Abstract
Background
Liver diseases are major global health problems. Ginseng extract has antioxidant, immune-modulatory and anti-inflammatory activities. This study investigated the effect of ginseng extract on carbon tetrachloride (CCl4)-induced liver fibrosis in rats.
Methods
Male Wistar rats were divided into four groups: control group, ginseng group, CCl4 group and CCl4 + ginseng group. Liver injury was induced by the intraperitoneal (I.P) injection of 3 ml/kg CCl4 (30% in olive oil) weekly for 8 weeks. The control group was I.P injected with olive oil. The expression of genes encoding transforming growth factor beta (TGF-β), type I TGF-β receptor (TβR-1), type II TGF-β receptor (TβR-II), mothers against decapentaplegic homolog 2 (Smad2), Smad3, Smad4, matrix metalloproteinase 2 (MMP2), MMP9, tissue inhibitor matrix metalloproteinase-1 (TIMP-1), Collagen 1a2 (Col1a2), Collagen 3a1 (Col3a1), interleukin-8 (IL-8) and interleukin -10 (IL-10) were measured by real-time PCR.
Results
Treatment with ginseng extract decreased hepatic fat deposition and lowered hepatic reticular fiber accumulation compared with the CCl4 group. The CCl4 group showed a significant increase in hepatotoxicity biomarkers and up-regulation of the expression of genes encoding TGF-β, TβR-I, TβR-II, MMP2, MMP9, Smad-2,-3, -4, and IL-8 compared with the control group. However, CCl4 administration resulted in the significant down-regulation of IL-10 mRNA expression compared with the control group. Interestingly, ginseng extract supplementation completely reversed the biochemical markers of hepatotoxicity and the gene expression alterations induced by CCl4.
Conclusion
ginseng extract had an anti‐fibrosis effect via the regulation of the TGF‐β1/Smad signaling pathway in the CCl4‐induced liver fibrosis model. The major target was the inhibition of the expression of TGF‐β1, Smad2, and Smad3.
Keywords: Ginseng extract, Carbon tetrachloride, Gene expression, Real time PCR
Background
The liver plays a key role in various pathological disorders such as fatty liver, hepatic virus infection, chemical hepatotoxins, and toxicity cases [1, 2]. Several hepatotoxicants including polycyclic aromatic hydrocarbons, nitrosamines, and carbon tetrachloride (CCl4) are transformed into intermediate reactive oxygen species that have hepatotoxic effects in humans and experimental animal models [3]. For models of human disease, the rat offers many advantages over mice and other organisms, including the size of its body and substructures in organs, as well as the ability to measure drug effects at specific anatomical areas [1].
Chronic liver diseases are worldwide health problems causing approximately 800,000 deaths per year [2, 3]. Of these, liver fibrosis is caused by inflammation and the excessive accumulation of the extracellular matrix. Subsequently, cirrhosis occurs and can cause hepatocellular carcinoma [4]. Although advanced fibrosis is reversible depending on the degree of fibrosis, end-stage cirrhosis is irreversible [5].
The inhibition of hepatic inflammation and fibrosis is crucial in preventing cirrhosis and HCC. However, the currently used methods for fibrosis evaluation are invasive such as liver biopsy or non-invasive including serum and genetic tests, and imaging techniques [6].
Carbon tetrachloride (CCl4) is a hepatotoxin that targets both the liver and kidneys. CCl4 is activated in the liver to highly reactive trichloromethyl radicals that initiate the free radical-mediated lipid peroxidation of membrane phospholipids, causing functional and morphological changes in the cell membrane, which stimulate hepatotoxicity, fibrosis [7], cirrhosis and HCC in animal species [8]. Reactive oxygen species results in oxidative stress that plays a significant role in liver fibrogenesis [9]. Tri-chloromethyl radicals produced from CCl4 metabolism initiate reactions that cause liver steatosis, fibrosis or cirrhosis [9] via the activation of cytokines such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β expression, and the inhibition of nitric oxide (NO) formation [10–12].
Activated TGF-β stimulated the over expression of many extracellular matrix (ECM) proteins and suppressed their degradation by matrix metalloproteinases (MMP) via the upregulation of tissue inhibitor of metalloproteinases (TIMP) [13]. Binding of TGF-β to TGF-β receptor–II (TβR-II) triggers signals mediated by the phosphorylation of receptor associated Smads (Smad2 and Smad3; R-Smads), which then form a complex with Smad4 that enters the nucleus and binds to the TGF-β promoter to regulate its expression [14].
Panax ginseng root is used in oriental medicine, diets or as a dietary supplement. Ginseng has a variety of beneficial biological properties including anti-diabetic, anti-carcinogenic, anti-inflammatory, and neuro-protective effects [15]. The pharmacological properties of ginseng are mainly attributed to ginsenosides, which are phenolic acids, flavonoids, and triterpenoid saponins [16]. Ginsenosides are bioactive compounds such as Rb1, Rb2, Rg1, Rd, and Re [10, 17] that have antioxidant and anti-inflammatory effects [18] via different mechanisms and pathways in vitro, in vivo, and in clinical models [19].
Many studies have shown that ginseng attenuates liver injury by inhibiting lipid peroxidation, as well as TNF-α, NO, prostaglandin E2 (PGE2), intercellular adhesion molecule (ICAM)-1 and nuclear factor-κB (NF-κB) activation [14, 20–22]. However, the pharmacological effects of ginseng/ginsenosides on liver disorders, especially liver fibrosis, are not clear. Therefore, the aim of this study was to investigate the effect of ginseng extract on CCl4-induced liver inflammation and fibrosis in rats.
Methods
The present study was performed and described according to the Animal Research: Reporting In Vivo Experiments (ARRIVE) statement [23].
