Abstract
Background
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most potent congener of the dioxin class of environmental contaminants. Exposure to TCDD causes a wide range of toxic outcomes, ranging from chloracne to acute lethality. The severity of toxicity is highly dependent on the aryl hydrocarbon receptor (AHR). Binding of TCDD to the AHR leads to changes in transcription of numerous genes. Studies evaluating the transcriptional changes brought on by TCDD may provide valuable insight into the role of the AHR in human health and disease. We therefore compiled a collection of transcriptomic datasets that can be used to aid the scientific community in better understanding the transcriptional effects of ligand-activated AHR.
Results
Specifically, we have created a datasets package – TCDD.Transcriptomics – for the R statistical environment, consisting of 63 unique experiments comprising 377 samples, including various combinations of 3 species (human derived cell lines, mouse and rat), 4 tissue types (liver, kidney, white adipose tissue and hypothalamus) and a wide range of TCDD exposure times and doses. These datasets have been fully standardized using consistent preprocessing and annotation packages (available as of September 14, 2015). To demonstrate the utility of this R package, a subset of “AHR-core” genes were evaluated across the included datasets. Ahrr, Nqo1 and members of the Cyp family were significantly induced following exposure to TCDD across the studies as expected while Aldh3a1 was induced specifically in rat liver. Inmt was altered only in liver tissue and primarily by rat-AHR.
Conclusions
Analysis of the “AHR-core” genes demonstrates a continued need for studies surrounding the impact of AHR-activity on the transcriptome; genes believed to be consistently regulated by ligand-activated AHR show surprisingly little overlap across species and tissues. Until now, a comprehensive assessment of the transcriptome across these studies was challenging due to differences in array platforms, processing methods and annotation versions. We believe that this package, which is freely available for download (http://labs.oicr.on.ca/boutros-lab/tcdd-transcriptomics) will prove to be a highly beneficial resource to the scientific community evaluating the effects of TCDD exposure as well as the variety of functions of the AHR.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-016-3446-z) contains supplementary material, which is available to authorized users.
Keywords: TCDD, AHR, Microarray datasets, R
Background
The aryl hydrocarbon receptor (AHR) is an evolutionarily conserved transcription factor [1] activated by small molecule binding. Prior to ligand-activation, the AHR resides in the cytoplasm bound to chaperone proteins, including heat shock protein 90 (HSP90) and the AHR-interacting protein (AIP) [2, 3]. Ligand-activation of this receptor leads to translocation into the nucleus, dissociation of chaperones and dimerization with the AHR nuclear translocator (ARNT) [4]. The AHR: ARNT complex is able to bind DNA at recognized motifs known as aryl hydrocarbon response elements (AHREs) whereby transcription of the associated genes is regulated [5]. Modulation of AHR activity has been linked to various diseases, including numerous in vitro studies of breast [6–8], endometrial [9], kidney [10], lung [11, 12] and prostate [13] cancers and inflammatory skin [14] and bowel [15, 16] diseases.
Activation of the AHR can occur by both endogenous molecules, such as tryptophan metabolites [17], and exogenous molecules, such as polycyclic aromatic hydrocarbons. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most potent congener of chlorinated dioxins, a large class of environmental contaminants produced as a by-product of various industrial processes [18]. Many of the toxic effects of TCDD exposure, including chloracne, immunosuppression, hepatotoxicity and cancer [19], are mediated by the AHR. Strong evidence for this relationship comes from studies of AHR-knockout mice [20–22], mice containing AHR-inactivating mutations [23–25] and conditional ARNT-null mice [26], all of which are unresponsive, or show reduced response, to TCDD. Differences in the toxic outcomes of TCDD occur across species and have been linked to polymorphisms in the AHR. A particularly TCDD-resistant strain of mice, DBA/2 J, presents with an Ala375Val mutation within the AHR gene; this leads to reduced affinity of the receptor for TCDD [27–29]. As another example, two strains of rat, Long-Evans (L-E) and Han/Wistar (H/W) show dramatic differences in TCDD susceptibility. These differences are primarily due to a point mutation that results in partial deletion of the transactivation domain of the AHR in the TCDD-resistant H/W rat [30]. Furthermore, inbred lines (Line A/B/C) derived from L-E x H/W crosses demonstrate intermediate susceptibility to TCDD depending on AHR genotype [31]. In humans, the AHR gene most closely resembles that of the DBA/2 J mouse [29]. However, a number of polymorphisms have been identified within both the ligand-binding and transactivation domains of the human AHR [32] but the overall functional consequences of these polymorphisms are not yet clear. In addition to the differences in TCDD-response phenotypes among different mammals, TCDD exposure results in tissue-specific responses. In rats, TCDD tends to accumulate in liver, spleen, adipose tissue and pancreas [33]. The broadest spectrum of transcriptional responses in rodents is detected in liver [34]. Further compounding the issue, there is a sex-dependent element to the transcriptomic alterations evoked by TCDD [35–38].
