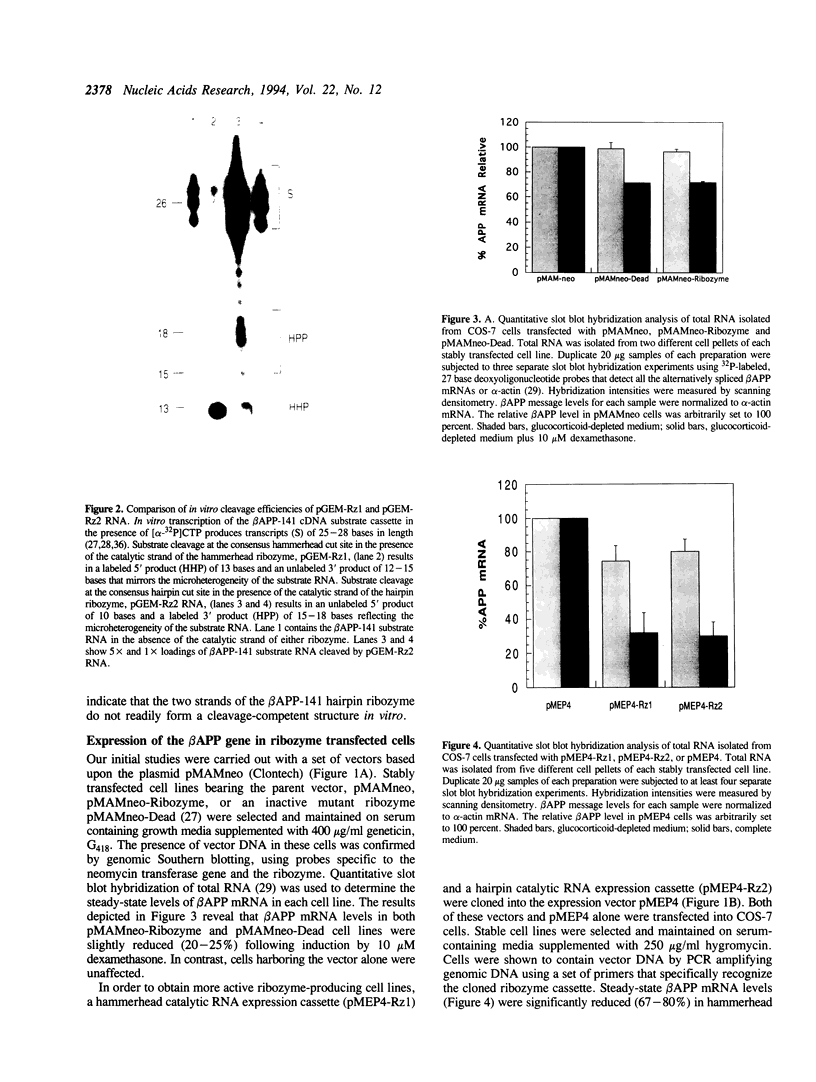
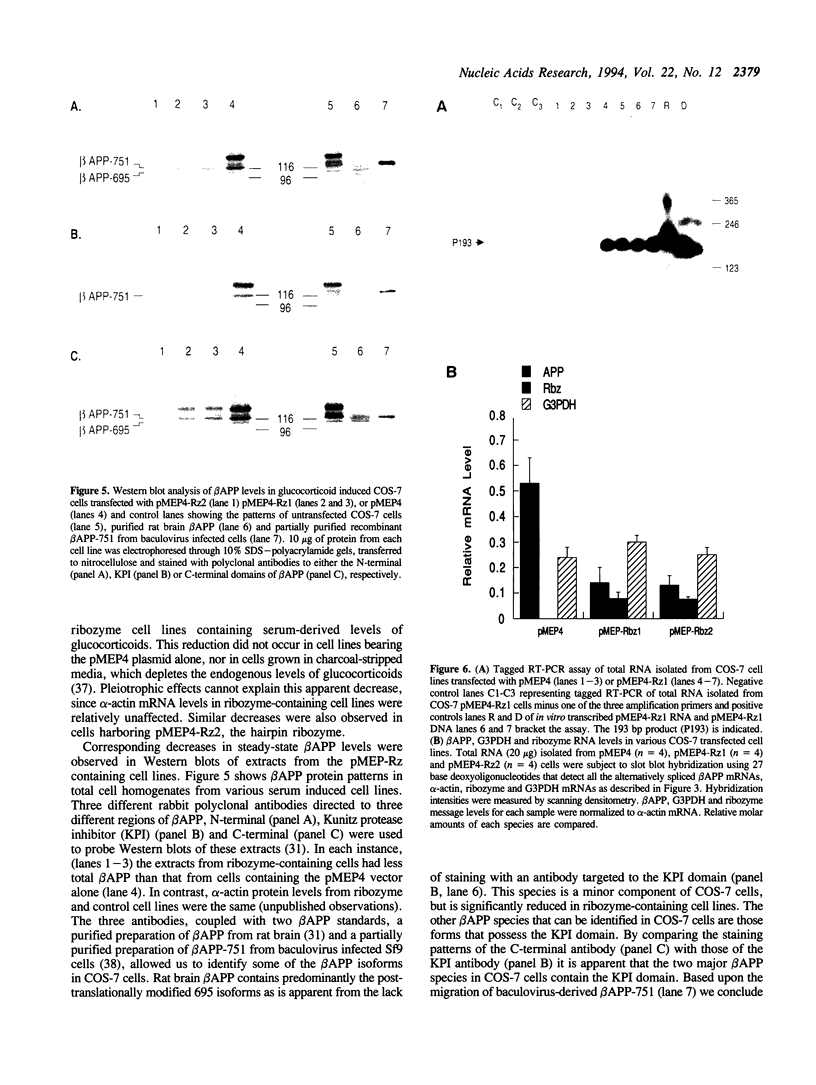
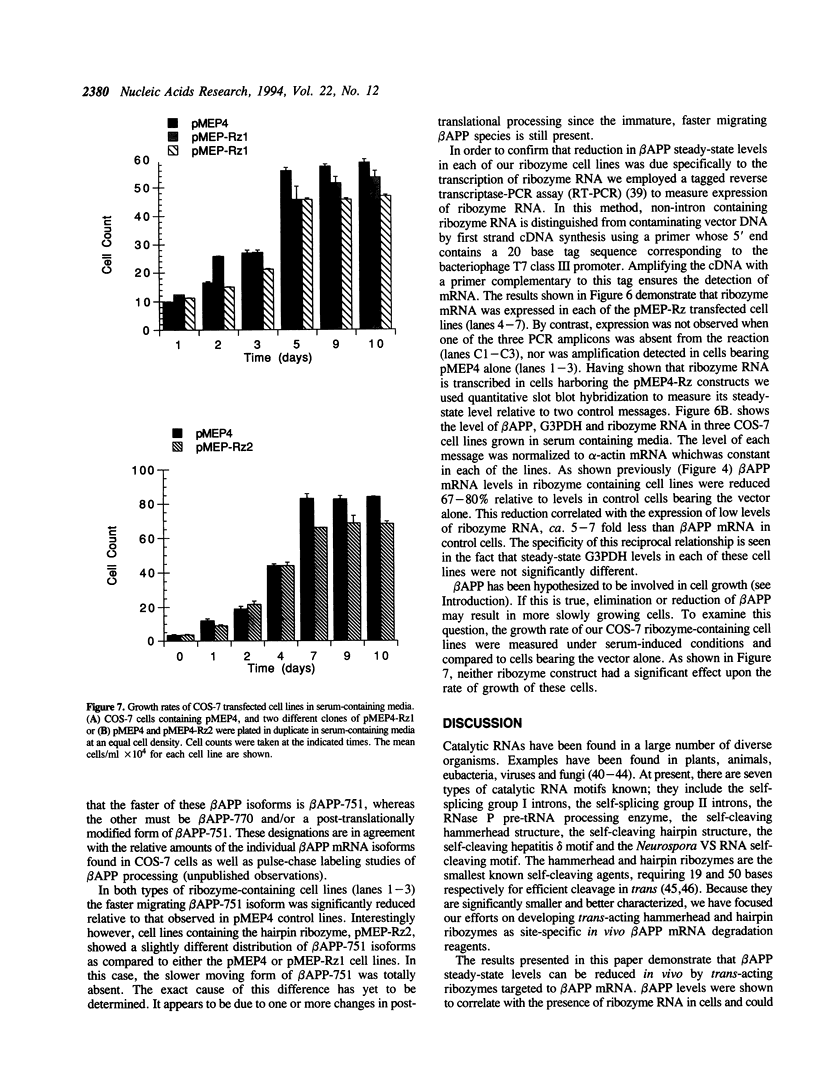
Abstract
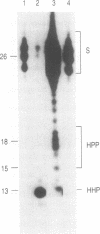
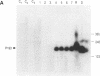
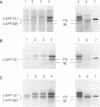
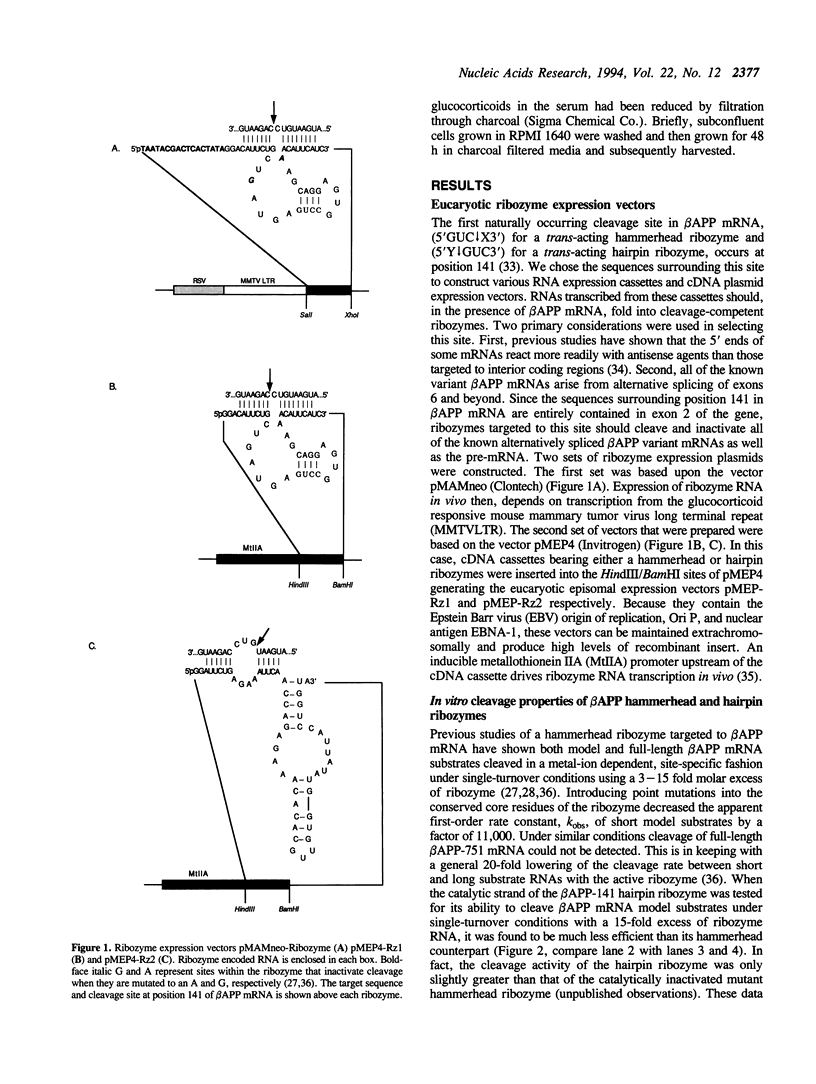
Two sets of eucaryotic expression vectors encoding trans-acting hammerhead ribozymes and trans-acting hairpin ribozymes were constructed. In one set of vectors ribozyme RNA transcription was placed under the control of a mouse mammary tumor virus long terminal repeat (MMTV-LTR). In the other set ribozyme expression was controlled by a metallothionein IIA (Mt-IIA) promoter. Each ribozyme was directed to the first target sequence in the Alzheimer amyloid peptide precursor mRNA (beta APP mRNA), 5' decreases GUC decreases 3'. Ribozyme RNA transcribed from these vectors, which should cleave all six alternatively spliced forms of beta APP mRNA as well as beta APP pre-mRNA, was shown to cleave a beta APP RNA substrate analog in vitro. Stably transfected COS-7 cell lines bearing both vector types were prepared. Steady-state levels of beta APP mRNA were reduced 25-30% in cells containing either active or mutant hammerhead ribozyme vectors driven by the MMTV-LTR promoter grown in the presence of glucocorticoids. In cell lines bearing Mt-IIA driven ribozymes steady-state levels of beta APP mRNA were reduced 67-80% in both hammerhead and hairpin ribozyme containing cell lines following promoter induction by glucocorticoids. These levels correlate with the appearance of low levels of induced ribozyme RNA. In contrast, steady-state alpha-actin mRNA and G3PDH mRNA levels in these cells remained constant. Western blotting of cell extracts revealed that all forms of beta APP were correspondingly reduced. Neither the RNA nor protein decreases observed in ribozyme transfected cell lines were observed in stably transfected control cells bearing the vector alone. These results suggest that ribozyme-mediated degradation of beta APP mRNA in COS-7 cells does not depend on ribozyme cleavage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Lambowitz A. M. A protein required for splicing group I introns in Neurospora mitochondria is mitochondrial tyrosyl-tRNA synthetase or a derivative thereof. Cell. 1987 Jul 31;50(3):331–345. doi: 10.1016/0092-8674(87)90488-0. [DOI] [PubMed] [Google Scholar]
