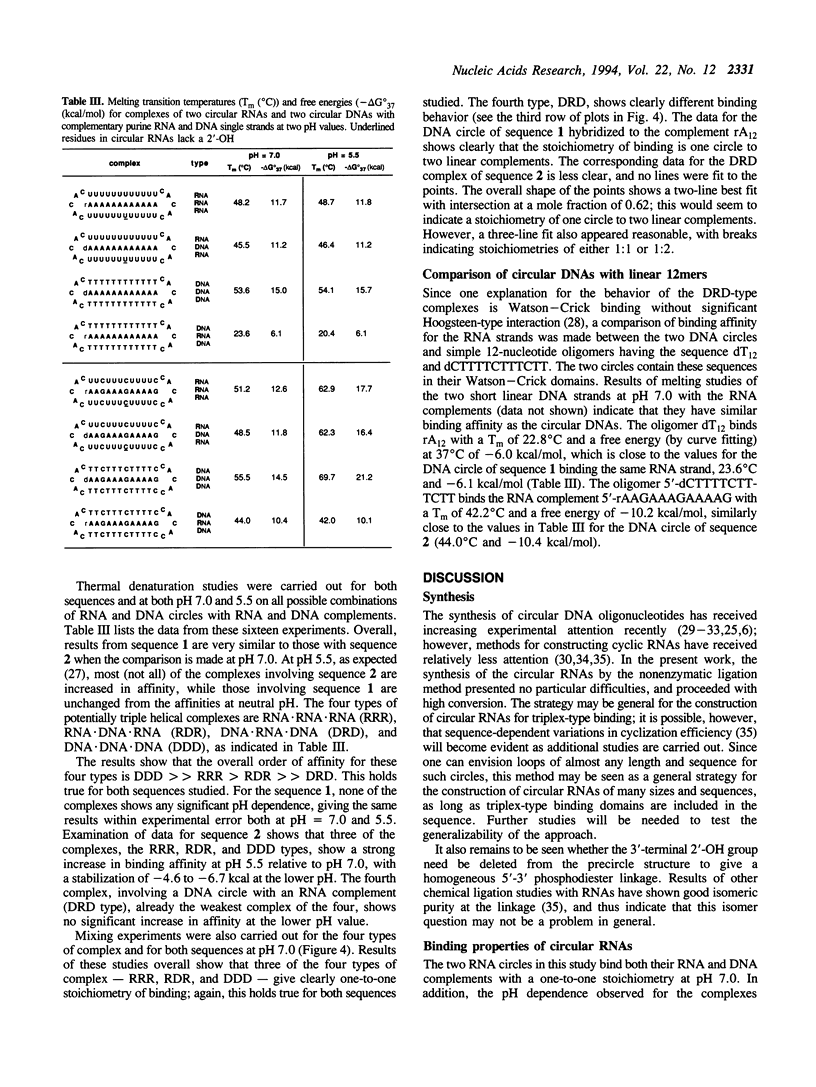
Abstract
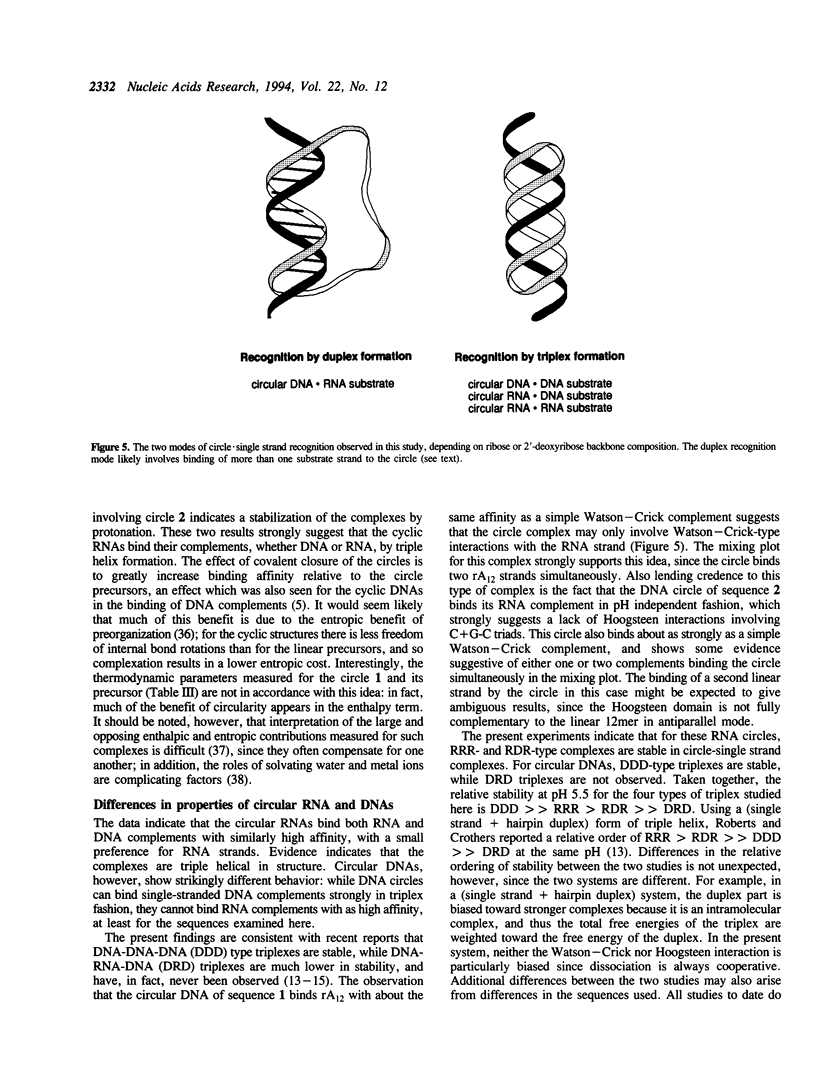
We report the synthesis and nucleic acid binding properties of two cyclic RNA oligonucleotides designed to bind single-stranded nucleic acids by pyr.pur.pyr-type triple helix formation. The circular RNAs are 34 nucleotides in size and were cyclized using a template-directed nonenzymatic ligation. To ensure isomeric 3'-5' purity in the ligation reaction, one nucleotide at the ligation site is a 2'-deoxyribose. One circle (1) is complementary to the sequence 5'-A12, and the second (2) is complementary to 5'-AAGAAAGAAAAG. Results of thermal denaturation experiments and mixing studies show that both circles bind complementary single-stranded DNA or RNA substrates by triple helix formation, in which two domains in a pyrimidine-rich circle sandwich a central purine-rich substrate. The affinities of these circles with their purine complements are much higher than the affinities of either the linear precursors or simple Watson-Crick DNA complements. For example, circle 1 binds rA12 (pH 7.0, 10 mM MgCl2, 100 mM NaCl) with a Tm of 48 degrees C and a Kd (37 degrees C) of 4.1 x 10(-9) M, while the linear precursor of the circle binds with a Tm of 34 degrees C and a Kd of 1.2 x 10(-6) M. The complexes of circle 2 are pH-dependent, as expected for triple helical complexes involving C(+)G.C triads, and mixing plots for both circles reveal one-to-one stoichiometry of binding either to RNA or DNA substrates. Comparison of circular RNAs with previously synthesized circular DNA oligonucleotides of the same sequence reveals similar behavior in the binding of DNA, but strikingly different behavior in the binding of RNA. The cyclic DNAs show high DNA-binding selectivity, giving relatively weaker duplex-type binding with complementary RNAs. The relative order of thermodynamic stability for the four types of triplex studied here is found to be DDD >> RRR > RDR >> DRD. The results are discussed in the context of recent reports of strong triplex dependence on RNA versus DNA backbones. Triplex-forming circular RNAs represent a novel and potentially useful strategy for high-affinity binding of RNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco M. L., Carcuro A., Tondelli L., Garbesi A., Bonora G. M. One pot solution synthesis of cyclic oligodeoxyribonucleotides. Nucleic Acids Res. 1990 May 11;18(9):2661–2669. doi: 10.1093/nar/18.9.