Abstract
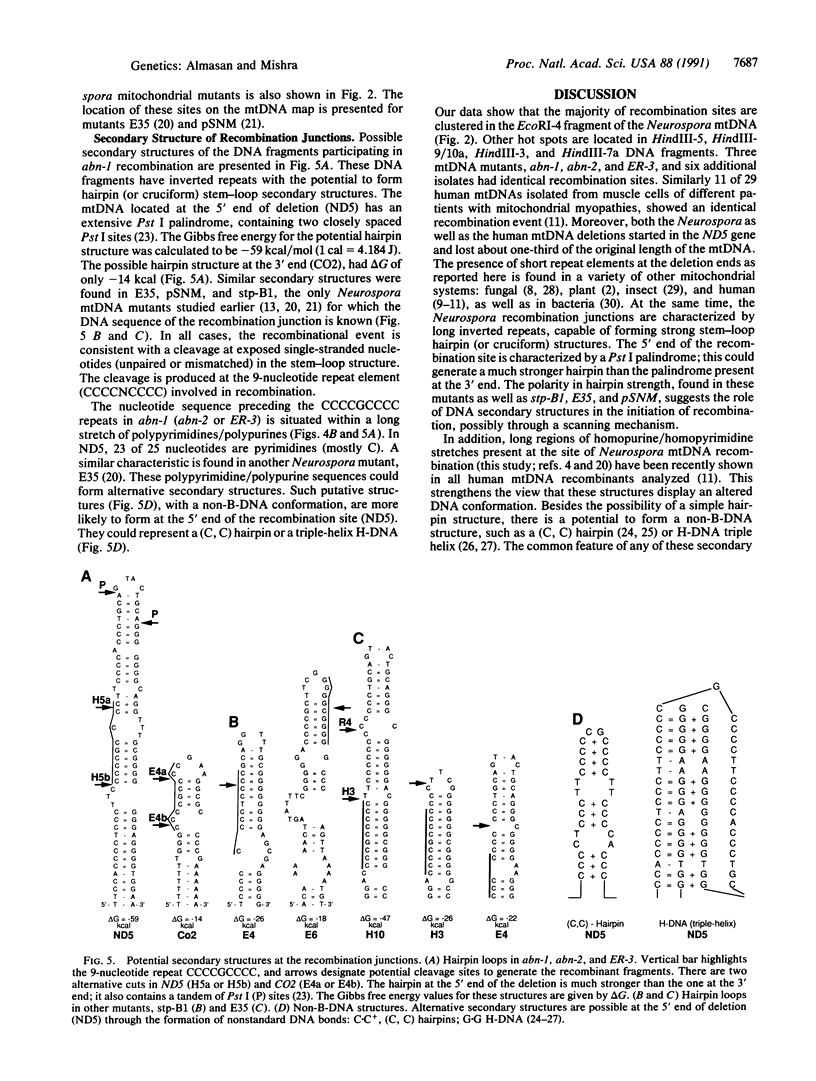
Recombination junctions of several Neurospora mitochondrial DNA (mtDNA) mutants and their revertants were identified. Their nucleotide sequences and putative secondary structures were determined in order to understand the nature of the elements involved in intramolecular recombination. Multiple deletions, involving the same portion of Neurospora mtDNA, were identified in six independently isolated mutants. A 9-nucleotide repeat element, CCCCNCCCC, was found to be involved in these and other Neurospora mitochondrial recombination events. The repeat elements were clustered as hot spots on the Neurospora mtDNA and were associated with palindromic DNA sequences. The palindromes have a potential to generate hairpin structures. A much lower free energy of the putative hairpins at the 5' end of the recombination site, and the possible formation of non-B-DNA structure by polypyrimidine tracks, may be important in the initiation of recombination. Using PCR, we found low levels of a specific mitochondrial deletion in certain Neurospora mutants. Their presence in low amounts in a population with a much larger number of normal mtDNA is unexpected. Contrary to earlier belief, this finding supports the view that deleted, smaller DNA molecules are not always suppressive relative to normal mtDNAs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Kelley R. L., Lambowitz A. M. Mitochondrial plasmids of Neurospora: integration into mitochondrial DNA and evidence for reverse transcription in mitochondria. Cell. 1986 Nov 21;47(4):505–516. doi: 10.1016/0092-8674(86)90615-x. [DOI] [PubMed] [Google Scholar]
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Almasan A., Mishra N. C. Characterization of a novel plasmid-like element in Neurospora crassa derived mostly from the mitochondrial DNA. Nucleic Acids Res. 1990 Oct 11;18(19):5871–5877. doi: 10.1093/nar/18.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasan A., Mishra N. C. Molecular characterization of the mitochondrial DNA of a new stopper mutant ER-3 of Neurospora crassa. Genetics. 1988 Dec;120(4):935–945. doi: 10.1093/genetics/120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y. S., Kawasaki I., Ikeda H., Liu L. F. Illegitimate recombination mediated by calf thymus DNA topoisomerase II in vitro. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2076–2080. doi: 10.1073/pnas.85.7.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand H., Collins R. A., Stohl L. L., Goewert R. R., Lambowitz A. M. Deletion mutants of Neurospora crassa mitochondrial DNA and their relationship to the "stop-start" growth phenotype. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6032–6036. doi: 10.1073/pnas.77.10.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein R., Lee E. Y., To H., Young L. J., Sery T. W., Hayes R. C., Friedmann T., Lee W. H. Human retinoblastoma susceptibility gene: genomic organization and analysis of heterozygous intragenic deletion mutants. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2210–2214. doi: 10.1073/pnas.85.7.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock N. Oxazaphosphorine cytostatics: past-present-future. Seventh Cain Memorial Award lecture. Cancer Res. 1989 Jan 1;49(1):1–7. [PubMed] [Google Scholar]