Chemicals
CCl4 and ginseng (Panax ginseng) were purchased from Sigma Chemicals (Sigma-Aldrich, St. Louis, MO, USA). Carbon tetrachloride was dissolved in olive oil, an emulsifying agent that allows CCl4 to dissolve sufficiently to induce liver damage. In addition, olive oil has no toxicity or other biological or pharmacological activity with regard to hepatotoxicity. Ginseng was dissolved in water and administrated by oral gavage. The SYBR® Green PCR Master Mix kit was purchased from Applied Biosystems (Life Technologies, Grand Island, NY, USA). Primers used in this study were designed using Primer Express 3.0 software (Applied Biosystem, Life Technologies, Grand Island, NY, USA) and synthesized by Metabion International AG (Planegg, Germany). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) measurement kits were purchased from Human (Human, Wiesbaden, Germany).
Study animals
Six-week-old male Wistar rats with a mean body weight of 180–200 g were obtained from the Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. The animals were kept under standard conditions of temperature (22 ± 1 °C), humidity (50–55%), and a 12 h light/dark cycle, with free access to standard laboratory feed and water according to the study protocol. All methods were conducted in accordance with the Guide for Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, National Institute of Health (NIH publication No. 80–23; 1996). The study was approved by the Experimental Animal Care Center Review Board (number E.A.C.P -6140/2016), King Saud University Riyadh, Saudi Arabia.
Experimental design
The experimental design was based on a previous protocol [24, 25]. Forty adult male Wistar rats were randomly divided into four groups of 10 animals each as follows.
Group I: the control group received 3 mL/kg olive oil twice a week for 8 weeks.
Group II: the ginseng group received 400 mg/kg/day ginseng dissolved in water administrated by oral gavage for 8 weeks.
Group III: the CCl4 group received 3 ml/kg CCl4 intraperitoneal (30% in olive oil) twice a week for 8 weeks and normal drinking water.
Group IV: the CCl4-ginseng group received 3 ml/kg CCl4 (30% in olive oil) twice a week for 8 weeks and 400 mg/kg/day ginseng dissolved in water and administrated by oral gavage one week before the first dose of CCl4 and continuously to the end of the protocol.
At least 24 h after the last treatment, blood samples were collected via cardiac puncture under anesthesia and centrifuged to separate the sera. Then the animals were euthanized by decapitation. The sera were separated and kept at −80 °C until used. The liver was immediately removed and divided into sections that were either washed with ice-cold saline solution, snap frozen in liquid nitrogen and stored until used for gene expression analysis, or cut into small pieces of 0.5–2.0 cm thick for histopathological study.
Histopathological study
Samples of livers from control and experimental groups were fixed in 10% neutral buffered formalin. The standard method of dehydration, clearing in xylene, and paraffin embedding was used. Sections of 5-μm thickness were cut by a rotary microtome and stained with Masson’s Trichrome (Bancroft and Gamble 2008). Sections were examined by light microscopy.
Liver biological activities
Blood from the heart was collected into coagulant tubes, left to coagulate, and then centrifuged at 3000 × g for 15 min at 4 °C. Serum ALT and AST activities were measured spectrophotometrically using a commercial kit (Human, Wiesbaden, Germany).
Serum and hepatic triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) concentrations were measured using commercial enzymatic kits (Randox® TR213 for triglycerides, Randox® CH201 for total cholesterol, Randox Laboratories Ltd., London, UK).
Liver samples were homogenized in a chloroform/methanol (2:1) solution to a final dilution 20 times the volume of tissues for at least 3 min and extracted with a chloroform/methanol/water (3:48:47) solution [26].
Measurement of gene expression by real-time PCR
Total RNA was extracted from liver tissues using a Total RNA Miniprep Kit (Axygen, Bioscience, Central Avenue, Union, USA) according to the manufacturer’s protocol. The RNA concentrations and purity were measured by NanoDrop (NanoDrop 8000, Thermo Scientific, USA). The extracted RNA had a 260/280 ratio of 1.9–2.1. cDNA was synthesized from 1 μg RNA using a high capacity cDNA reverse transcription kit as described in the manufacturer’s protocol (Applied Biosystems, Life Technologies, Grand Island, NY, USA).
Real-time quantitative PCR (SYBR® Green PCR Master Mix kit, Applied Biosystems, Life Technologies, Grand Island, NY, USA) was used to detect the expression levels of transforming growth factor beta (TGF-β), type I TGF-β receptor (TβR-1), type II TGF-β receptor (TβR-II), mothers against decapentaplegic homolog 2 (Smad2), Smad3, Smad4, matrix metalloproteinase2 (MMP2), MMP9, tissue inhibitor matrix metalloproteinase-1 (TIMP-1), Collagen 1a2 (Col1a2), Collagen 3a1 (Col3a1), IL-8 (IL-8) and IL-10 (IL-10) genes in liver tissues. GAPDH was used as an internal control. The reaction was performed on ABI 7500 Detection System (Applied Biosystems, Life Technologies, Grand Island, NY, USA). The program was set to run for one cycle at 95 °C for 2 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The specificity of amplification was confirmed by melting curve analysis. The primer sequences used in this study are shown in Table 1. The gene expression results were analyzed using the 2-ΔΔCT method [27]. Data were expressed as the mean fold change ± standard error for three independent amplifications.
Table 1.
Primers used in this study
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| TGF-β | 5’-TAGGCTGACAGCTTTGCGAA-3’ | 5’-GAACAACCGGCCTCCAAAAC-3’ |
| TβFI | 5’-GAAATCGCTCGACGCTGTTC-3’ | 5’-TTCGCAAAGCTGTCAGCCTA-3’ |
| TβFII | 5’-CGTGTGGAGGAAGAACGACA-3’ | 5’-CGTGGGAGAAGTGGCATCTT-3’ |
| Col1a2 | 5’-GCCAAGAATGCATACAGCCG-3’ | 5’-GACACCCCTTCTGCGTTGTA-3’ |
| Col3a1 | 5’-CCCAGGGATTCCAGGACCTA-3’ | 5’-ACTCCTCTAGGTCCTGCAGG-3’ |
| IL-8 | 5’-TGACCATGAGACACTGTGGC-3’ | 5’-GAAGAGCACGGGTCCTTTGA-3’ |
| IL-10 | 5’-GCGACGCTGTCATCGATTTC-3’ | 5’-GTAGATGCCGGGTGGTTCAA-3’ |
| MMP2 | 5’-CCCCATGTGTCTTCCCCTTC-3’ | 5’-AGCTCCTGGATCCCCTTGAT-3’ |
| MMP9 | 5’-AGGGCCCCTTTCTTATTGCC-3’ | 5’-CGAGTAACGCTCTGGGGATC-3’ |
| TIMP1 | 5’-TGTGCACAGTGTTTCCCTGT-3’ | 5’-TAGCCCTTCTCAGAGCCCAT-3’ |
| GAPDH | 5’-AACTCCCATTCCTCCACCTT-3’ | 5’-GAGGGCCTCTCTCTTGCTCT-3’ |
Statistical analyses
The differences between the obtained values (mean ± SEM, n = 10) were assessed by one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparison using Graph Pad Prism five software (GraphPad Software, Inc., La Jolla, CA, USA). The differences were considered statistically significant when p <0.05.