Despite considerable study into the transcriptomic changes mediated by the TCDD: AHR complex that lead to the observed toxic outcomes, the specific genes and pathways responsible for these outcomes remain unknown. As such, a global resource describing transcriptomic changes following activation of the AHR across a wide variety of tissues and species would prove very useful to the scientific community. Therefore, we have generated such a resource consisting of transcriptomic data from numerous studies in our laboratory and others (a total of 377 samples) and we introduce the freely-available TCDD.Transcriptomics package for the R statistical environment.
Implementation
Experimental methods
The experimental design, animal handling and sample preparation for individual experiments are described elsewhere [34, 37–43]. Data from human Multipotent Adipose-Derived Stem (hMADS) cells (differentiated and undifferentiated; TCDD treated and control) were downloaded from NCBI’s GEO repository (GSE32026) [42], as were data for primary human and female Sprague–Dawley (SD) rat hepatocyte cell lines (GSE14555) [44]. Array data for DBA/2 J mouse liver were generated in our laboratories as follows: briefly, adult male DBA/2 J mice were bred in the colonies of the National Public Health Institute, Division of Environmental Health, Kuopio, Finland. Study plans were approved by the Finnish National Animal Experiment Board (Eläinkoelautakunta, ELLA; permit code: ESLH-2008-07223/Ym-23). Animals were housed singly in Makrolon cages with aspen chip bedding (Tapvei Oy, Kaavi, Finland) and provided with Altromin 1314 pellet feed (Altromin Spezialfutter GmbH & Co. KG, Lage, Germany) and water available ad libitum. The housing environment was maintained at 21 ± 1 °C, with a relative humidity of 50 ± 10% and a 12 h light cycle. TCDD (5 or 500 μg/kg dissolved in corn oil) or corn oil vehicle alone were administered by oral gavage (10 mL/kg). Animals were euthanized by carbon dioxide, followed immediately by cardiac exsanguination 19 h following treatment. Livers were excised and frozen in liquid nitrogen. Tissue samples were shipped on dry ice to the analytic laboratory and stored at −80 °C.
Similarly, adult male transgenic “AHR-ratonized” mice, ages 12–23 weeks, were bred as above. Mice were housed singly in suspended, wire-mesh stainless-steel or Makrolon cages, with the housing environment maintained as described above. Animals were provided with R36 pellet feed (Lactamin, Stockholm, Sweden) and tap water available ad libitum. Animals (n = 83) were divided into 5 groups per Ahr isoform (INS/DEL/rWT) and TCDD (125, 250, 500, or 1000 μg/kg dissolved in corn oil) or corn oil vehicle alone were administered by oral gavage (10 mL/kg). Animals were euthanized by cervical dislocation 4 days following exposure. Tissue was collected and stored as above.
Animal handling and reporting comply with ARRIVE guidelines [45]. RNA isolation and microarray hybridization were performed as described elsewhere [37, 38, 46]. Remaining data were generated and deposited in the GEO repository as described in the original publications [34, 37–41, 43].
Computational methods
Human cell lines
For hMADS cells, raw GenePix image data (.gpr files) were loaded into the R statistical environment (v3.2.1) using the limma (v3.24.13) package. Efforts were made to emulate the processing procedures conducted by the original authors: images were first cleaned by filtering out bad spots (flagged as “bad”, “not found” or “absent” or known as −100, −50 and −75 respectively) by assigning those spots a weight of zero. Normalization was performed within arrays by applying global LOESS. Agilent Feature IDs were annotated with EntrezGene IDs and gene names using an annotation table obtained from NCBI's GEO repository for the array type (GPL4133). Linear modelling was conducted to identify genes with statistically significant differential mRNA abundances between TCDD-treated and reference samples. An empirical Bayes method was applied following model fitting to reduce standard error and moderated t-tests were used to assess statistical significance [47]. All p-values were adjusted for multiple testing using a 5% false discovery rate (FDR) [48]. Genes with multiple mapped Agilent Feature IDs were trimmed by keeping the feature ID with the lowest p-value.