- Amini S., DeSeau V., Reddy S., Shalloway D., Bolen J. B. Regulation of pp60c-src synthesis by inducible RNA complementary to c-src mRNA in polyomavirus-transformed rat cells. Mol Cell Biol. 1986 Jul;6(7):2305–2316. doi: 10.1128/mcb.6.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E., Pictet R., Grange T. Can hammerhead ribozymes be efficient tools to inactivate gene function? Nucleic Acids Res. 1994 Feb 11;22(3):293–300. doi: 10.1093/nar/22.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H. P., DeArmond S. J., Prusiner S. B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992 Apr 16;356(6370):577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Denman R. B. Cleavage of full-length beta APP mRNA by hammerhead ribozymes. Nucleic Acids Res. 1993 Aug 25;21(17):4119–4125. doi: 10.1093/nar/21.17.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman R. B., Purow B., Rubenstein R., Miller D. L. Hammerhead ribozyme cleavage of hamster prion pre-mRNA in complex cell-free model systems. Biochem Biophys Res Commun. 1992 Jul 31;186(2):1171–1177. doi: 10.1016/0006-291x(92)90870-q. [DOI] [PubMed] [Google Scholar]
- Denman R. B., Rosenzcwaig R., Miller D. L. A system for studying the effect(s) of familial Alzheimer disease mutations on the processing of the beta-amyloid peptide precursor. Biochem Biophys Res Commun. 1993 Apr 15;192(1):96–103. doi: 10.1006/bbrc.1993.1386. [DOI] [PubMed] [Google Scholar]
- Denman R., Potempska A., Wolfe G., Ramakrishna N., Miller D. L. Distribution and activity of alternatively spliced Alzheimer amyloid peptide precursor and scrapie PrP mRNAs on rat brain polysomes. Arch Biochem Biophys. 1991 Jul;288(1):29–38. doi: 10.1016/0003-9861(91)90161-b. [DOI] [PubMed] [Google Scholar]
