7611. doi: 10.1073/pnas.85.20.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Martin A., Osmani A., Sofer W. A transient expression assay for tissue-specific gene expression of alcohol dehydrogenase in Drosophila. Dev Biol. 1986 Oct;117(2):574–580. doi: 10.1016/0012-1606(86)90326-x. [DOI] [PubMed] [Google Scholar]
- Moses K., Heberlein U., Ashburner M. The Adh gene promoters of Drosophila melanogaster and Drosophila orena are functionally conserved and share features of sequence structure and nuclease-protected sites. Mol Cell Biol. 1990 Feb;10(2):539–548. doi: 10.1128/mcb.10.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan R. G., Brennan M. D., Dickinson W. J. Developmentally regulated RNA transcripts coding for alcohol dehydrogenase in Drosophila affinidisjuncta. Genetics. 1986 Oct;114(2):405–433. doi: 10.1093/genetics/114.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan R. G., Dickinson W. J. Nucleotide sequence of the genomic region encoding alcohol dehydrogenase in Drosophila affinidisjuncta. J Mol Evol. 1988 Dec;28(1-2):43–54. doi: 10.1007/BF02143496. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N. L., Hotaling E. C., Subrahmanyam G., Martin P. F., Sofer W. Analysis of sequences regulating larval expression of the Adh gene of Drosophila melanogaster. Genetics. 1991 Nov;129(3):763–771. doi: 10.1093/genetics/129.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N. L., Subrahmanyam G., Clark W., Martin P., Sofer W. Analysis of Adh gene regulation in Drosophila: studies using somatic transformation. Dev Genet. 1989;10(3):210–219. doi: 10.1002/dvg.1020100310. [DOI] [PubMed] [Google Scholar]
- Sofer W., Martin P. F. Analysis of alcohol dehydrogenase gene expression in Drosophila. Annu Rev Genet. 1987;21:203–225. doi: 10.1146/annurev.ge.21.120187.001223. [DOI] [PubMed] [Google Scholar]
- Sofer W., Ursprung H. Drosophila alcohol dehydrogenase. Purification and partial characterization. J Biol Chem. 1968 Jun 10;243(11):3110–3115. [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Technau G. M. A single cell approach to problems of cell lineage and commitment during embryogenesis of Drosophila melanogaster. Development. 1987 May;100(1):1–12. doi: 10.1242/dev.100.1.1. [DOI] [PubMed] [Google Scholar]
- Wu C. Y., Brennan M. D. Similar tissue-specific expression of the Adh genes from different Drosophila species is mediated by distinct arrangements of cis-acting sequences. Mol Gen Genet. 1993 Jul;240(1):58–64. doi: 10.1007/BF00276884. [DOI] [PubMed] [Google Scholar]
- Wu C. Y., Mote J., Jr, Brennan M. D. Tissue-specific expression phenotypes of Hawaiian Drosophila Adh genes in Drosophila melanogaster transformants. Genetics. 1990 Jul;125(3):599–610. doi: 10.1093/genetics/125.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Porton B., Shen L. Y., Eckhardt L. A. Role of the octamer motif in hybrid cell extinction of immunoglobulin gene expression: extinction is dominant in a two enhancer system. Cell. 1989 Aug 11;58(3):441–448. doi: 10.1016/0092-8674(89)90425-x. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]