For primary human hepatocyte cell line data, CEL files were downloaded from GEO (GSE14555) and loaded into the R statistical environment (v3.3.1) using the affy package (v1.48.0) of the BioConductor library [49]. Due to limited sample size, data for both sexes and all dose points were processed together. Data were normalized using the RMA algorithm [50] and probe annotations were obtained using the custom CDF [51] hgu133ahsentrezgcdf (v19.0.0) and database hgu133ahsentrezg_db (v19.0.0) packages. Probes were filtered using a background intensity threshold established by evaluating chromosome Y associated probes in female samples. Linear modelling was performed using the limma (v3.28.21) package, with contrasts fit to identify differences between treatment and control groups for each treatment dose. An empirical Bayes moderation of the standard error [47] was applied, and model-based t-tests were used to assess significance, accompanied by FDR correction for multiple testing [48].
Mouse data
Raw CEL files for livers from male and female C57BL/6 mice treated with TCDD or corn oil along a time-course (GSE61037) were loaded in the R statistical environment (v3.2.1) using the affy package (v1.46.1)as described above. Data for both sexes and all time points were preprocessed together and normalized using the RMA algorithm [50]. Probe annotations were obtained using the custom CDF [51] mogene11stmmentrezgcdf (v19.0.0) and database mogene11stmmentrezg_db (v19.0.0) packages. Chromosome Y probe filtering was performed as described above. Linear modelling was performed separately for each time point with both male and female samples using the limma (v3.24.13) package. An empirical Bayes moderation of the standard error [47] was applied, and model-based t-tests were used to assess significance, accompanied by FDR correction for multiple testing [48].
Data from the corresponding dose–response study were processed similarly with the following exceptions: male and female samples were preprocessed and modelled separately to avoid masking sex-specific effects at low dose treatments. As such, no additional probe filtering was performed. Similarly, transgenic “AHR-ratonized” mouse data were preprocessed and modelled independently for each AHR genotype with no additional probe filtering. Dose–response data from DBA/2 J mouse liver were processed and modelled as a single dataset using the above methods.
Rat data
For each experiment, raw CEL files were loaded in the R statistical environment (v3.2.1) using the affy package (v1.46.1) of the BioConductor library [49]. Rat adipose data (GSE18301) were preprocessed as a whole, including both strains and time points, using the RMA algorithm [50] with the custom rat2302rnentrezgcdf (v19.0.0) package [51]. Probes were further annotated with gene symbols and named using the rat2302rnentrezg_db (v19.0.0) package. Linear modelling was performed on all samples using the limma (v3.24.13) package, with contrasts fit to specify individual comparisons for both strains. Experiments at the day-1 time-point evaluated TCDD relative to corn oil, whereas the 4-day experiment compared TCDD with feed-restricted, corn oil treated controls. As above, an empirical Bayes moderation of the standard error [47] was applied, and model-based t-tests were used to assess significance, accompanied by FDR correction for multiple testing [48]. Identical processing was performed for data generated from rat hypothalamus at 23 h (GSE61039) and rat liver at 4 and 10 day time points (GSE13513). Data from rat liver, 3 and 19 h time points (GSE10083), were processed together as described above using the custom rae230arnentrezgcdf (v19.0.0) package [51].
CEL files for the primary rat hepatocyte cell line dataset were downloaded from GEO (GSE14555) and loaded into the R statistical environment (v3.3.1) using the affy package (v1.48.0) of the BioConductor library [49]. Data were normalized using the RMA algorithm [50] and probe annotations were obtained using the custom CDF [51] rgu34arnentrezgcdf (v19.0.0) and database rgu34arnentrezg_db (v19.0.0) packages. As this specifc array type does not include probes for genes located on chromosome Y, no additional filtering was performed. Linear modelling was performed using the limma (v3.28.21) package, with contrasts fit to identify differences between treatment and control groups for each treatment dose. An empirical Bayes moderation of the standard error [47] was applied, and model-based t-tests were used to assess significance, accompanied by FDR correction for multiple testing [48].
Interspecies comparisons
All interspecies comparisons may be facilitated using homologene IDs provided by NCBI. A function is provided in the TCDD.Transcriptomics package to load filtered HomoloGene data (build 68) for comparison of mouse, rat and human transcriptomic responses to TCDD.
