Abstract
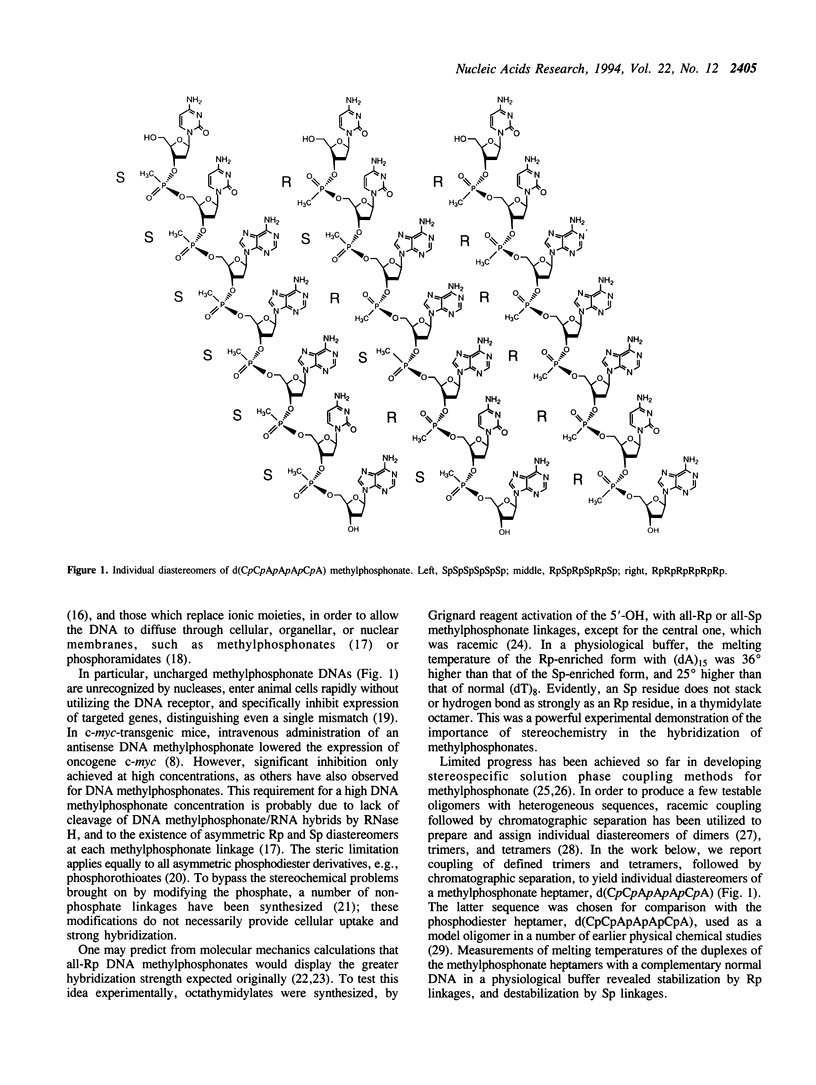
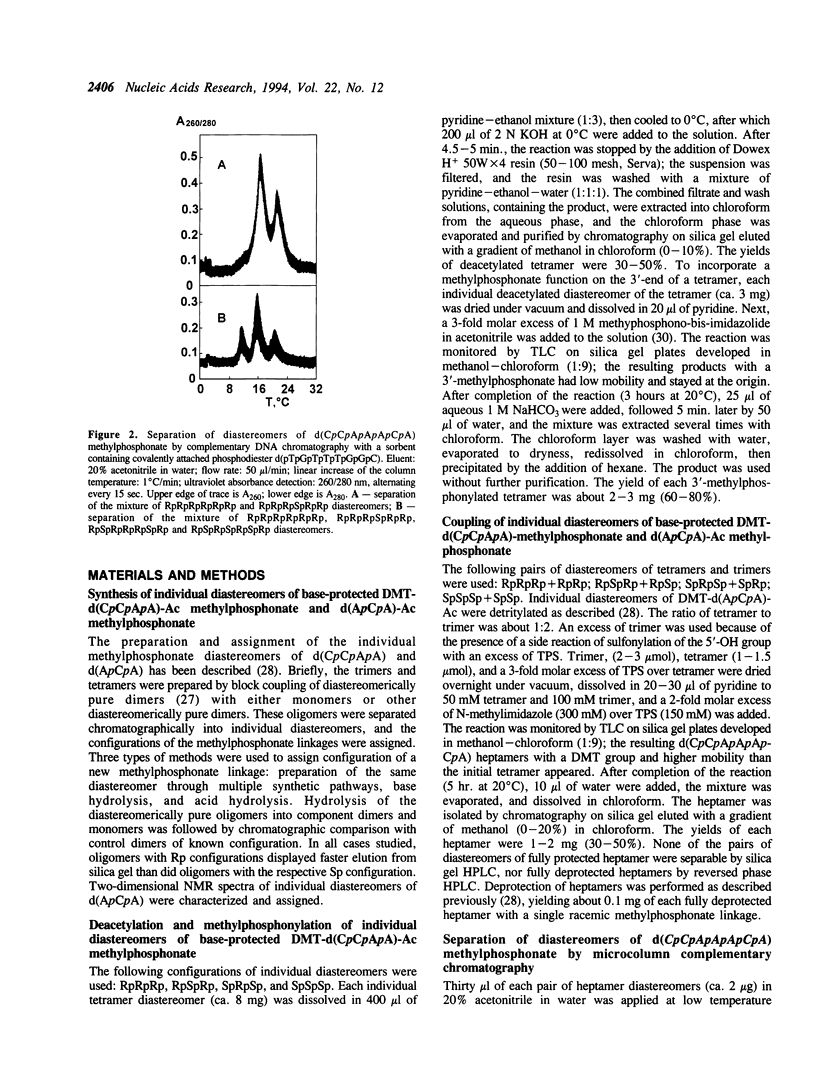
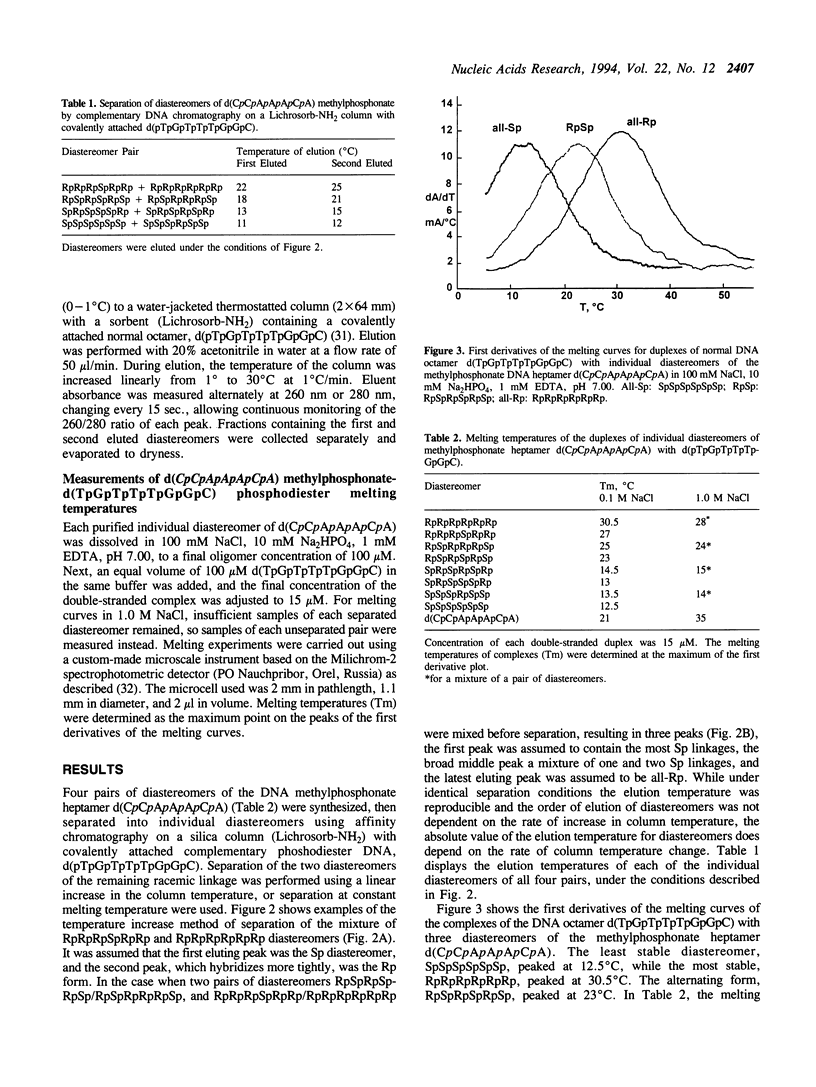
DNA methylphosphonates are candidate derivatives for use in antisense DNA therapy. Their efficacy is limited by weak hybridization. One hypothesis to explain this phenomenon holds that one configuration of the chiral methylphosphonate linkage, Rp, permits stronger base pairing than the other configuration, Sp. To test this hypothesis, four specific pairs of Rp and Sp diastereomers of the DNA methylphosphonate heptamer d(CpCpApApApCpA) were prepared by block coupling of different combinations of individual diastereomers of d(CpCpApA) and d(ApCpA). Each pair of the diastereomers of the heptamer was separated into individual diastereomes using affinity chromatography on a Lichrosorb-NH2 silica column with a covalently attached complementary normal DNA octamer, d(pTpGpTpTpTpGpGpC). The stabilities of complementary complexes of phosphodiester d(TpGpTpTpTpGpGpC) with 8 individual diastereomers of methylphosphonate d(CpCpApApApCpA) were studied by measuring their melting temperatures (Tm). A direct correlation of Tm values with the number of Rp methylphosphonate centers in the heptamer was found: the more Rp centers, the higher the stability of the complex. Tm values for the diastereomers with 6 all-Rp or all-Sp methylphosphonate centers were found to be 30.5 degrees and 12.5 degrees C, respectively, in 100 mM NaCl, 10 mM Na2HPO4, 1 mM EDTA, pH 7.0 with 15 microM of each oligomer. On the average, each substitution of one Rp-center to an Sp-center in the heptamer decreased the Tm by 3 degrees C. Under the same conditions, the Tm of