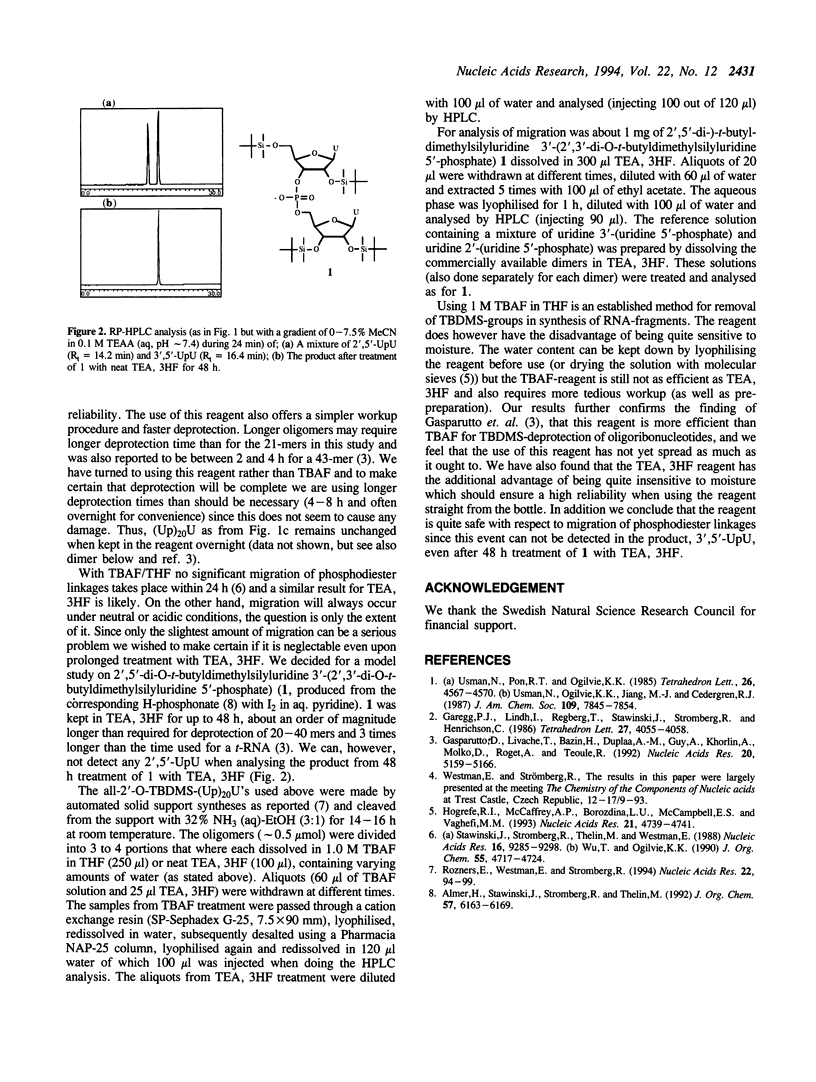
Full text
PDF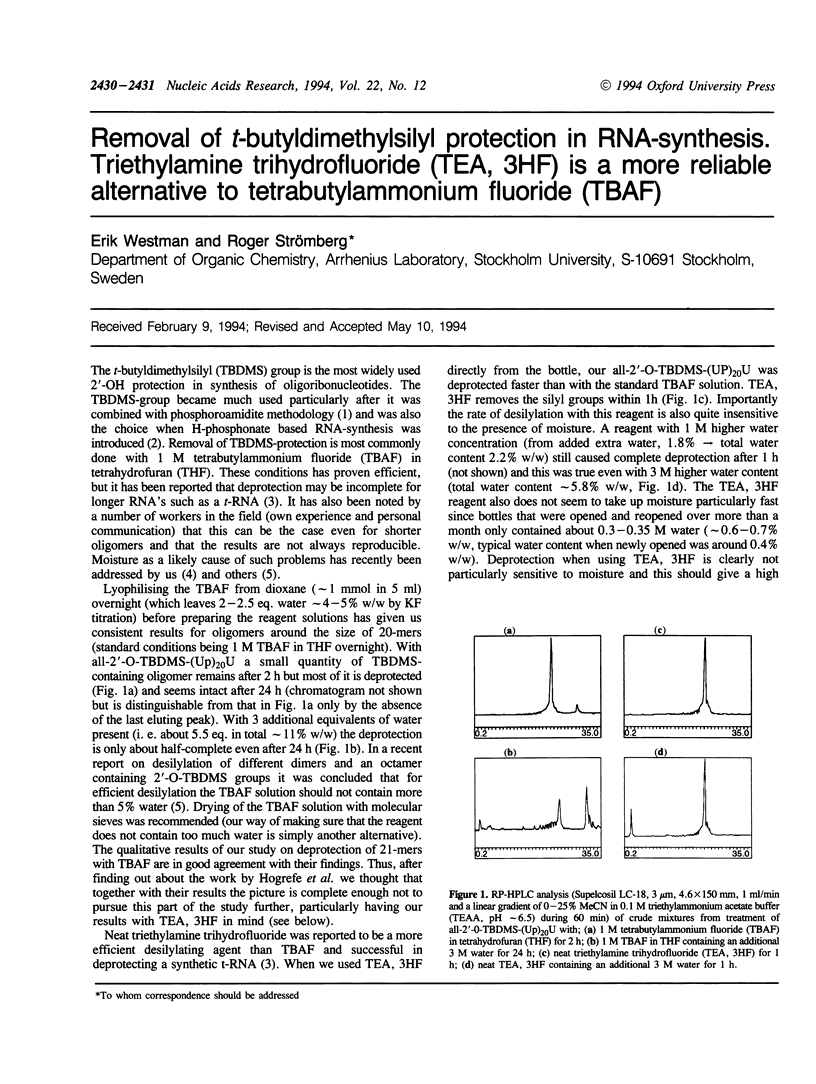
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gasparutto D., Livache T., Bazin H., Duplaa A. M., Guy A., Khorlin A., Molko D., Roget A., Téoule R. Chemical synthesis of a biologically active natural tRNA with its minor bases. Nucleic Acids Res. 1992 Oct 11;20(19):5159–5166. doi: 10.1093/nar/20.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe R. I., McCaffrey A. P., Borozdina L. U., McCampbell E. S., Vaghefi M. M. Effect of excess water on the desilylation of oligoribonucleotides using tetrabutylammonium fluoride. Nucleic Acids Res. 1993 Oct 11;21(20):4739–4741. doi: 10.1093/nar/21.20.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozners E., Westman E., Strömberg R. Evaluation of 2'-hydroxyl protection in RNA-synthesis using the H-phosphonate approach. Nucleic Acids Res. 1994 Jan 11;22(1):94–99. doi: 10.1093/nar/22.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawinski J., Strömberg R., Thelin M., Westman E. Studies on the t-butyldimethylsilyl group as 2'-O-protection in oligoribonucleotide synthesis via the H-phosphonate approach. Nucleic Acids Res. 1988 Oct 11;16(19):9285–9298. doi: 10.1093/nar/16.19.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]