Package access
The TCDD.Transcriptomics package is available for download from http://labs.oicr.on.ca/boutros-lab/tcdd-transcriptomics, along with instructions for direct download and installation within the R statistical environment. TCDD.Transcriptomics is designed for use with the R statistical environment (≥ v2.10.1) and is dependent on the BoutrosLab.plotting.general (BPG) package (≥ v3.10.2) (P’ng et al., submitted) for production of plotting covariates and easy integration with data visualizations. The BPG package can be found here: http://labs.oicr.on.ca/boutros-lab/software/bpg.
Results and Discussion
In recent years, activation of the AHR has become the focus of myriad studies across a wide range of fields. In particular, significant effort has gone into elucidating the mechanism by which TCDD activation of the AHR leads to a diversity of toxic outcomes. As the ligand-activated AHR is a transcription factor, the transcriptome has been the primary focus of these studies. As such, we have collated a number of transcriptomic datasets arising from various TCDD exposure studies in rats, mice and human cell lines (Table 1, Additional file 1) into a datasets package for the R statistical environment. Specifically, microarray data from 12 separate studies covering 63 unique experimental conditions were collected. Data were generated using two microarray platforms and seven unique array types: Agilent’s Whole Human Genome Microarray and Affymetrix’s Human Genome U133A Array, Rat Expression Array (230A), Rat Genome U34 and 230 2.0 Arrays, Mouse Genome 430 2.0 Array and Mouse Gene 1.1 ST Array. Data from Affymetrix arrays were processed using the RMA algorithm with the most recent probe to EntrezGeneID map (as described in Materials and Methods), analyzed using linear modelling, FDR-corrected for multiple testing and results format-standardized.
Table 1.
Summary of TCDD Datasets
| Species | Tissue | Strain/AHR Genotype | Sex | Number of Samples | Dose(s) (μg/kg) | Time(s) (hours) | GEO Accession |
|---|---|---|---|---|---|---|---|
| Human | adipose-derived stem cells | hWT | N/A | 8 | 25nM | 48 | GSE32026 |
| primary hepatocyte cell line | hWT | male and female | 3 | 0.00001, 0.001, 0.01, 0.1, 1, 10, 316 (nM) | 48 | GSE14555 | |
| Mouse | kidney | C57BL/6 | male | 12 | 0, 1000 | 19 | GSE15857 |
| AHR-KO | male | 6 | |||||
| liver | C57BL/6 | male | 62 | 0, 125, 250, 500, 1000 | 6, 19, 24, 72, 96, 144 |
GSE15858 GSE61037 GSE61038 |
|
| female | 47 | 6, 24, 72, 96, 144 |
GSE61037 GSE61038 |
||||
| DBA/2 | male | 12 | 0, 5, 500 | 19 | NA | ||
| AHR-KO | male | 6 | 0, 1000 | 19 | GSE15858 | ||
| rWT (L-E) | male | 17 | 0, 125, 250, 500, 1000 | 96 | GSE72270 | ||
| DEL (H/W) | male | 21 | |||||
| INS (H/W) | male | 22 | |||||
| Rat | adipose | L-E | male | 20 | 0, 100 | 24, 96 | GSE18301 |
| H/W | male | 8 | 24 | ||||
| hypothalamus | L-E | male | 7 | 23 | GSE61039 | ||
| H/W | male | 8 | |||||
| liver | L-E | male | 39 | 3, 19, 96, 240 |
GSE10083 GSE13513 |
||
| H/W | male | 30 | |||||
| Ln-A (H/W) | male | 8 | 19 | GSE10083 | |||
| Ln-C (L-E) | male | 8 | |||||
| primary hepatocyte cell line | rWT (SD) | female | 14 | 0.00001, 0.001, 0.01, 0.1, 1, 10, 316 (nM) | 48 | GSE14555 |
A total of 63 datasets are included in this package consisting of 377 samples across 3 species, 4 tissue types and a wide range of AHR genotypes, TCDD exposure times and doses
Each dataset is labelled with a highly descriptive title, indicating the species, strain, tissue type, length of exposure (hours), TCDD dose (μg/kg) and sex (male if unlabelled) from which the data were generated. Furthermore, each dataset contains three parts: the sample information, preprocessed data and analysis results. First, the sample information file outlines the type and treatment conditions for each sample in the experiment. The second file contains the expression data and consists of the normalized array data for all samples listed in the sample information file. Finally, the results file outlines the magnitude and significance of change as determined by linear modelling for each gene available on the array. In this file, all genes are annotated with EntrezGene IDs, gene symbol and full gene name.
