Abstract
Background
Due to the prevalence of HIV-1 group M and the endemicity of HIV-1 group O infections in Cameroon, patients may be infected with both viruses and/or with HIV-1/MO recombinant forms. Such atypical infections may be deleterious in terms of diagnosis and therapeutic management due to the high divergence of HIV-1/O. The aim of this study was to identify prospectively such atypical infections in Cameroon.
Results
Based on serological screening by env-V3 serotyping and a molecular strategy using group-specific (RT)-PCRs, we identified 10 Cameroonian patients harboring three different profiles of infection: (1) 4 HIV-1/M + O dual infections without evidence of recombinant; (2) 5 recombinants associated with one or both parental strains; and (3) 1 new recombinant form without parental strains.
Conclusions
This work highlights the dynamic co-evolution of these two HIV groups in Cameroon that could lead to the emergence of a circulating recombinant form MO, and the need for accurate identification of such atypical infections for precise diagnosis, virological monitoring and therapeutic management with adapted tools.
Keywords: HIV genetic diversity, M and O inter-group recombinant forms, Dual infections
Background
The Human Immunodeficiency Virus type 1 (HIV-1) displays an extraordinary genetic diversity, divided into four groups (M to P) [1–4] due to simian origins, errors in reverse transcription, and a high recombinogenic potential. This latter accentuates diversity and evolution through the dynamic generation of multiple recombinant forms. At least 79 circulating recombinant forms (CRFs) and numerous unique recombinant forms (URFs) are now described for HIV-1 group M (HIV-1/M), accounting for almost 20% of all HIV infections (http://www.hiv.lanl.gov/, accessed in October 2016) [5]. Recombination is not restricted to HIV-1/M, since one intra-HIV-1 group O (HIV-1/O) recombinant as well as one HIV-2 CRF have also been reported [6, 7]. CRFs/URFs emerge in epidemiological conditions where two (or more) different strains predominate. The resulting dual (or multiple) infections [8] can generate recombinants that potentially spread in the population.
In Cameroon, besides the pandemic group M, all HIV-1 divergent groups described to date (N, O and P) are in circulation, with HIV-1/O representing about 1% of all HIV infections [9]. Despite the genetic distance between groups M and O, inter-group recombination is possible; three HIV-1/MO recombinant forms were thus reported in 1999 and 2004 in Cameroonian patients, also infected by the parental HIV-1/O and/or HIV-1/M strains [10–12]. The transmission potential of such recombinant forms was demonstrated by the description in 2010 of a fourth recombinant detected in the absence of parental strains in a Cameroonian patient living in France [13], and more recently by the transmission of a unique HIV-1/MO recombinant form in a Cameroonian couple [14].
These limited observations do not enable assessment of either the prevalence of these dual infections or the dynamic evolution and impact of their associated recombinant forms. But, it is known that presence of HIV-1/O in some part of the genome and/or in dual infections can have serious consequences at the individual level, raising future public health concerns. Indeed, HIV-1/O strains are characterized by high genetic diversity compared to HIV-1/M [6, 15] implying: (1) antigenic consequences that may result in false negative serological testing [16–18]; (2) genetic consequences that may lead to misquantification of the RNA viral load using group-M specific kits [19–21], and the need for specific assays for anti-retroviral (ARV) resistance genotyping [9, 22]; and (3) therapeutic consequences, with a natural resistance to Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) and poor predictive value of the genotypic resistance interpretation algorithms used for HIV-1/M [22–25].
Because HIV-1/O prevalence in Cameroon appears to have remained stable for many decades while HIV-1/M has expanded rapidly [26, 27], the co-circulation of these two different groups in the Cameroonian population may facilitate HIV-1/M + O dual infections and the genesis of HIV-1/MO recombinants.
The aim of this work was to identify prospectively such atypical infections in Cameroon, with an effective strategy combining a serological screening based on V3-serotyping and a molecular characterization using group-specific (RT)-PCRs.
Methods
Characteristics of samples
Between March 2006 and July 2009, 6796 serum samples were prospectively diagnosed HIV positive using a diagnosis strategy previously described as part of the routine laboratory activities of the Centre Pasteur du Cameroun (CPC) [28]. In the same period, 15,000 HIV plasma samples were received for viral load (VL) monitoring of previously diagnosed HIV positive patients. Buffy coats were also available for some patients.
Serotyping screening of potential HIV-1/M + O dual infections
To discriminate between HIV-1/M or HIV-1/O mono-infections and HIV-1/M + O dual infections, all serum and plasma samples were screened with a serotyping ELISA test using a previously described method based on specific antigenic peptides of the env -V3 loop from both HIV-1/O (V3-O) and HIV-1/M (V3-M) [28].