Results
Liver enzyme (ALT and AST) levels in sera were used as biochemical markers for early acute hepatotoxicity. The CCl4 group showed a significant increase in the levels of AST (112.3 ± 3.5 U/L) (Fig. 1a) and ALT (135.6 ± 5.2 U/L) (Fig. 1b) compared with the control group (48.9 ± 2.8 U/L and 52.2 ± 2.2 U/L, respectively) (p <0.001). However, ginseng restored liver enzyme levels to values similar to the control group.
Fig. 1.
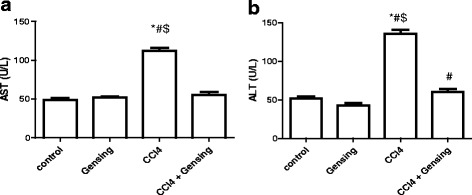
Effect of CCl4, Ginseng, and CCl4 + Ginseng on the serum levels of AST (a) and ALT (b). Data are presented as the mean ± SEM (n = 10). *, # and $ indicate a significant change from control, Ginseng and CCl4 + Ginseng groups, respectively (p <0.05 using ANOVA followed by Tukey—Kramer as a post ANOVA test)
CCl4 significantly increased serum lipid parameters including TG, TC and LDL levels and nonsignificantly decreased the HDL level compared with the control group. Ginseng supplementation in combination with CCl4 significantly decreased TC and LDL levels compared with the CCl4 group. No effect of ginseng alone on TG, TC, HDL, and LDL levels was observed (Table 2).
Table 2.
Correlation between lipid profile and the studied groups
| Group | TG (mg/dl) | Total Cholesterol(mg/dl) | HDL(mg/dl) | LDL(mg/dl) |
|---|---|---|---|---|
| Control | 62.3 ± 3.99 | 55.6 ± 2.2 | 28.4 ± 1.32 | 44.3 ± 2.4 |
| ginseng | 58.5 ± 4.6 | 61.7 ± 3.5 | 33.2 ± 2.1 | 48.1 ± 2.8 |
| CCl4 | 98.2 ± 3.5*#$ | 127.5 ± 6.5*#$ | 23.2 ± 1.54 | 93.5 ± 2.8*#$ |
| CCl4+ ginseng | 70.2 ± 3.54 | 70.67 ± 5.3 | 33.2 ± 3.32 | 50.2 ± 2.1 |
Values are the means ± SE (n = 10). *, # and $ indicate a significant change from control, ginseng, and CCl4 + ginseng groups, respectively (p <0.05 using ANOVA followed by Tukey—Kramer as a post ANOVA test)
Histopathological examination of the liver
Histopathological abnormalities in the liver were studied at the end of the experiment using Masson’s trichrome staining. The effects of CCl4, ginseng, and CCl4 + ginseng on histopathological changes in the liver, especially in liver fibrosis, are shown in Fig. 2.
Fig. 2.
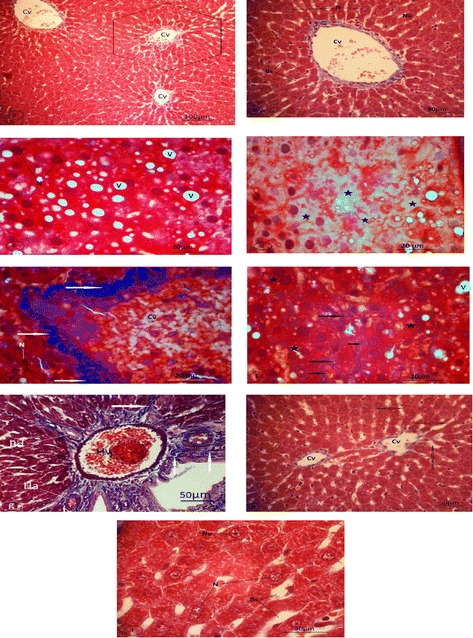
Light micrographs of liver sections of rat. Specimens were fixed in 10% neutral formalin and stained with Masson Trichrome. a-b Control rat (GI). a Control rat (GI) with poorly defined classical hepatic lobules and central veins (Cv) (magnification 100x). b: A hexagonal hepatocyte with central nucleus (N) and prominent nucleolus (Nu) arranged in strands around the central vein (Cv) and blood sinusoids (Bs) (magnification 400x). c-g Rats treated with CCl4 (GIII). e Hydropic degeneration and vacuolated hepatocytes. (N) Nuclei, (V) vacuoles. A marked increase in collagen fibers (arrows) around a congested central vein (Cv) and degenerated hepatocytes with nuclei (N) (magnification 1000x). f Fatty changes liver (stars) and collapsed sinusoids. f: Collagen fibers (arrows) around collapsed sinusoids. An increase in binucleated hepatocytes (stars) and vacuoles (V) was observed (magnification 1000x). g Fibrosis in the portal area (arrows). (Hv) hepatic portal vein, (Ha) hepatic artery, and (Bd) proliferated bile duct (magnification 400x). h Ginseng supplementation with CCl4 (GIV) reduced the accumulation of collagen fibers after CCl4 administration. (Cv) central veins, (arrows) blood sinusoids (magnification 400x). i Nearly normal hepatic strands and hepatocytes of GIV with nuclei (N) and nucleoli (Nu), and the reduction of perisinusoidal collagen fibers. Bs: blood sinusoids (magnification 1000x)
In the control group (GI), all features of the liver were observed to be normal (hepatic lobules with central veins, hepatic strands of hepatocytes, portal area, and blood sinusoids) (Fig. 2a, b). Animals in group II who received ginseng dissolved in water showed no histological evidence of pathological alterations (Fig. 2c, d,).