To provide an example of the usefulness of this R package, a set of widely recognized “AHR-core” genes, genes with mRNA transcription known to be regulated by the AHR transcription factor in a wide range of species and tissues [52–57], were visualized across the available datasets (Fig. 1). Data was extracted according to Homologene ID in order to ensure the suitability of comparisons across species. Interestingly, while most of the selected genes show some degree of TCDD-mediated change in transcript levels across the datasets, some genes demonstrate a species and/or tissue specific effect. In particular, ALDH3A1 appears to be induced only in rat liver and primary human hepatocytes. Similarly, INMT is repressed in livers of rats and TCDD-sensitive “AHR-ratonized” mice. The variability in response to the “AHR-core” genes highlights the need for further study into the effects of TCDD on various organisms.
Fig. 1.
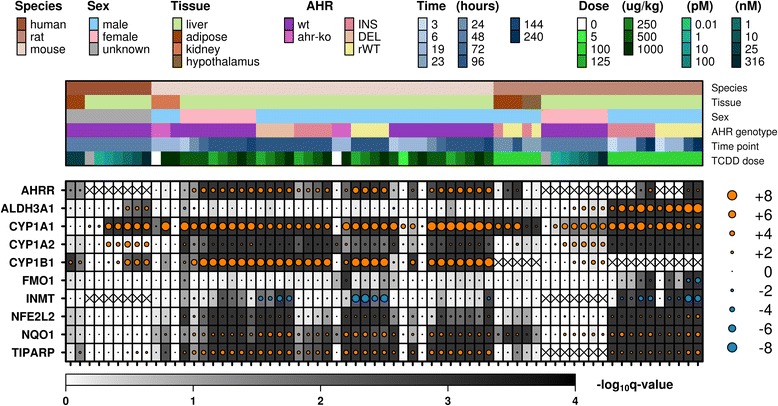
“AHR-core” genes. Transcriptional response (treatment relative to control) of a subset of 10 “AHR-core” genes across the available datasets demonstrates unexpected variability. Dot size indicates magnitude of change in mRNA abundance; colour represents direction of change; background shading demonstrates significance of change. Boxes containing an ‘X’ indicate that data for the given gene was not available for the indicated dataset
While the TCDD.Transcriptomics package provides access to a wide range of datasets, it is by no means fully comprehensive. The GEO repository contains a number of additional datasets relating to array-based mRNA abundance studies of TCDD-mediated transcriptional regulation, however these typically pertain to gestational exposure in mice and rats, human cancer-derived cell lines and zebra fish – all of which are beyond the current scope of this package but may be incorporated at a later date.
Conclusion
Here we have produced a standardized compendium of TCDD-mediated transcriptional changes for use in the R statistical environment. This package, titled TCDD.Transcripomics, contains data from 63 experiments including 377 samples, incorporating data from 3 species, 4 tissue types, both sexes and a wide range of AHR genotypes, TCDD exposure times and doses, along with an up-to-date HomoloGene dataset for interspecies comparison, and is freely available for download (http://labs.oicr.on.ca/boutros-lab/tcdd-transcriptomics). This datasets package will provide a significant resource for the scientific community encompassing a variety of fields of study, from toxicological studies concerning TCDD and dioxins to studies of the AHR and its role in normal physiology and human disease.
Availability and requirements
Project name: TCDD Transcriptomics
Project home page: http://labs.oicr.on.ca/boutros-lab/tcdd-transcriptomics
Operating system(s): tested on Ubuntu and Debian
Programming language: R
Other requirements: depends on BPG (http://labs.oicr.on.ca/boutros-lab/software/bpg)
Licence: GPL-2
Acknowledgements
The authors thank all members of the Boutros Lab, as well as Dr. Patricia Harper, Kirsten Bielefeld and Hanbo Chen, for excellent technical assistance and support.
Funding
This work was supported by the Canadian Institutes of Health Research (grant number MOP-57903 to ABO and PCB), the Academy of Finland (grant number 261232 to RP), and with the support of the Ontario Institute for Cancer Research to PCB through funding provided by the Government of Ontario. PCB was supported by a Terry Fox Research Institute New Investigator Award and a CIHR New Investigator Award. The study sponsors had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Availability of data and material
The TCDD Transcriptomics datasets package is freely available for download (http://labs.oicr.on.ca/boutros-lab/tcdd-transcriptomics). Additionally, the original datasets supporting the results of this article are publicly available on NCBI’s GEO repository: L-E and H/W rat hypothalamus (GSE61039), adipose (GSE18301) and liver at 3 and 19 h (GSE10083), 4 and 10 days (GSE13513); human multipotent adipose-derived stem cells (GSE32026); AHR-null and wild-type mouse liver (GSE15858) and kidney (GSE15857); male and female C57BL/6 mice along a time-course (GSE61037) and dose response (GSE61038); AHR-ratonized mice (GSE72270); primary human hepatocyte and primary rat hepatocyte cell lines (GSE14555).