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza D. J., Kool E. T. Strong binding of single-stranded DNA by stem-loop oligonucleotides. J Biomol Struct Dyn. 1992 Aug;10(1):141–152. doi: 10.1080/07391102.1992.10508634. [DOI] [PubMed] [Google Scholar]
- Dolinnaya N. G., Blumenfeld M., Merenkova I. N., Oretskaya T. S., Krynetskaya N. F., Ivanovskaya M. G., Vasseur M., Shabarova Z. A. Oligonucleotide circularization by template-directed chemical ligation. Nucleic Acids Res. 1993 Nov 25;21(23):5403–5407. doi: 10.1093/nar/21.23.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinnaya N. G., Sokolova N. I., Ashirbekova D. T., Shabarova Z. A. The use of BrCN for assembling modified DNA duplexes and DNA-RNA hybrids; comparison with water-soluble carbodiimide. Nucleic Acids Res. 1991 Jun 11;19(11):3067–3072. doi: 10.1093/nar/19.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erie D. A., Jones R. A., Olson W. K., Sinha N. K., Breslauer K. J. Melting behavior of a covalently closed, single-stranded, circular DNA. Biochemistry. 1989 Jan 10;28(1):268–273. doi: 10.1021/bi00427a037. [DOI] [PubMed] [Google Scholar]
- Escudé C., François J. C., Sun J. S., Ott G., Sprinzl M., Garestier T., Hélène C. Stability of triple helices containing RNA and DNA strands: experimental and molecular modeling studies. Nucleic Acids Res. 1993 Dec 11;21(24):5547–5553. doi: 10.1093/nar/21.24.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannangeli C., Thuong N. T., Hélène C. Oligonucleotide clamps arrest DNA synthesis on a single-stranded DNA target. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10013–10017. doi: 10.1073/pnas.90.21.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Gryaznov S. M., Lloyd D. H. Modulation of oligonucleotide duplex and triplex stability via hydrophobic interactions. Nucleic Acids Res. 1993 Dec 25;21(25):5909–5915. doi: 10.1093/nar/21.25.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Dervan P. B. Sequence-specific recognition of double helical RNA and RNA.DNA by triple helix formation. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3806–3810. doi: 10.1073/pnas.90.9.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe R. I., McCaffrey A. P., Borozdina L. U., McCampbell E. S., Vaghefi M. M. Effect of excess water on the desilylation of oligoribonucleotides using tetrabutylammonium fluoride. Nucleic Acids Res. 1993 Oct 11;21(20):4739–4741. doi: 10.1093/nar/21.20.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya E., Yanagawa H. Template-directed polymerization of oligoadenylates using cyanogen bromide. Biochemistry. 1986 Nov 18;25(23):7423–7430. doi: 10.1021/bi00371a026. [DOI] [PubMed] [Google Scholar]
- LIPSETT M. N. COMPLEX FORMATION BETWEEN POLYCYTIDYLIC ACID AND GUANINE OLIGONUCLEOTIDES. J Biol Chem. 1964 Apr;239:1256–1260. [PubMed] [Google Scholar]
- Pan T., Gutell R. R., Uhlenbeck O. C. Folding of circularly permuted transfer RNAs. Science. 1991 Nov 29;254(5036):1361–1364. doi: 10.1126/science.1720569. [DOI] [PubMed] [Google Scholar]
- Petersheim M., Turner D. H. Base-stacking and base-pairing contributions to helix stability: thermodynamics of double-helix formation with CCGG, CCGGp, CCGGAp, ACCGGp, CCGGUp, and ACCGGUp. Biochemistry. 1983 Jan 18;22(2):256–263. doi: 10.1021/bi00271a004. [DOI] [PubMed] [Google Scholar]
- Roberts R. W., Crothers D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992 Nov 27;258(5087):1463–1466. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- Searle M. S., Williams D. H. On the stability of nucleic acid structures in solution: enthalpy-entropy compensations, internal rotations and reversibility. Nucleic Acids Res. 1993 May 11;21(9):2051–2056. doi: 10.1093/nar/21.9.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xodo L. E., Manzini G., Quadrifoglio F. Spectroscopic and calorimetric investigation on the DNA triplex formed by d(CTCTTCTTTCTTTTCTTTCTTCTC) and d(GAGAAGAAAGA) at acidic pH. Nucleic Acids Res. 1990 Jun 25;18(12):3557–3564. doi: 10.1093/nar/18.12.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vroom E., Broxterman H. J., Sliedregt L. A., van der Marel G. A., van Boom J. H. Synthesis of cyclic oligonucleotides by a modified phosphotriester approach. Nucleic Acids Res. 1988 May 25;16(10):4607–4620. doi: 10.1093/nar/16.10.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]