- Bullock P., Champoux J. J., Botchan M. Association of crossover points with topoisomerase I cleavage sites: a model for nonhomologous recombination. Science. 1985 Nov 22;230(4728):954–958. doi: 10.1126/science.2997924. [DOI] [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Mitochondrial DNA from Podospora anserina. I. Isolation and characterization. Mol Gen Genet. 1979 Mar 27;171(3):229–238. doi: 10.1007/BF00267577. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M. J., Hatahet Z., Huang X. T. The actions of Neurospora endo-exonuclease on double strand DNAs. J Biol Chem. 1989 Aug 5;264(22):13093–13101. [PubMed] [Google Scholar]
- Garnjobst L., Wilson J. F., Tatum E. L. Studies on a cytoplasmic character in Neurospora crassa. J Cell Biol. 1965 Aug;26(2):413–425. doi: 10.1083/jcb.26.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. R., Levine P. H., Metzger S., Glaser G. Recombination and replication of plasmid-like derivatives of a short section of the mitochondrial chromosome of Neurospora crassa. Genetics. 1989 Apr;121(4):693–701. doi: 10.1093/genetics/121.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Htun H., Dahlberg J. E. Topology and formation of triple-stranded H-DNA. Science. 1989 Mar 24;243(4898):1571–1576. doi: 10.1126/science.2648571. [DOI] [PubMed] [Google Scholar]
- Kohwi Y., Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3781–3785. doi: 10.1073/pnas.85.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M. Preparation and analysis of mitochondrial ribosomes. Methods Enzymol. 1979;59:421–433. doi: 10.1016/0076-6879(79)59103-4. [DOI] [PubMed] [Google Scholar]
- Lazarus C. M., Earl A. J., Turner G., Küntzel H. Amplification of a mitochondrial DNA sequence in the cytoplasmically inherited 'ragged' mutant of Aspergillus amstelodami. Eur J Biochem. 1980 May;106(2):633–641. doi: 10.1111/j.1432-1033.1980.tb04611.x. [DOI] [PubMed] [Google Scholar]
- Lestienne P., Ponsot G. Kearns-Sayre syndrome with muscle mitochondrial DNA deletion. Lancet. 1988 Apr 16;1(8590):885–885. doi: 10.1016/s0140-6736(88)91632-7. [DOI] [PubMed] [Google Scholar]
- Lindlöf M., Kiuru A., Käriäinen H., Kalimo H., Lang H., Pihko H., Rapola J., Somer H., Somer M., Savontaus M. L. Gene deletions in X-linked muscular dystrophy. Am J Hum Genet. 1989 Apr;44(4):496–503. [PMC free article] [PubMed] [Google Scholar]
- Locker J., Lewin A., Rabinowitz M. The structure and organization of mitochondrial DNA from petite yeast. Plasmid. 1979 Apr;2(2):155–181. doi: 10.1016/0147-619x(79)90036-2. [DOI] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Danilevskaya O. N., Voloshin O. N., Balatskaya S. V., Dobrynin V. N., Filippov S. A., Frank-Kamenetskii M. D. An unusual DNA structure detected in a telomeric sequence under superhelical stress and at low pH. Nature. 1989 Jun 22;339(6226):634–637. doi: 10.1038/339634a0. [DOI] [PubMed] [Google Scholar]