- Dropulić B., Lin N. H., Martin M. A., Jeang K. T. Functional characterization of a U5 ribozyme: intracellular suppression of human immunodeficiency virus type 1 expression. J Virol. 1992 Mar;66(3):1432–1441. doi: 10.1128/jvi.66.3.1432-1441.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. M., Coats S. R. Tissue-specific permutations of self-cleaving newt satellite-2 transcripts. Gene. 1991 Nov 15;107(2):213–218. doi: 10.1016/0378-1119(91)90321-2. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Usiak M., Younkin L. H., Younkin S. G. Expression of beta amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer's disease using PCR. Neuron. 1990 Feb;4(2):253–267. doi: 10.1016/0896-6273(90)90100-t. [DOI] [PubMed] [Google Scholar]
- Gustincich S., Manfioletti G., Del Sal G., Schneider C., Carninci P. A fast method for high-quality genomic DNA extraction from whole human blood. Biotechniques. 1991 Sep;11(3):298-300, 302. [PubMed] [Google Scholar]
- Hampel A., Tritz R. RNA catalytic properties of the minimum (-)sTRSV sequence. Biochemistry. 1989 Jun 13;28(12):4929–4933. doi: 10.1021/bi00438a002. [DOI] [PubMed] [Google Scholar]
- Herrin D. L., Chen Y. F., Schmidt G. W. RNA splicing in Chlamydomonas chloroplasts. Self-splicing of 23 S preRNA. J Biol Chem. 1990 Dec 5;265(34):21134–21140. [PubMed] [Google Scholar]
- Jahroudi N., Foster R., Price-Haughey J., Beitel G., Gedamu L. Cell-type specific and differential regulation of the human metallothionein genes. Correlation with DNA methylation and chromatin structure. J Biol Chem. 1990 Apr 15;265(11):6506–6511. [PubMed] [Google Scholar]
- Joachim C. L., Selkoe D. J. The seminal role of beta-amyloid in the pathogenesis of Alzheimer disease. Alzheimer Dis Assoc Disord. 1992 Spring;6(1):7–34. doi: 10.1097/00002093-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Johnstone E. M., Chaney M. O., Moore R. E., Ward K. E., Norris F. H., Little S. P. Alzheimer's disease amyloid peptide is encoded by two exons and shows similarity to soybean trypsin inhibitor. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1248–1255. doi: 10.1016/0006-291x(89)91112-1. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Sharmeen L., Dinter-Gottlieb G., Taylor J. Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988 Dec;62(12):4439–4444. doi: 10.1128/jvi.62.12.4439-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König G., Mönning U., Czech C., Prior R., Banati R., Schreiter-Gasser U., Bauer J., Masters C. L., Beyreuther K. Identification and differential expression of a novel alternative splice isoform of the beta A4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells. J Biol Chem. 1992 May 25;267(15):10804–10809. [PubMed] [Google Scholar]