Molecular confirmation of HIV-1/M + O dual infections
All samples considered as dual seroreactive were further investigated using group-O and group-M specific PCRs or RT-PCRs, followed by sequencing. Due to the recombinant patterns previously published, four regions were targeted: the Protease (PR), Reverse Transcriptase (RT), and Integrase (IN) in pol; and the gp41 in env. The position of the different primers on the HIV-1 genome is summarized in Fig. 1. Primer sequences, amplified fragment lengths, and PCR conditions were previously detailed in former publications [6, 9, 22, 29–31].
Fig. 1.
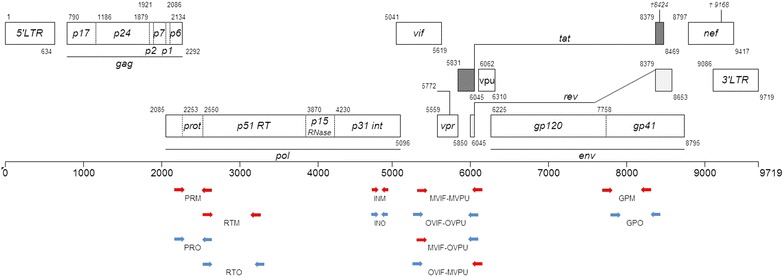
Position of HIV-1/O and HIV-1/M specific primers [cf materials and methods]. In blue, HIV-1/O target regions, in red, HIV-1/M. The lengths of the PCR fragment are respectively: PRM 507 pb, RTM 805 pb, PRO 452 pb, RTO 883 pb, INM 239 pb, INO 192 pb, MVIF-MVPU 891 pb, OVIF-OVPU 816 pb, MVIF-OVPU 815 pb, OVIF-MPVU 895 pb, GPM 656 pb, and GPO 674 pb. The position numbers are relative to HXB2 (GenBank accession number K03455)
Molecular detection of HIV-1 M/O recombinant in vpr
As characterization of the partial or complete genomes of the three recombinant forms (described before the start of this work), revealed a breakpoint in vpr for two of them [10, 11], we hypothesized that this gene could be considered as a hotspot and it was included in our molecular strategy. Putative recombinants with a breakpoint in the Vpr region (designated as vpr recombinants) were investigated with a nested PCR protocol [10] that covered the region between the middle of vif to the middle of vpu (Fig. 1). Identification of vpr recombinants in both [M–O] and [O–M] patterns were made possible by the use of heterologous primers: MVIF/OVPU and OVIF/MVPU respectively. Presence of parental HIV-1/M and HIV-1/O strains was revealed by using the homologous primers MVIF/MVPU and OVIF/OVPU.
Near full-length genome sequencing
Near full-length genome characterization was obtained from RNA extracted from two samples by the amplification of overlapping fragments using group-specific RT-PCR, followed by nested PCR and sequencing as previously described [14].
Phylogenetic and recombination analyses
Group specific PCR fragments and vpr fragments were sequenced and aligned along with a set of different HIV-1/M and HIV-1/O reference sequences from the LANL database using MEGA 5.05 software [32]. vpr sequences from the recombinants described by Peeters et al. [10], Yamaguchi et al. [12], Vessière et al. [13] and Ngoupo et al. [14] were also included in the alignment (GenBank access No AJ239083, AY489738, GQ351296, KM438031 and KM438032). Genotyping was performed using HIV BLAST (http://lanl.hiv.org), Genotyping Retrovirus Tool (http://www.ncbi.nlm.nih.gov/retroviruses/) and REGA HIV-1 Subtyping Tool (http://dbpartners.stanford.edu/RegaSubtyping/). For recombination analyses, we performed: Similarity analysis, allowing localization of the recombination points, with SimPlot software [33]; and the Recombinant Identification Program (RIP) available on-line through the Los Alamos Database [34].
Construction of phylogenetic trees was performed using MEGA; genetic distances were calculated with the Kimura two-parameter method, and trees were obtained by the neighbor-joining method. The reliability of the branching order was estimated by 1000 bootstrap replicates.
Results
HIV-1/O mono-reactivities and HIV-1/M + O dual reactivities
During the 40-month period of the study, 61 of the 6796 HIV positive sera and 81 of the 15,000 plasma for viral load were reactive against at least the V3-O antigen. Among these 142 V3-O positive samples, 53 (37.6%) presented V3-O + V3-M dual seroreactivity.
Molecular confirmation of HIV-1/M + O dual infections and detection of HIV-1/MO recombinant forms
HIV-1/M + O dual infection was defined as the presence of both HIV-1/O and M genomes in a sample; it was explored with group specific PCRs in 39/53 samples with dual seroreactivity. Fourteen samples were not tested because of a lack of material (plasma/serum or buffy coat). Molecular analysis showed that most dual reactivities with serological tests corresponded to non-specific cross-reactivities with V3-M or V3-O antigens. Among the 39 dual seroreactivities, 23 (59%) were positive only with group-O specific PCR in all of the four genes, and sequencing confirmed HIV-1/O fragments. These samples were considered as HIV-1/O mono-infections, i.e. presence of group O species only. Conversely, 6 (15%) were considered as HIV-1/M mono-infections, i.e. presence of group M species only.