the normal DNA heptamer with its complement was 21 degrees C. These results are consistent with the model that all-Rp methylphosphonate DNAs hybridize much more tightly to complementary normal DNA than do racemic methylphosphonate DNAs, and may therefore exhibit greater potency as antisense inhibitors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S. Antisense oligonucleotides as antiviral agents. Trends Biotechnol. 1992 May;10(5):152–158. doi: 10.1016/0167-7799(92)90203-8. [DOI] [PubMed] [Google Scholar]
- Belikova A. M., Zarytova V. F., Grineva N. I. Synthesis of ribonucleosides and diribonucleoside phosphates containing 2-chloroethylamine and nitrogen mustard residues. Tetrahedron Lett. 1967 Sep;37:3557–3562. doi: 10.1016/s0040-4039(01)89794-x. [DOI] [PubMed] [Google Scholar]
- Bichenkova E. V., Abramova T. V., Vorob'ev YuN, Zarytova V. F., Ivanova E. M., Maltseva T. V., Lebedev A. V. 2D NMR and molecular mechanics structural studies on the oligonucleotide duplex d[p(TGTTTGGC)].d[p(CCAAACA)] and its modified derivatives. Nucleic Acids Symp Ser. 1991;(24):115–116. [PubMed] [Google Scholar]
- Cooney M., Czernuszewicz G., Postel E. H., Flint S. J., Hogan M. E. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988 Jul 22;241(4864):456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- Crooke S. T. Therapeutic applications of oligonucleotides. Annu Rev Pharmacol Toxicol. 1992;32:329–376. doi: 10.1146/annurev.pa.32.040192.001553. [DOI] [PubMed] [Google Scholar]
- Ferguson D. M., Kollman P. A. Application of free-energy decomposition to determine the relative stability of R and S oligodeoxyribonucleotide methylphosphonates. Antisense Res Dev. 1991 Fall;1(3):243–254. [PubMed] [Google Scholar]