- L'Huillier P. J., Davis S. R., Bellamy A. R. Cytoplasmic delivery of ribozymes leads to efficient reduction in alpha-lactalbumin mRNA levels in C127I mouse cells. EMBO J. 1992 Dec;11(12):4411–4418. doi: 10.1002/j.1460-2075.1992.tb05541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fauci G., Lahiri D. K., Salton S. R., Robakis N. K. Characterization of the 5'-end region and the first two exons of the beta-protein precursor gene. Biochem Biophys Res Commun. 1989 Feb 28;159(1):297–304. doi: 10.1016/0006-291x(89)92437-6. [DOI] [PubMed] [Google Scholar]
- LeBlanc A. C., Kovacs D. M., Chen H. Y., Villaré F., Tykocinski M., Autilio-Gambetti L., Gambetti P. Role of amyloid precursor protein (APP): study with antisense transfection of human neuroblastoma cells. J Neurosci Res. 1992 Apr;31(4):635–645. doi: 10.1002/jnr.490310407. [DOI] [PubMed] [Google Scholar]
- Lemaire H. G., Salbaum J. M., Multhaup G., Kang J., Bayney R. M., Unterbeck A., Beyreuther K., Müller-Hill B. The PreA4(695) precursor protein of Alzheimer's disease A4 amyloid is encoded by 16 exons. Nucleic Acids Res. 1989 Jan 25;17(2):517–522. doi: 10.1093/nar/17.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Tully T., White K. Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron. 1992 Oct;9(4):595–605. doi: 10.1016/0896-6273(92)90024-8. [DOI] [PubMed] [Google Scholar]
- Ninomiya H., Roch J. M., Sundsmo M. P., Otero D. A., Saitoh T. Amino acid sequence RERMS represents the active domain of amyloid beta/A4 protein precursor that promotes fibroblast growth. J Cell Biol. 1993 May;121(4):879–886. doi: 10.1083/jcb.121.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., Sinha S. The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989 Sep 14;341(6238):144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Potempska A., Styles J., Mehta P., Kim K. S., Miller D. L. Purification and tissue level of the beta-amyloid peptide precursor of rat brain. J Biol Chem. 1991 May 5;266(13):8464–8469. [PubMed] [Google Scholar]
