Abstract
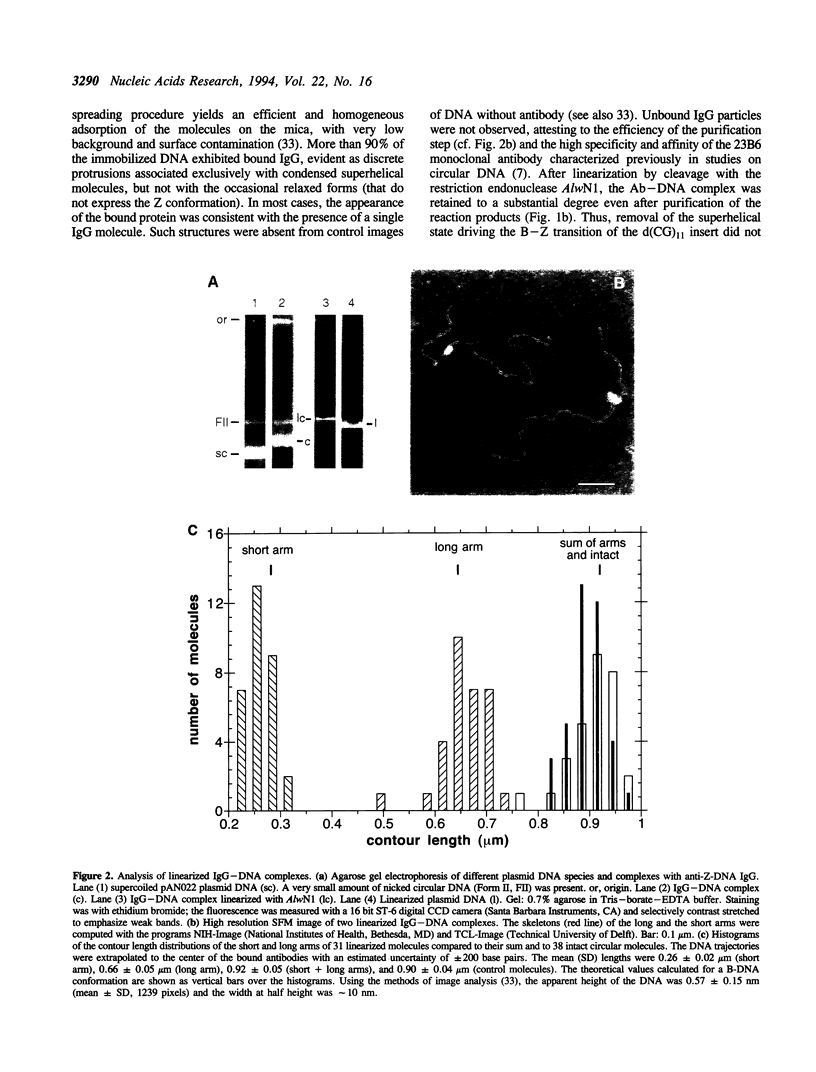
An anti-Z-DNA IgG antibody was used to probe for the left-handed Z-DNA conformation of a d(CG)11 insert in a negatively supercoiled plasmid DNA (pAN022). The complexes were spread on mica in the presence of a quaternary ammonium detergent benzyldimethylalkylammonium chloride and imaged with a scanning force microscope (SFM). The high affinity anti-Z-DNA antibody was retained even after restriction endonuclease cleavage of the DNA. The two arms in the product molecules had unequal lengths in conformity with the known location of the Z-DNA forming insert. Most complexes exhibited one IgG per DNA molecule. The bound antibodies were up to approximately 35 nm in diameter and extended approximately 2 nm from the mica surface. They were generally in a lateral orientation relative to the DNA, in accordance with prior chemical modification experimental data indicating a bipedal mode of binding for an anti-Z-DNA IgG. However, the SFM images also suggest that the DNA bends to accommodate the two Fab combining regions of the antibody. This study demonstrates the utility of the SFM for investigating conformation-dependent molecular recognition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian W. J., Spraker T. R., Davies R. B. Epornitics of aspergillosis in mallards (Anas platyrhynchos) in north central Colorado. J Wildl Dis. 1978 Apr;14(2):212–217. doi: 10.7589/0090-3558-14.2.212. [DOI] [PubMed] [Google Scholar]
- Allen M. J., Lee C., Lee J. D., 4th, Pogany G. C., Balooch M., Siekhaus W. J., Balhorn R. Atomic force microscopy of mammalian sperm chromatin. Chromosoma. 1993 Nov;102(9):623–630. doi: 10.1007/BF00352310. [DOI] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Udvardy A., Garner M. M., Ritter S., Jovin T. M. Z-DNA binding and inhibition by GTP of Drosophila topoisomerase II. Biochemistry. 1993 May 11;32(18):4862–4872. doi: 10.1021/bi00069a023. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Bustamante C., Vesenka J., Tang C. L., Rees W., Guthold M., Keller R. Circular DNA molecules imaged in air by scanning force microscopy. Biochemistry. 1992 Jan 14;31(1):22–26. doi: 10.1021/bi00116a005. [DOI] [PubMed] [Google Scholar]
- Castleman H., Erlanger B. F. Electron microscopy of "Z-DNA". Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):133–142. doi: 10.1101/sqb.1983.047.01.018. [DOI] [PubMed] [Google Scholar]
- Castleman H., Hanau L. H., Zacharias W., Erlanger B. F. Z DNA and loop structures by immunoelectronmicroscopy of supercoiled pRW751, a plasmid containing left-handed helices. Nucleic Acids Res. 1988 May 11;16(9):3977–3996. doi: 10.1093/nar/16.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Capua E., Stasiak A., Koller T., Brahms S., Thomae R., Pohl F. M. Torsional stress induces left-handed helical stretches in DNA of natural base sequence: circular dichroism and antibody binding. EMBO J. 1983;2(9):1531–1535. doi: 10.1002/j.1460-2075.1983.tb01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. Biological applications of scanning probe microscopes. Annu Rev Biophys Biophys Chem. 1991;20:79–108. doi: 10.1146/annurev.bb.20.060191.000455. [DOI] [PubMed] [Google Scholar]