- Gray G. D., Hernandez O. M., Hebel D., Root M., Pow-Sang J. M., Wickstrom E. Antisense DNA inhibition of tumor growth induced by c-Ha-ras oncogene in nude mice. Cancer Res. 1993 Feb 1;53(3):577–580. [PubMed] [Google Scholar]
- Lesnikowski Z. J., Jaworska M., Stec W. J. Octa(thymidine methanephosphonates) of partially defined stereochemistry: synthesis and effect of chirality at phosphorus on binding to pentadecadeoxyriboadenylic acid. Nucleic Acids Res. 1990 Apr 25;18(8):2109–2115. doi: 10.1093/nar/18.8.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhov S. G., Podyminogin M. A., Sergeev D. S., Silnikov V. N., Kutyavin I. V., Shishkin G. V., Zarytova V. P. Synthesis and high stability of complementary complexes of N-(2-hydroxyethyl)phenazinium derivatives of oligonucleotides. Bioconjug Chem. 1992 Sep-Oct;3(5):414–419. doi: 10.1021/bc00017a010. [DOI] [PubMed] [Google Scholar]
- Löschner T., Engels J. W. Diastereomeric dinucleoside-methylphosphonates: determination of configuration with the 2-D NMR ROESY technique. Nucleic Acids Res. 1990 Sep 11;18(17):5083–5088. doi: 10.1093/nar/18.17.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991 Dec 6;254(5037):1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- Ratajczak M. Z., Kant J. A., Luger S. M., Hijiya N., Zhang J., Zon G., Gewirtz A. M. In vivo treatment of human leukemia in a scid mouse model with c-myb antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11823–11827. doi: 10.1073/pnas.89.24.11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec W. J., Grajkowski A., Koziolkiewicz M., Uznanski B. Novel route to oligo(deoxyribonucleoside phosphorothioates). Stereocontrolled synthesis of P-chiral oligo(deoxyribonucleoside phosphorothioates). Nucleic Acids Res. 1991 Nov 11;19(21):5883–5888. doi: 10.1093/nar/19.21.