- Potter P. M., Harris L. C., Remack J. S., Edwards C. C., Brent T. P. Ribozyme-mediated modulation of human O6-methylguanine-DNA methyltransferase expression. Cancer Res. 1993 Apr 15;53(8):1731–1734. [PubMed] [Google Scholar]
- Price D. L., Borchelt D. R., Sisodia S. S. Alzheimer disease and the prion disorders amyloid beta-protein and prion protein amyloidoses. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6381–6384. doi: 10.1073/pnas.90.14.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna N., Saikumar P., Potempska A., Wisniewski H. M., Miller D. L. Expression of human Alzheimer amyloid precursor protein in insect cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):983–989. doi: 10.1016/0006-291x(91)91515-e. [DOI] [PubMed] [Google Scholar]
- Roch J. M., Shapiro I. P., Sundsmo M. P., Otero D. A., Refolo L. M., Robakis N. K., Saitoh T. Bacterial expression, purification, and functional mapping of the amyloid beta/A4 protein precursor. J Biol Chem. 1992 Feb 5;267(4):2214–2221. [PubMed] [Google Scholar]
- Rosen D. R., Martin-Morris L., Luo L. Q., White K. A Drosophila gene encoding a protein resembling the human beta-amyloid protein precursor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2478–2482. doi: 10.1073/pnas.86.7.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Sundsmo M., Roch J. M., Kimura N., Cole G., Schubert D., Oltersdorf T., Schenk D. B. Secreted form of amyloid beta protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989 Aug 25;58(4):615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Scanlon K. J., Jiao L., Funato T., Wang W., Tone T., Rossi J. J., Kashani-Sabet M. Ribozyme-mediated cleavage of c-fos mRNA reduces gene expression of DNA synthesis enzymes and metallothionein. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10591–10595. doi: 10.1073/pnas.88.23.10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Cole G., Saitoh T., Oltersdorf T. Amyloid beta protein precursor is a mitogen. Biochem Biophys Res Commun. 1989 Jul 14;162(1):83–88. doi: 10.1016/0006-291x(89)91965-7. [DOI] [PubMed] [Google Scholar]