Both HIV-1/M and O genomes were detected in 10 of the 39 (26%) patients. Molecular profiles and phylogenetic analyses are detailed in Table 1 and Fig. 2. vpr amplification and sequencing data allowed us to define three different profiles of infections:
dual infections without evidence of a vpr recombinant
Table 1.
Molecular profiles of the 10 samples presenting a dual infection HIV-1/M+O and/or a HIV-1/MO recombinant form
| Samples | Sample collection date | Protease | RT | Integrase | Envelope | HIV-1/O | HIV-1/M | vpr | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroupa | Subtype | HIV-1/O | HIV-1/M | HIV-1/MO | HIV-1/OM | ||||||
| YBF221 | 10/19/2006 | M + O | M + O | M + O | M + O | H | CRF02 | + | + | − | − |
| YBF280 | NA | M + O | M + O | M + O | M + O | T | CRF02 | + | + | − | − |
| YBF301b | 10/16/2007 | M + O | M + O | M + O | M + O | H | CRF02 | + | + | − | − |
| YBF320b | NA | M + O | M + O | M + O | M + O | H | CRF02 | + | + | − | − |
| YBF211b | 05/04/2009 | M + O | M + O | M + O | M + O | H | CRF02 | + | + | − | + |
| YBF212b | 12/11/2006 | M + O | M + O | M + O | M + O | H | CRF02 | + | + | − | + |
| YBF205b | 09/13/2007 | M + O | O | M + O | M + O | H | NT | + | − | − | + |
| YBF274 | 05/14/2008 | O | O | O | M + O | H | D | + | − | − | + |
| YBF298b | 11/06/2007 | M | M | M | M + O | H | CRF02 | − | + | + | − |
| YBF282 | 04/07/2007 | M | M | M | O | H | CRF02 | − | + | − | − |
NT non typable, NA not available
aAccording to classification described by Leoz & al PLoS Pathog, 2015 (16)
bAmplification and sequencing of DNA from buffy coat
Fig. 2.
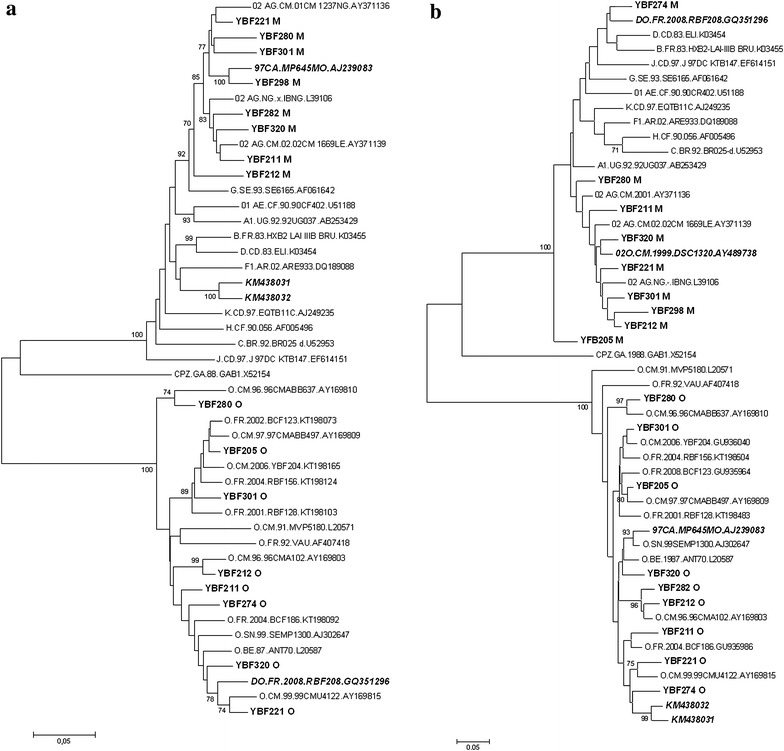
Phylogenetic trees of a the Protease-RT (899 bp) and b the gp41 (443 pb) regions. Sequences representative of HIV-1/M and HIV-1/O were downloaded from the Los Alamos database (hptt://www.hiv.lanl.gov). The four HIV-1/MO recombinants: (1) 97CA.MP645MO [10], (2) 02O.CM.1999.DSC1320 [12], (3) DO.FR.2008.RBF208 [13], and (4) KM438031 and KM438032 [14] are represented in bold italics. The new sequences obtained here are represented in bold. The gp41 HIV-1/O sequence of YBF298 could not be used for phylogenetic analysis, because of the relatively short length of the strain. SIVcpzGAB was used as the outgroup. Phylogenetic analyses were performed using MEGA software [32]: evolutionary distances were computed using the Kimura 2-parameter method; 1000 bootstrap replicates were performed to assess the reliability of the branching order. Bootstrap values are shown only when significant (>70)
Both HIV-1/M and O genomes were detected in four samples (YBF221, YBF280, YBF301, and YBF320). Both strains were characterized in the four genes using plasma (YBF221, YBF280) or buffy coat (YBF301, YBF320) (Table 1). The vpr PCR confirmed the results obtained with group specific PCRs, i.e. only the O–O and M–M homologous primers yielded an amplification fragment.