In the CCl4-treated group (GIII), the liver showed classical fibrosis around the congested central vein (Fig. 2e) and portal area, which was accompanied by a variable degree of proliferated bile duct (Fig. 2g). The normal architecture of the hepatic lobules were lost (Fig. 2f), and fibroid collapsed blood sinusoids were demonstrated. Hydropic degeneration, vacuolization (Fig. 2c), and fatty hepatocytes were observed (Fig. 2d).
In the CCl4 and ginseng group (GIV), the normal architecture of hepatic lobules was observed (Fig. 2h). The hepatic strands were composed of atypical polyhedral hepatocytes with a distinct nucleus and prominent nucleolus. A reduction of fatty hepatocytes, vacuoles, and collagen fibers especially around the central vein were detected (Fig. 2i).
The effect of CCl4, ginseng, and CCl4 + ginseng on TGF-β, TβF-I, and TβF-II expression is shown in Fig. 3. The expressions of TGF-β, TβF-I, and TβF-II in the CCl4 group were increased significantly by 7.6, 9.2 and 7.3-fold, respectively, compared with the control group (p <0.001). Interestingly, the administration of CCl4 + ginseng resulted in a significant decrease in the expression of TGF-β and its receptors (p <0.002).
Fig. 3.
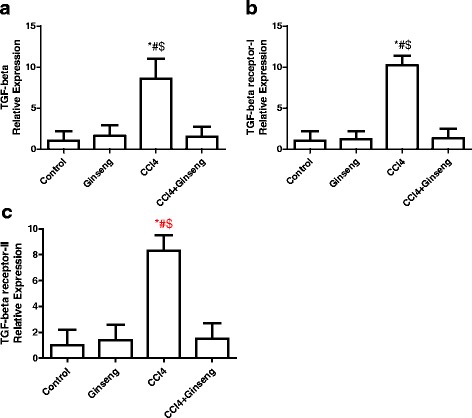
Effect of CCl4, ginseng, and CCl4 + ginseng on the expression of TGF-β (A), TβF-I (B) and TβF-II (C) genes in the liver. Data are the mean ± SEM (n = 10). *, # and $ indicate a significant change from the control, ginseng, and CCl4 + ginseng groups, respectively (p <0.05 using ANOVA followed by Tukey—Kramer as a post ANOVA test)
The effect of CCl4, ginseng, and CCl4 + ginseng on Smad2, Smad3, and Smad4 expression is shown in Fig. 4. The administration of CCl4 for 8 weeks increased the expression of Smad2, Smad3 and Smad4 by 2.2-, 5.8- and 4.8-fold, respectively, compared with the control group (p <0.001). Interestingly, the administration of ginseng in combination with CCl4 significantly decreased the expression of Smad2, Smad3 and Smad4 by 7-, 3.5- and 5.1-fold, respectively, compared with the CCl4 group (p <0.001).
Fig. 4.
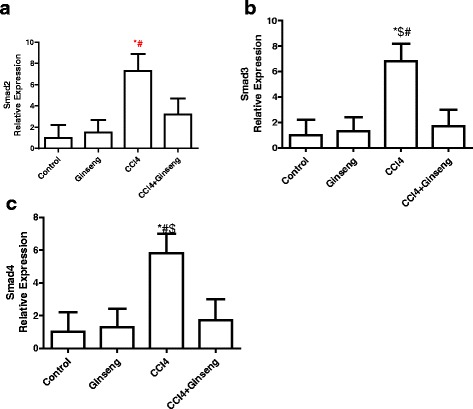
Effect of CCl4, ginseng, and CCl4 + ginseng on the expression of Smad2 (A), Smad3 (B) and Smad4 (C) in the liver. Data are the mean ± SEM (n = 10). *, # and $ indicate a significant change from the control, ginseng, and CCl4 + ginseng, groups, respectively (p <0.05 using ANOVA followed by Tukey—Kramer as a post ANOVA test)
The effects of CCl4, ginseng, and CCl4 + ginseng on MMP2, MMP9, and TIMP-1 gene expression are shown in Fig. 5. The administration of CCl4 for 8 weeks increased the expression of MMP2, MMP9, and TIMP-1 by 5.4-, 4.6- and 7.2-fold, respectively, compared with the control group (p <0.001). However, the administration of CCl4 + ginseng significant decreased the expression of MMP2, MMP9, and TIMP-1 by 5-, 4.3- and 6.7-fold, respectively, compared with the CCl4 group (p <0.0001). No significant difference was observed for gene expression in the CCl4 + ginseng group compared with the control group.
Fig. 5.
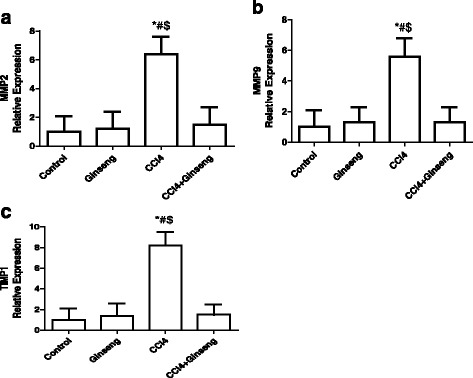
Effect of CCl4, ginseng, and CCl4 + ginseng on the expression of MMP2 (a), MMP9 (b) and TIMP-1 (c) genes in the liver. Data are the mean ± SEM (n = 10). *, # and $ indicate a significant change from the control, ginseng, and CCl4 + ginseng groups, respectively (p <0.05 using ANOVA followed by Tukey—Kramer as a post ANOVA test)
The effect of CCl4, ginseng, and CCl4 + ginseng on Col1a2 and Col3a1 gene expression is shown in Fig. 6a and b. The administration of CCl4 for 8 weeks significantly increased the expression of Col1a2 9.5-fold compared with the control group (p <0.001). The administration of CCl4 increased the expression of Col3a1 8.3-fold (p <0.001). However, the administration of CCl4 + ginseng significantly restored the alteration in these genes compared with the CCl4 group.