Authors’ contributions
JL, SL and RP carried out and supervised animal experiments. IDM, JDW, SDP and ABS performed sample preparation for mouse and rat transcriptomic analyses. SDP, KEH, RXS and JL performed statistical and bioinformatics analyses. SDP wrote the first draft of the manuscript. CQY, CP, RPang, LCC and NJH generated tools and reagents. ABO, RP and PCB initiated the project and supervised research. All authors approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable
Abbreviations
- AHR
Aryl hydrocarbon receptor
- H/W
Han/Wistar (Kuopio)
- hMADS
Human Multipotent Adipose-Derived Stem
- L-E
Long-Evans (Turku/AB)
- SD
Sprague Dawley
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
Additional file
Overview of Included TCDD Datasets. (XLSX 16 kb)
References
- 1.Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc Natl Acad Sci U S A. 1997;94(25):13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27(2):109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 3.Petrulis JR, Perdew GH. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact. 2002;141(1–2):25–40. doi: 10.1016/S0009-2797(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 4.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 5.Shen ES, Whitlock JP., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J Biol Chem. 1992;267(10):6815–6819. [PubMed] [Google Scholar]
- 6.Goode GD, Ballard BR, Manning HC, Freeman ML, Kang Y, Eltom SE. Knockdown of aberrantly upregulated aryl hydrocarbon receptor reduces tumor growth and metastasis of MDA-MB-231 human breast cancer cell line. Int J Cancer. 2013;133(12):2769–2780. doi: 10.1002/ijc.28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito R, Miki Y, Hata S, Takagi K, Iida S, Oba Y, Ono K, Ishida T, Suzuki T, Ohuchi N, et al. Aryl hydrocarbon receptor in breast cancer-a newly defined prognostic marker. Horm Cancer. 2014;5(1):11–21. doi: 10.1007/s12672-013-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salisbury TB, Tomblin JK, Primerano DA, Boskovic G, Fan J, Mehmi I, Fletcher J, Santanam N, Hurn E, Morris GZ, et al. Endogenous aryl hydrocarbon receptor promotes basal and inducible expression of tumor necrosis factor target genes in MCF-7 cancer cells. Biochem Pharmacol. 2014;91(3):390–399. doi: 10.1016/j.bcp.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu XL, Wang SY, Jia YJ, Guan Y. Aryl hydrocarbon receptor exogenous ligand 3-methylchoranthrene inhibited endometrial cancer cells proliferation. Eur Rev Med Pharmacol Sci. 2014;18(8):1269–1276. [PubMed] [Google Scholar]
- 10.Ishida M, Mikami S, Shinojima T, Kosaka T, Mizuno R, Kikuchi E, Miyajima A, Okada Y, Oya M. Activation of aryl hydrocarbon receptor promotes invasion of clear cell renal cell carcinoma and is associated with poor prognosis and cigarette smoke. Int J Cancer. 2015;137(2):299–310. doi: 10.1002/ijc.29398. [DOI] [PubMed] [Google Scholar]
- 11.Su JM, Lin P, Chang H. Prognostic value of nuclear translocation of aryl hydrocarbon receptor for non-small cell lung cancer. Anticancer Res. 2013;33(9):3953–3961. [PubMed] [Google Scholar]
- 12.Spink BC, Bloom MS, Wu S, Sell S, Schneider E, Ding X, Spink DC. Analysis of the AHR gene proximal promoter GGGGC-repeat polymorphism in lung, breast, and colon cancer. Toxicol Appl Pharmacol. 2015;282(1):30–41. doi: 10.1016/j.taap.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richmond O, Ghotbaddini M, Allen C, Walker A, Zahir S, Powell JB. The aryl hydrocarbon receptor is constitutively active in advanced prostate cancer cells. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HO, Kim JH, Chung BY, Choi MG, Park CW. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23(4):278–281. doi: 10.1111/exd.12350. [DOI] [PubMed] [Google Scholar]