- Glikin G. C., Jovin T. M., Arndt-Jovin D. J. Interactions of Drosophila DNA topoisomerase II with left-handed Z-DNA in supercoiled minicircles. Nucleic Acids Res. 1991 Dec;19(25):7139–7144. doi: 10.1093/nar/19.25.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen F. K., Zarling D. A., Jovin T. M. Electron microscopy of SV40 DNA cross-linked by anti-Z DNA IgG. EMBO J. 1985 Mar;4(3):837–844. doi: 10.1002/j.1460-2075.1985.tb03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma H. G., Bezanilla M., Zenhausern F., Adrian M., Sinsheimer R. L. Atomic force microscopy of DNA in aqueous solutions. Nucleic Acids Res. 1993 Feb 11;21(3):505–512. doi: 10.1093/nar/21.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma H. G., Sinsheimer R. L., Groppe J., Bruice T. C., Elings V., Gurley G., Bezanilla M., Mastrangelo I. A., Hough P. V., Hansma P. K. Recent advances in atomic force microscopy of DNA. Scanning. 1993 Sep-Oct;15(5):296–299. doi: 10.1002/sca.4950150509. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., McIntosh L. P., Arndt-Jovin D. J., Zarling D. A., Robert-Nicoud M., van de Sande J. H., Jorgenson K. F., Eckstein F. Left-handed DNA: from synthetic polymers to chromosomes. J Biomol Struct Dyn. 1983 Oct;1(1):21–57. doi: 10.1080/07391102.1983.10507425. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang M. C., Malfoy B., Freund A. M., Daune M., Leng M. Visualization of Z sequences in form V of pBR322 by immuno-electron microscopy. EMBO J. 1982;1(10):1149–1153. doi: 10.1002/j.1460-2075.1982.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart M., Deisenhofer J., Huber R., Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980 Aug 25;141(4):369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Meese K. Topoisomer gel retardation: detection of anti-Z-DNA antibodies bound to Z-DNA within supercoiled DNA minicircles. Nucleic Acids Res. 1988 Jan 11;16(1):21–37. doi: 10.1093/nar/16.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989 Oct 20;246(4928):358–363. doi: 10.1126/science.2678475. [DOI] [PubMed] [Google Scholar]
- Rees W. A., Keller R. W., Vesenka J. P., Yang G., Bustamante C. Evidence of DNA bending in transcription complexes imaged by scanning force microscopy. Science. 1993 Jun 11;260(5114):1646–1649. doi: 10.1126/science.8503010. [DOI] [PubMed] [Google Scholar]