Fig. 6.
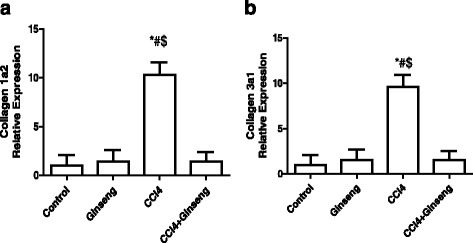
Effect of CCl4, ginseng, and CCl4 + ginseng on the expression of Collagen 1a2 (a) and Collagen 3a1 (b) genes in the rat liver. Data are the mean ± SEM (n = 10). *, # and $ indicate a significant change from the control, ginseng, and CCl4 + ginseng groups, respectively (p <0.05 using ANOVA followed by Tukey—Kramer as a post ANOVA test)
The effect of CCl4, ginseng, and CCl4 + ginseng on IL-10 and IL-8 gene expression is shown in Fig. 7a and b. The administration of CCl4 for 8 weeks significantly decreased the expression of IL-10 4-fold compared with the control group (p <0.05). The administration of CCl4 increased the expression of IL-8 5.4-fold (p <0.01). However, the administration of CCl4 + ginseng significantly restored the alteration in these genes compared with the CCl4 group. No significant difference in gene expression was observed in the CCl4 + ginseng group compared with the control group.
Fig. 7.
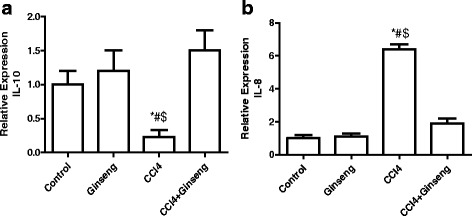
Effect of CCl4, ginseng, and CCl4 + ginseng on the expression of IL-10 (a) and IL-8 (b) genes in the liver. Data are the mean ± SEM (n = 10). *, # and $ indicate a significant change from the control, ginseng, and CCl4 + ginseng groups, respectively (p <0.05 using ANOVA followed by Tukey—Kramer as a post ANOVA test)
Discussion
The liver is the first line of protection against hepatic damage induced by xenobiotics and drugs, which can cause hepatic necrosis and apoptosis [28]. Reactive oxygen species cause direct tissue damage and initiate inflammation through the activation of various cytokines [29]. Lipid peroxidation and free radicals cause the necrosis of hepatocytes, induce inflammation, and promote the progression of hepatic fibrogenesis [30].
CCl4 is used to induce hepatic fibrosis in experimental rat models, and several studies have focused on the prevention of CCl4-induced hepatotoxicity [31–33]. CCl4 is used to investigate the liver injury associated with oxidative stress and free radicals. Ginseng extract contains Rb1, Rb2, Rc, Rd, Re, and Rg1 that have a major role in hepato-protection by suppressing oxidative stress and lipid peroxides via inhibition of the expression and activity of cytochrome P450 in the liver [34].
The leakage of hepatocellular enzymes is used as a hepatotoxicity marker. The current study showed significant increases in the serum levels of ALT, AST, total cholesterol, triglyceride, LDL, and a significant decrease in HDL following CCl4 administration. However, in the CCl4 + ginseng group, the levels of ALT and AST were restored to their normal levels. These results indicate that ginseng protects against CCl4-induced hepatic damage. Similar previous studies demonstrated that ginseng extract had antioxidant activity and acted as a free radical scavenger [34, 35]. Importantly, the increased levels of triglycerides, total cholesterol, and LDL and the decreased level of HDL were restored to their normal values with CCl4 + ginseng.
IL-8 is as a major factor of acute inflammation [36]. High IL-8 levels in the liver are observed during acute liver injury [37, 38]. In the present study, CCl4 increased the expression of IL-8. A similar study found that serum IL-8 levels were significantly elevated in chronic liver disease patients. IL-8 serum levels were also associated with the progression of fibrosis [39]. Therefore, IL-8 overexpression might be related to tissue damage [40, 41]. In the current study, a high expression of IL-8 was observed in association with CCl4, and the administration of ginseng extract decreased IL-8 levels to normal values indicating the anti-inflammatory effects of ginseng extract.
IL-10 is a cytokine produced by cells of the immune system as well as liver cells including hepatocytes, sinusoidal cells, Kupffer cells, stellate cells, and liver-associated lymphocytes [42]. It was reported that endogenous IL-10 decreased intrahepatic inflammatory responses and fibrosis in several models of liver injury [43]. In the current study, the down-regulation of Il-10 induced by CCl4 lead to the overexpression of collagen types I and III. Another study found that collagen types I and III were significantly decreased to normal values at 3 weeks after IL-10 treatment. Furthermore, the high expressions of MMP2 and TIMP-1 induced by CCl4 were significantly decreased after 3 weeks of IL-10 treatment, indicating that collagen types I and III might be associated with a decrease in MMP-2 and TIMP-1 levels[44]. In the current study, ginseng extract increased the expression of IL-10, which was associated with a decrease in MMP-2, -9 and MIT-1 and a decrease in the expression of collagen types I and III. These results indicated that exogenous IL-10 might have a therapeutic effect on advanced liver fibrosis.
Hydroxyproline, a major constituent of collagen, is a good marker for ECM accumulation [45]. The overexpression of type I, III and IV collagen are early events in the development of CCl4-induced hepatic fibrosis. Type I and III collagen fibers are localized around the portal area and are markedly increased around the tributaries of portal veins. In the current study, histological analysis of liver from the CCl4 group showed fibrosis around the central veins, portal area, and perisinusoidal space. In the current study, livers in the CCl4 group showed fibrosis of the central veins as indicated by collagen fibers in the portal area and highly proliferated bile duct, necrotic disorganized hepatic strands, hyalinization of cytoplasm and vacuolated hepatocytes. In addition, the results of the present study are in agreement with a study by Iredale et al., that reported mild fatty change and the vacuolization of hepatocytes after the intraperitoneal injection of rats with CCl4 [46].