- 15.Furumatsu K, Nishiumi S, Kawano Y, Ooi M, Yoshie T, Shiomi Y, Kutsumi H, Ashida H, Fujii-Kuriyama Y, Azuma T, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56(9):2532–2544. doi: 10.1007/s10620-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 16.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(1):237–248. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37(33):11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- 18.Schecter A, Birnbaum L, Ryan JJ, Constable JD. Dioxins: an overview. Environ Res. 2006;101(3):419–428. doi: 10.1016/j.envres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Kransler KM, McGarrigle BP, Olson JR. Comparative developmental toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the hamster, rat and guinea pig. Toxicology. 2007;229(3):214–225. doi: 10.1016/j.tox.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93(13):6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 22.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2(10):645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 23.Bunger MK, Moran SM, Glover E, Thomae TL, Lahvis GP, Lin BC, Bradfield CA. Resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity and abnormal liver development in mice carrying a mutation in the nuclear localization sequence of the aryl hydrocarbon receptor. J Biol Chem. 2003;278(20):17767–17774. doi: 10.1074/jbc.M209594200. [DOI] [PubMed] [Google Scholar]
- 24.Walisser JA, Bunger MK, Glover E, Harstad EB, Bradfield CA. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem. 2004;279(16):16326–16331. doi: 10.1074/jbc.M400784200. [DOI] [PubMed] [Google Scholar]
- 25.Bunger MK, Glover E, Moran SM, Walisser JA, Lahvis GP, Hsu EL, Bradfield CA. Abnormal liver development and resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in mice carrying a mutation in the DNA-binding domain of the aryl hydrocarbon receptor. Toxicol Sci. 2008;106(1):83–92. doi: 10.1093/toxsci/kfn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nukaya M, Walisser JA, Moran SM, Kennedy GD, Bradfield CA. Aryl hydrocarbon receptor nuclear translocator in hepatocytes is required for aryl hydrocarbon receptor-mediated adaptive and toxic responses in liver. Toxicol Sci. 2010;118(2):554–563. doi: 10.1093/toxsci/kfq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman DE, Schiller CM. Dose-related effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57BL/6 J and DBA/2 J mice. Toxicol Appl Pharmacol. 1985;78(1):147–157. doi: 10.1016/0041-008X(85)90314-X. [DOI] [PubMed] [Google Scholar]
- 28.Okey AB, Vella LM, Harper PA. Detection and characterization of a low affinity form of cytosolic Ah receptor in livers of mice nonresponsive to induction of cytochrome P1-450 by 3-methylcholanthrene. Mol Pharmacol. 1989;35(6):823–830. [PubMed] [Google Scholar]
- 29.Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, Fujii-Kuriyama Y. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem. 1994;269(44):27337–27343. [PubMed] [Google Scholar]
- 30.Pohjanvirta R, Wong JM, Li W, Harper PA, Tuomisto J, Okey AB. Point mutation in intron sequence causes altered carboxyl-terminal structure in the aryl hydrocarbon receptor of the most 2,3,7,8-tetrachlorodibenzo-p-dioxin-resistant rat strain. Mol Pharmacol. 1998;54(1):86–93. doi: 10.1124/mol.54.1.86. [DOI] [PubMed] [Google Scholar]
- 31.Tuomisto JT, Viluksela M, Pohjanvirta R, Tuomisto J. The AH receptor and a novel gene determine acute toxic responses to TCDD: segregation of the resistant alleles to different rat lines. Toxicol Appl Pharmacol. 1999;155(1):71–81. doi: 10.1006/taap.1998.8564. [DOI] [PubMed] [Google Scholar]
- 32.Rowlands CJ, Staskal DF, Gollapudi B, Budinsky R. The human AHR: identification of single nucleotide polymorphisms from six ethnic populations. Pharmacogenet Genomics. 2010;20(5):283–290. doi: 10.1097/FPC.0b013e32833605f8. [DOI] [PubMed] [Google Scholar]
- 33.Pohjanvirta R, Vartiainen T, Uusi-Rauva A, Monkkonen J, Tuomisto J. Tissue distribution, metabolism, and excretion of 14C-TCDD in a TCDD-susceptible and a TCDD-resistant rat strain. Pharmacol Toxicol. 1990;66(2):93–100. doi: 10.1111/j.1600-0773.1990.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 34.Houlahan KE, Prokopec SD, Sun RX, Moffat ID, Linden J, Lensu S, Okey AB, Pohjanvirta R, Boutros PC. Transcriptional profiling of rat white adipose tissue response to 2,3,7,8-tetrachlorodibenzo-rho-dioxin. Toxicol Appl Pharmacol. 2015. [DOI] [PubMed]
- 35.Enan E, Overstreet JW, Matsumura F, VandeVoort CA, Lasley BL. Gender differences in the mechanism of dioxin toxicity in rodents and in nonhuman primates. Reprod Toxicol. 1996;10(5):401–411. doi: 10.1016/0890-6238(96)83995-5. [DOI] [PubMed] [Google Scholar]