- Schubert D., Jin L. W., Saitoh T., Cole G. The regulation of amyloid beta protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989 Dec;3(6):689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Schubert D., Schroeder R., LaCorbiere M., Saitoh T., Cole G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science. 1988 Jul 8;241(4862):223–226. doi: 10.1126/science.2968652. [DOI] [PubMed] [Google Scholar]
- Shuldiner A. R., Tanner K., Moore C. A., Roth J. RNA template-specific PCR: an improved method that dramatically reduces false positives in RT-PCR. Biotechniques. 1991 Dec;11(6):760–763. [PubMed] [Google Scholar]
- Snyder D. S., Wu Y., Wang J. L., Rossi J. J., Swiderski P., Kaplan B. E., Forman S. J. Ribozyme-mediated inhibition of bcr-abl gene expression in a Philadelphia chromosome-positive cell line. Blood. 1993 Jul 15;82(2):600–605. [PubMed] [Google Scholar]
- Tanzi R. E., George-Hyslop P. S., Gusella J. F. Molecular genetics of Alzheimer disease amyloid. J Biol Chem. 1991 Nov 5;266(31):20579–20582. [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Van Nostrand W. E., Cunningham D. D. Purification of protease nexin II from human fibroblasts. J Biol Chem. 1987 Jun 25;262(18):8508–8514. [PubMed] [Google Scholar]
- Van Nostrand W. E., Wagner S. L., Suzuki M., Choi B. H., Farrow J. S., Geddes J. W., Cotman C. W., Cunningham D. D. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989 Oct 12;341(6242):546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- Xing Z., Whitton J. L. An anti-lymphocytic choriomeningitis virus ribozyme expressed in tissue culture cells diminishes viral RNA levels and leads to a reduction in infectious virus yield. J Virol. 1993 Apr;67(4):1840–1847. doi: 10.1128/jvi.67.4.1840-1847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. Q., Kathe S. D., Goodrich-Blair H., Nierzwicki-Bauer S. A., Shub D. A. Bacterial origin of a chloroplast intron: conserved self-splicing group I introns in cyanobacteria. Science. 1990 Dec 14;250(4987):1566–1570. doi: 10.1126/science.2125747. [DOI] [PubMed] [Google Scholar]
- Yu M., Ojwang J., Yamada O., Hampel A., Rapapport J., Looney D., Wong-Staal F. A hairpin ribozyme inhibits expression of diverse strains of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6340–6344. doi: 10.1073/pnas.90.13.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]