In the present study, CCl4 increased the expression of collagen 1a1 and collagen 1a III. A similar study reported the increased expression of collagen types I and II after CCl4 administration [47]. These findings were consistent with those of Yu et al., who reported that the overexpression of type I, III and IV collagen were early events in the development of CCl4-induced hepatic fibrosis in rats. Type I and III collagen fibers are localized around the portal area [45] and are markedly increased around the tributaries of portal veins [47]. The overexpression of COL1A1 and CL1AIII leads to enhanced collagenous matrix deposition in liver. The CCl4 + ginseng group had a reduced accumulation of collagen fibers compared with the CCl4 group. Similarly, in the present study, the administration of ginseng extract reduced the expression of collagens type I and II.
Extracellular matrix (ECM) accumulation is a common phenomenon in liver fibrosis. MMPs and TIMPs are a class of secreted enzymes with important functions in ECM degradation [48] and MMPs are associated with liver fibrosis. MMPs and their tissue inhibitors were elevated quickly after CCl4-induced liver injury in rats, and the hepatic expression of MMP-3 was detected as early as 6 h after CCl4 administration in rats. In the current study, the administration of CCl4 caused an overexpression of MMP-2, -9, and TIMP-1. Similarly, Knittel et al., reported MMP and TIMP overexpression during liver injury and fibrosis after a single dose of CCl4, and that MMP-2, MMP-3, MMP-9, MMP-10, MMP-13, and MT1-MMP were all overexpressed [49]. In a rat model of hepatic fibrosis induced by bile duct ligation (BDL), MMP-2 and MMP-9 were increased suggesting that continued tissue damage and inflammation induced MMP expression [50]. Therefore, MMPs might be associated with liver fibrosis. In the present study, ginseng administration with CCl4 decreased the expressions of MMP-2, -9 and TIMP-1 to their normal levels similar to that in the control group. Similarly, Lo et al., found that ginsenoside significantly reduced the expression of MMP-2, -9 and TIMP-1 in HSC-T6 cells after induction with H2O2. They suggested that ginsenoside had an anti-fibrotic effect on HSCs by inhibiting the activation, proliferation, and expression of collagen, TGF-β1, MMP-2, and TIMP-1 [51].
The pathogenesis of fibrosis involves several mechanisms such as the inflammatory, growth factor signaling, and lipid signaling pathways. The inflammatory pathway and the growth factor signaling pathway mediated by TGF-β are the most important pathways for fibrosis [52]. Transforming growth factor-β is involved in various physiologic processes; therefore, understanding the molecular mechanisms involved in TGF-β signaling in diseases is important for the development of its therapies. The regulation of extra-cellular matrix accumulation by fibrogenic transforming growth factor (TGF)-β signals involves different mechanisms dependent upon whether there is acute or chronic liver damage. Hepatic stellate cells (HSC) are the principal effector cell type in this pathway. After acute liver injury, TGF-β enhances collagen synthesis by activating hepatic stellate cells via the Smad pathways. Activated TGF-β mediates the activation of Smad2, Smad3, and Smad4 in a fibrotic rat model [53]. In the present study, CCl4 increased Smad2, Smad3, and Smad4 expression, and ginseng extract restored their expression to normal levels and decreased histological fibrosis. These results indicate that Smad2, Smad3, and Smad4 have a significant role in the progression of liver fibrosis. A similar study found that the high expression of Smad-2 and Smad-4 was associated with liver fibrosis in rats using in situ hybridization [54].
TGF-β1 is a pro-fibrogenic cytokine in hepatic fibrosis [47]. Activated TGF‐β1 signal to the cells through its trans-membrane receptors TβR‐I and TβR‐II. In the present study, CCl4 administration increased the expression of TGF-β1 and its receptors TβR-1, and TβR-1I. The increased TGF-β1, TβR-1, and TβR-II expressions were restored to their normal values after ginseng treatment. A similar study found that CCl4 administration increased TGF-β1 protein levels, and its receptor levels, and this alteration in protein expression was restored by Gypenosides [55]. Another study reported that TGF-β1 activity was increased in rats administered a single dose of 20% CCl4 in olive oil, and this increase was restored by a low-dose of herbal extract [56]. Another study proposed that suppressing TGF-β-induced Smad2/Smad3 phosphorylation and nuclear translocation in HSCs attenuated fibrosis [55].
When TGF‐β1 binds to it receptors (TβR‐I and TβR‐II), it catalyzes Smad2/Smad3, which enters the nucleus, binds to transcriptional factors and regulates the expression of collagen and TIMP genes [24, 25]. In the current study, the expressions of TGF‐β1, TβR‐I, TβR‐II, Smad2, and Smad3 were significantly increased in the CCl4 group compared with the normal group. This indicated that the TGF‐β1/Smad signaling pathway was activated during liver fibrosis. ginseng extract administration decreased Smad2, Smad3, TβR‐I, TβR‐II, and TGF‐β1 expression to their normal values.
Conclusions
Ginseng extract had an anti‐fibrosis effect in a CCl4‐induced liver fibrosis model by regulating the TGF‐β1/Smad signaling pathway via the inhibition of Smad3, Smad2, and TGF‐β1 expression.
Acknowledgments
The authors thank the Deanship of Scientific Research at KSU for funding this work (research group project no. RGP-142).
Funding
This work was funded by the Deanship of Scientific Research at KSU (research group project no. RGP-142).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Authors’ contribution
MMH participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. SSH participated histopathological work, interpreted the histopathological data, and helped to draft the manuscript. MFE participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. ZKH participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. SSA participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. MMSA participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. NOA participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. KAA participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. MMA participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. ARA participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. OAA participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. All authors contributed equally. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors consent to this manuscript’s publication.
Ethics approval
The study was approved by the Experimental Animal Care Center Review Board (number E.A.C.P -6140/2016), King Saud University Riyadh, Saudi Arabia.