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vtorushina I. A., Gorbunov Iu A., Zarytova V. F., Komarova N. I., Lebedev A. V., Lokhov S. G., Manaenko A. V., Siniakov A. N., Shishkina I. G. Issedovanie diastereomerov neionnykh analogov oligonukleotidov. VI. Razdelenie diastereomerov étilovykh fosfotriéfirov oktanukleotida d(GCCAAACA) metodom vysokoéffektivnoi affinnoi khromatografii. Bioorg Khim. 1992 Jan;18(1):92–99. [PubMed] [Google Scholar]
- Vyazovkina E. V., Rife J. P., Lebedev A. V., Wickstrom E. Preparation of trimers and tetramers of mixed sequence oligodeoxynucleoside methylphosphonates and assignment of configurations at the chiral phosphorus. Nucleic Acids Res. 1993 Dec 25;21(25):5957–5963. doi: 10.1093/nar/21.25.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L., Rosolen A., Neckers L. M. In vivo modulation of N-myc expression by continuous perfusion with an antisense oligonucleotide. Antisense Res Dev. 1991 Winter;1(4):343–350. doi: 10.1089/ard.1991.1.343. [DOI] [PubMed] [Google Scholar]
- Wickstrom E., Bacon T. A., Wickstrom E. L. Down-regulation of c-MYC antigen expression in lymphocytes of Emu-c-myc transgenic mice treated with anti-c-myc DNA methylphosphonates. Cancer Res. 1992 Dec 15;52(24):6741–6745. [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. Strategies for administering targeted therapeutic oligodeoxynucleotides. Trends Biotechnol. 1992 Aug;10(8):281–287. doi: 10.1016/0167-7799(92)90245-q. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]