- Michel B., Ehrlich S. D. Illegitimate recombination at the replication origin of bacteriophage M13. Proc Natl Acad Sci U S A. 1986 May;83(10):3386–3390. doi: 10.1073/pnas.83.10.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niagro F. D., Mishra N. C. An ethidium bromide induced mutant of Neurospora crassa defective in mitochondrial DNA. Curr Genet. 1989 Oct;16(4):303–305. doi: 10.1007/BF00422117. [DOI] [PubMed] [Google Scholar]
- Rand D. M., Harrison R. G. Molecular population genetics of mtDNA size variation in crickets. Genetics. 1989 Mar;121(3):551–569. doi: 10.1093/genetics/121.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- Shapiro L. J., Yen P., Pomerantz D., Martin E., Rolewic L., Mohandas T. Molecular studies of deletions at the human steroid sulfatase locus. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8477–8481. doi: 10.1073/pnas.86.21.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S. Vaccinia DNA topoisomerase I promotes illegitimate recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1989 May;86(10):3489–3493. doi: 10.1073/pnas.86.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Turker M. S., Domenico J. M., Cummings D. J. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. A potential role for an 11 base-pair consensus sequence in the excision process. J Mol Biol. 1987 Nov 20;198(2):171–185. doi: 10.1016/0022-2836(87)90304-4. [DOI] [PubMed] [Google Scholar]
- Voloshin O. N., Mirkin S. M., Lyamichev V. I., Belotserkovskii B. P., Frank-Kamenetskii M. D. Chemical probing of homopurine-homopyrimidine mirror repeats in supercoiled DNA. Nature. 1988 Jun 2;333(6172):475–476. doi: 10.1038/333475a0. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends Genet. 1989 Jan;5(1):9–13. doi: 10.1016/0168-9525(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Patel P. I., Chinault A. C., Stout J. T., Jackson L. G., Hildebrand B. M., Caskey C. T. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984 Aug 2;310(5976):412–414. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]
- Yin S., Heckman J., RajBhandary U. L. Highly conserved GC-rich palindromic DNA sequences flank tRNA genes in Neurospora crassa mitochondria. Cell. 1981 Nov;26(3 Pt 1):325–332. doi: 10.1016/0092-8674(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Servidei S., Gellera C., Bertini E., DiMauro S., DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989 May 25;339(6222):309–311. doi: 10.1038/339309a0. [DOI] [PubMed] [Google Scholar]
- de Vries H., Alzner-DeWeerd B., Breitenberger C. A., Chang D. D., de Jonge J. C., RajBhandary U. L. The E35 stopper mutant of Neurospora crassa: precise localization of deletion endpoints in mitochondrial DNA and evidence that the deleted DNA codes for a subunit of NADH dehydrogenase. EMBO J. 1986 Apr;5(4):779–785. doi: 10.1002/j.1460-2075.1986.tb04281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Gene. 1983 Mar;21(3):193–202. doi: 10.1016/0378-1119(83)90002-1. [DOI] [PubMed] [Google Scholar]