Abbreviations
- (ALT)
Alanine aminotransferase
- (AST)
Aspartate aminotransferase
- (CCl4)
Carbon tetrachloride
- (Col1a2)
Collagen 1a2
- (Col3a1)
Collagen 3a1
- (HDL)
High-density lipoprotein
- (I.P)
Intraperitoneal
- (ICAM)-1
Intercellular adhesion molecule
- (IL-10)
interleukin -10
- (IL-8)
interleukin-8
- (LDL)
Low-density lipoprotein
- (MMP2)
Matrix metalloproteinase 2
- (MMP9)
Matrix metalloproteinase 9
- (NF-κB)
Nuclear factor-κB
- (NO)
Nitric oxide
- (PGE2)
Prostaglandin E2
- (real-time PCR)
real time polymerase chain reaction
- (Smad2)
- (Smad3)
- (Smad4)
- (TC)
Total cholesterol
- (TG)
Triglycerides
- (TGF-β)
Transforming growth factor beta
- (TIMP-1)
Tissue inhibitor matrix metalloproteinase-1
- (TNF)-α
Tumor necrosis factor
- (TβR-1)
Transforming growth factor beta receptor type I
- (TβR-II)
Transforming growth factor beta receptor type II
Contributor Information
Mohamed M. Hafez, Email: Mohhafez_2000@yahoo.com
Sherifa S. Hamed, Email: hamed6782000@yahoo.com
Manal F. El-Khadragy, Email: manalelkhadragy@yahoo.com
Zeinab K. Hassan, Email: hildahafez@hotmail.com
Salim S. Al Rejaie, Email: rejaie@hotmail.com
Mohamed M. Sayed-Ahmed, Email: sayedahmedmm@hotmail.com
Naif O. Al-Harbi, Email: nharbi1@ksu.edu.sa
Khalid A. Al-Hosaini, Email: kalhosaini@ksu.edu.sa
Mohamed M. Al-Harbi, Email: mmzharbi@ksu.edu.sa
Ali R. Alhoshani, Email: ahoshani@ksu.edu.sa
Othman A. Al-Shabanah, Email: shabanah@ksu.edu.sa
Shakir Dekhal Alsharari, Email: shakir9556@gmail.com.
References
- 1.Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011;50(5):600–613. [PMC free article] [PubMed] [Google Scholar]
- 2.Labarga P, Fernandez-Montero JV, de Mendoza C, Barreiro P, Pinilla J, Soriano V. Liver fibrosis progression despite HCV cure with antiviral therapy in HIV-HCV-coinfected patients. Antivir Ther. 2015;20(3):329–34. doi: 10.3851/IMP2909. [DOI] [PubMed] [Google Scholar]
- 3.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng YC, Lin CL, Hsu WY, Chang CS, Yeh HZ, Kao CH. Risk of Liver Cirrhosis in Patients with Tuberculosis: A Nationwide Cohort Study. Eur J Clin Investig 2015;45(7):663–9 [DOI] [PubMed]
- 5.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi: 10.1016/S0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105–117. doi: 10.4137/BMI.S10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh N, Kamath V, Narasimhamurthy K, Rajini PS. Protective effect of potato peel extract against carbon tetrachloride-induced liver injury in rats. Environ Toxicol Pharmacol. 2008;26(2):241–246. doi: 10.1016/j.etap.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, Trebicka J, Weiskirchen R. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6(1):19. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegmund SV, Brenner DA. Molecular pathogenesis of alcohol-induced hepatic fibrosis. Alcohol Clin Exp Res. 2005;29(11 Suppl):102S–109S. doi: 10.1097/01.alc.0000189275.97419.58. [DOI] [PubMed] [Google Scholar]
- 10.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 11.Lu DH, Guo XY, Qin SY, Luo W, Huang XL, Chen M, Wang JX, Ma SJ, Yang XW, Jiang HX. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol. 2015;21(5):1531–1545. doi: 10.3748/wjg.v21.i5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Liu W, Liu X, Sheng M, Pei Y, Lei R, Zhang S, Tao R. Role of liver in modulating the release of inflammatory cytokines involved in lung and multiple organ dysfunction in severe acute pancreatitis. Cell Biochem Biophys. 2015;71(2):765–776. doi: 10.1007/s12013-014-0261-5. [DOI] [PubMed] [Google Scholar]
- 13.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–d807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Qiao L, Li Y, Yang G. Ginsenoside Rb1 attenuates intestinal ischemia-reperfusion- induced liver injury by inhibiting NF-kappaB activation. Exp Mol Med. 2008;40(6):686–698. doi: 10.3858/emm.2008.40.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung CH, Seog HM, Choi IW, Choi HD, Cho HY. Effects of wild ginseng (panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J Ethnopharmacol. 2005;98(3):245–250. doi: 10.1016/j.jep.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Huang YC, Chen CT, Chen SC, Lai PH, Liang HC, Chang Y, Yu LC, Sung HW. A natural compound (ginsenoside Re) isolated from panax ginseng as a novel angiogenic agent for tissue regeneration. Pharm Res. 2005;22(4):636–646. doi: 10.1007/s11095-005-2500-3. [DOI] [PubMed] [Google Scholar]
- 17.Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28(3):467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cicero AF, Vitale G, Savino G, Arletti R. Panax notoginseng (burk.) effects on fibrinogen and lipid plasma level in rats fed on a high-fat diet. Phytother Res. 2003;17(2):174–178. doi: 10.1002/ptr.1262. [DOI] [PubMed] [Google Scholar]
- 19.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean panax ginseng C a Meyer. Acta Pharmacol Sin. 2008;29(9):1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang XW, Hattori M, Namba T, Chen DF, Xu GJ. Anti-lipid peroxidative effect of an extract of the stems of kadsura heteroclita and its major constituent, kadsurin, in mice. Chem Pharm Bull. 1992;40(2):406–409. doi: 10.1248/cpb.40.406. [DOI] [PubMed] [Google Scholar]
- 21.Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005;25(5):1069–1073. doi: 10.1111/j.1478-3231.2005.01068.x. [DOI] [PubMed] [Google Scholar]