- Revet B., Zarling D. A., Jovin T. M., Delain E. Different Z DNA forming sequences are revealed in phi X174 RFI by high resolution darkfield immuno-electron microscopy. EMBO J. 1984 Dec 20;3(13):3353–3358. doi: 10.1002/j.1460-2075.1984.tb02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Runkel L., Nordheim A. Chemical footprinting of the interaction between left-handed Z-DNA and anti-Z-DNA antibodies by diethylpyrocarbonate carbethoxylation. J Mol Biol. 1986 Jun 5;189(3):487–501. doi: 10.1016/0022-2836(86)90319-0. [DOI] [PubMed] [Google Scholar]
- Schaper A., Pietrasanta L. I., Jovin T. M. Scanning force microscopy of circular and linear plasmid DNA spread on mica with a quaternary ammonium salt. Nucleic Acids Res. 1993 Dec 25;21(25):6004–6009. doi: 10.1093/nar/21.25.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaiu W. L., Larson D. D., Vesenka J., Henderson E. Atomic force microscopy of oriented linear DNA molecules labeled with 5nm gold spheres. Nucleic Acids Res. 1993 Jan 11;21(1):99–103. doi: 10.1093/nar/21.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverton E. W., Navia M. A., Davies D. R. Three-dimensional structure of an intact human immunoglobulin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5140–5144. doi: 10.1073/pnas.74.11.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton J. F., Miller F. D., Jorgenson K. F., Zarling D. A., Morgan A. R., Rattner J. B., van de Sande J. H. Left-handed Z-DNA regions are present in negatively supercoiled bacteriophage PM2 DNA. EMBO J. 1983;2(12):2123–2128. doi: 10.1002/j.1460-2075.1983.tb01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesenka J., Guthold M., Tang C. L., Keller D., Delaine E., Bustamante C. Substrate preparation for reliable imaging of DNA molecules with the scanning force microscope. Ultramicroscopy. 1992 Jul;42-44(Pt B):1243–1249. doi: 10.1016/0304-3991(92)90430-r. [DOI] [PubMed] [Google Scholar]
- Wittig B., Dorbic T., Rich A. Transcription is associated with Z-DNA formation in metabolically active permeabilized mammalian cell nuclei. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2259–2263. doi: 10.1073/pnas.88.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Shao Z. Effect of probe force on the resolution of atomic force microscopy of DNA. Ultramicroscopy. 1993 Jul;50(2):157–170. doi: 10.1016/0304-3991(93)90006-j. [DOI] [PubMed] [Google Scholar]
- Yang J., Tamm L. K., Somlyo A. P., Shao Z. Promises and problems of biological atomic force microscopy. J Microsc. 1993 Sep;171(Pt 3):183–198. doi: 10.1111/j.1365-2818.1993.tb03375.x. [DOI] [PubMed] [Google Scholar]
- Zarling D. A., Arndt-Jovin D. J., Robert-Nicoud M., McIntosh L. P., Thomae R., Jovin T. M. Immunoglobulin recognition of synthetic and natural left-handed Z DNA conformations and sequences. J Mol Biol. 1984 Jul 5;176(3):369–415. doi: 10.1016/0022-2836(84)90495-9. [DOI] [PubMed] [Google Scholar]