- 36.Pohjanvirta R. Transgenic mouse lines expressing rat AH receptor variants--a new animal model for research on AH receptor function and dioxin toxicity mechanisms. Toxicol Appl Pharmacol. 2009;236(2):166–182. doi: 10.1016/j.taap.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Prokopec SD, Watson JD, Lee J, Pohjanvirta R, Boutros PC. Sex-related differences in murine hepatic transcriptional and proteomic responses to TCDD. Toxicol Appl Pharmacol. 2015;284(2):188–196. doi: 10.1016/j.taap.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Prokopec SD, Watson JD, Sun RX, Pohjanvirta R, Boutros PC. Male and female mice show significant differences in hepatic transcriptomic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. BMC Genomics. 2015;16(1):625. doi: 10.1186/s12864-015-1840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boutros PC, Bielefeld KA, Pohjanvirta R, Harper PA. Dioxin-dependent and dioxin-independent gene batteries: comparison of liver and kidney in AHR-null mice. Toxicol Sci. 2009;112(1):245–256. doi: 10.1093/toxsci/kfp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moffat ID, Boutros PC, Chen H, Okey AB, Pohjanvirta R. Aryl hydrocarbon receptor (AHR)-regulated transcriptomic changes in rats sensitive or resistant to major dioxin toxicities. BMC Genomics. 2010;11:263. doi: 10.1186/1471-2164-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boutros PC, Yao CQ, Watson JD, Wu AH, Moffat ID, Prokopec SD, Smith AB, Okey AB, Pohjanvirta R. Hepatic transcriptomic responses to TCDD in dioxin-sensitive and dioxin-resistant rats during the onset of toxicity. Toxicol Appl Pharmacol. 2011;251(2):119–129. doi: 10.1016/j.taap.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui LC, Chevallier A, Forest C, Benelli C, Clement K, Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ Health Perspect. 2012;120(4):508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houlahan KE, Prokopec SD, Moffat ID, Linden J, Lensu S, Okey AB, Pohjanvirta R, Boutros PC. Transcriptional profiling of rat hypothalamus response to 2,3,7,8-tetrachlorodibenzo-rho-dioxin. Toxicology. 2014;328C:93–101. doi: 10.1016/j.tox.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Carlson EA, McCulloch C, Koganti A, Goodwin SB, Sutter TR, Silkworth JB. Divergent transcriptomic responses to aryl hydrocarbon receptor agonists between rat and human primary hepatocytes. Toxicol Sci. 2009;112(1):257–272. doi: 10.1093/toxsci/kfp200. [DOI] [PubMed] [Google Scholar]
- 45.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prokopec SD, Buchner NB, Fox NS, Chong LC, Mak DY, Watson JD, Petronis A, Pohjanvirta R, Boutros PC. Validating reference genes within a mouse model system of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxicity. Chem Biol Interact. 2013;205(1):63–71. doi: 10.1016/j.cbi.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 48.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100(16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 51.Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33(20) doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boutros PC, Yan R, Moffat ID, Pohjanvirta R, Okey AB. Transcriptomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in liver: comparison of rat and mouse. BMC Genomics. 2008;9:419. doi: 10.1186/1471-2164-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forgacs AL, Dere E, Angrish MM, Zacharewski TR. Comparative analysis of temporal and dose-dependent TCDD-elicited gene expression in human, mouse, and rat primary hepatocytes. Toxicol Sci. 2013;133(1):54–66. doi: 10.1093/toxsci/kft028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59(1):65–85. doi: 10.1016/S0006-2952(99)00310-X. [DOI] [PubMed] [Google Scholar]
- 55.Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol Pharmacol. 2006;69(1):140–153. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- 56.Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the “TCDD-inducible AhR-Nrf2 gene battery”. Toxicol Sci. 2009;111(2):238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson JD, Prokopec SD, Smith AB, Okey AB, Pohjanvirta R, Boutros PC. TCDD dysregulation of 13 AHR-target genes in rat liver. Toxicol Appl Pharmacol. 2014;274(3):445–454. doi: 10.1016/j.taap.2013.12.004. [DOI] [PubMed] [Google Scholar]