- 22.Park EK, Shin YW, Lee HU, Kim SS, Lee YC, Lee BY, Kim DH. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull. 2005;28(4):652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 23.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan RA, Khan MR, Sahreen S. CCl4-induced hepatotoxicity: protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement Altern Med. 2012;12:178. doi: 10.1186/1472-6882-12-S1-P178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karadeniz A, Cemek M, Simsek N. The effects of panax ginseng and spirulina platensis on hepatotoxicity induced by cadmium in rats. Ecotoxicol Environ Saf. 2009;72(1):231–235. doi: 10.1016/j.ecoenv.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 27.Hafez MM, Al-Shabanah OA, Al-Harbi NO, Al-Harbi MM, Al-Rejaie SS, Alsurayea SM, Sayed-Ahmed MM. Association between paraoxonases gene expression and oxidative stress in hepatotoxicity induced by CCl4. Oxid Med Cell Longev. 2014;2014:893212. doi: 10.1155/2014/893212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol. 2009;36(1):4–12. doi: 10.1007/s12016-008-8091-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189(1-2):113–127. doi: 10.1016/S0300-483X(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 31.Aruoma OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res. 2003;523–524:9–20. doi: 10.1016/S0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 32.Emzhik M, Rahimi-Moghaddam P, Ebrahimi SA, Keyhanfar F, Moazzam AS. Commentary on prevention a possible drug-drug interaction: is concurrent administration of orlistat and pioglitazone increase the risk of durg-induced hepatotoxicity? Int J Prev Med. 2015;6:16. doi: 10.4103/2008-7802.151825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda T. Idiosyncratic drug hepatotoxicity: strategy for prevention and proposed mechanism. Curr Med Chem. 2015;22(4):528–537. doi: 10.2174/0929867321666140916122628. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Chun YJ, Park JD, Kim SI, Roh JK, Jeong TC. Protection of rat liver microsomes against carbon tetrachloride-induced lipid peroxidation by red ginseng saponin through cytochrome P450 inhibition. Planta Med. 1997;63(5):415–418. doi: 10.1055/s-2006-957724. [DOI] [PubMed] [Google Scholar]
- 35.Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a north american ginseng extract. Mol Cell Biochem. 2000;203(1–2):1–10. doi: 10.1023/A:1007078414639. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 37.Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 38.Jaeschke H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol. 2002, 27(1):23-27. [DOI] [PubMed]
- 39.Zimmermann HW, Seidler S, Gassler N, Nattermann J, Luedde T, Trautwein C, Tacke F. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One. 2011;6(6):e21381. doi: 10.1371/journal.pone.0021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35(6):757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 41.Escudero-Lourdes C, Wu T, Camarillo JM, Gandolfi AJ. Interleukin-8 (IL-8) over-production and autocrine cell activation are key factors in monomethylarsonous acid [MMA(III)]-induced malignant transformation of urothelial cells. Toxicol Appl Pharmacol. 2012;258(1):10–18. doi: 10.1016/j.taap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller AM, Wang H, Bertola A, Park O, Horiguchi N, Ki SH, Yin S, Lafdil F, Gao B. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology. 2011;54(3):846–856. doi: 10.1002/hep.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28(6):1597–1606. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- 44.Huang YH, Shi MN, Zheng WD, Zhang LJ, Chen ZX, Wang XZ. Therapeutic effect of interleukin-10 on CCl4-induced hepatic fibrosis in rats. World J Gastroenterol. 2006;12(9):1386–1391. doi: 10.3748/wjg.v12.i9.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu J, Wu CW, Chu ES, Hui AY, Cheng AS, Go MY, Ching AK, Chui YL, Chan HL, Sung JJ. Elucidation of the role of COX-2 in liver fibrogenesis using transgenic mice. Biochem Biophys Res Commun. 2008;372(4):571–577. doi: 10.1016/j.bbrc.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 46.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102(3):538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan X, Zhang Q, Li S, Lv Y, Su H, Jiang H, Hao Z. Attenuation of CCl4-induced hepatic fibrosis in mice by vaccinating against TGF-beta1. PLoS One. 2013;8(12):e82190. doi: 10.1371/journal.pone.0082190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44-46C:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113(6):443–453. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- 50.Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL, Phillips BW, Zhang Y, Urbanski SJ. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153(6):1895–1902. doi: 10.1016/S0002-9440(10)65703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo YT, Tsai YH, Wu SJ, Chen JR, Chao JC. Ginsenoside Rb1 inhibits cell activation and liver fibrosis in rat hepatic stellate cells. J Med Food. 2011;14(10):1135–1143. doi: 10.1089/jmf.2010.1485. [DOI] [PubMed] [Google Scholar]
- 52.Sadasivan SK, Siddaraju N, Khan KM, Vasamsetti B, Kumar NR, Haridas V, Reddy MB, Baggavalli S, Oommen AM, Pralhada Rao R. Developing an in vitro screening assay platform for evaluation of antifibrotic drugs using precision-cut liver slices. Fibrogenesis Tissue Repair. 2015;8(1):1. doi: 10.1186/s13069-014-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Wang LF, Zou HF, Song XY, Xu HF, Lin P, Zheng HH, Yu XG. Expression and location of Smad2, 4 mRNAs during and after liver fibrogenesis of rats. World J Gastroenterol. 2006;12(10):1577–1582. doi: 10.3748/wjg.v12.i10.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Li X-m, Chen L, Feng Q, Xu L, Hu Y-y, Peng J-h. The effect of gypenosides on TGF-β1/smad pathway in liver FibrosisInduced by carbon tetrachloride in rats. Int J Integr Med. 2003;1(23):1–6. [Google Scholar]
- 56.Chang HF, Lin YH, Chu CC, Wu SJ, Tsai YH, Chao JC. Protective effects of ginkgo biloba, panax ginseng, and schizandra chinensis extract on liver injury in rats. Am J Chin Med. 2007;35(6):995–1009. doi: 10.1142/S0192415X07005466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