vpr recombinants associated with one or both parental strains
We identified two samples (YBF211 and YBF212) for which both HIV-1/M and O strains were detected in the four genes. The sequence analysis of vpr fragments obtained with heterologous primers showed a recombination point in this gene (Fig. 3). For a third sample, YBF205, the results of the group-specific PCRs performed on the buffy coat indicated the presence of HIV-1/O sequences in all regions, and of HIV-1/M sequences only in the PR, IN and gp41 regions. The heterologous vpr PCR revealed the presence of an HIV-1 [OM] recombinant. The absence of amplification of HIV-1/M in two genes prevented us from concluding between a dual infection associated with a vpr recombinant or an HIV-1/O single infection associated with a recombinant with a complex pattern.
Fig. 3.
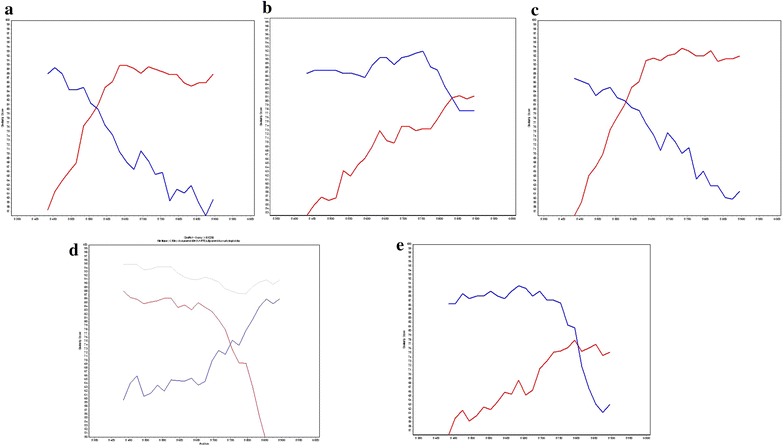
Similarity plots for vpr sequences of the 5 recombinant forms. Plots were generated by SimPlot software with 200 nucleotide (nt) windows, 20 nt increments, and the Kimura 2-parameter method with a transition-transversion (Ts/Tv) ratio of 2.0. Each of the 5 samples (YBF211 (a), YBF212 (b), YBF205 (c), YBF298 (d), and YBF274 (e)) was queried against an HIV-1/M subtype CRF02-AG (02_AG.IBNG.L39106) or subtype D (D.UG.92.92UG001.AJ320484) in red, and an HIV-1/O (O.BE.87.ANT70.L20587) in blue. YBF298 (d) was also queried against the recombinant form 97CA.MP645 (grey line) previously described by Peeters et al. [10]
For two other patients (YBF274 and YBF298), the results of the group specific PCRs or RT-PCRs of the pol and env genes were discordant, with only one strain amplified in pol (HIV-1/O strain for the YBF274 sample and HIV-1/M strain for YBF298), and the two populations, M and O, in env. These profiles associated with the results of vpr amplification indicated a vpr recombinant, associated with only one parental virus (Table 1). For YBF274, sequencing of the complete genome of the recombinant form, showed a mosaic pattern with gag-pol belonging to group O (subgroup H) and env belonging to group M (subtype D), with two breakpoints, in vpr and in LTR (Fig. 4a). For YBF298, similarity analyses showed homology with the recombinant form 97CA.MP645 previously described by Peeters et al. [10]. Indeed, an HIV-1/M fragment was detected in the four regions as well as a group O fragment in the envelope (Table 1). The sequence alignment and the recombinant [MO] profile in the vpr gene showed a recombination breakpoint at the same localization as for 97CA.MP645 (Figs. 2 and 4). This lead us to conclude to a single HIV-1/M infection associated with the presence of an [MO] recombinant pattern (Table 1), as for 97CA.MP645. Phylogenetic analysis showed a strong link between YBF298 and 97CA.MP645 in the HIV-1/M pol fragment (Fig. 2).
Recombinant form only with breakpoint outside vpr
Fig. 4.
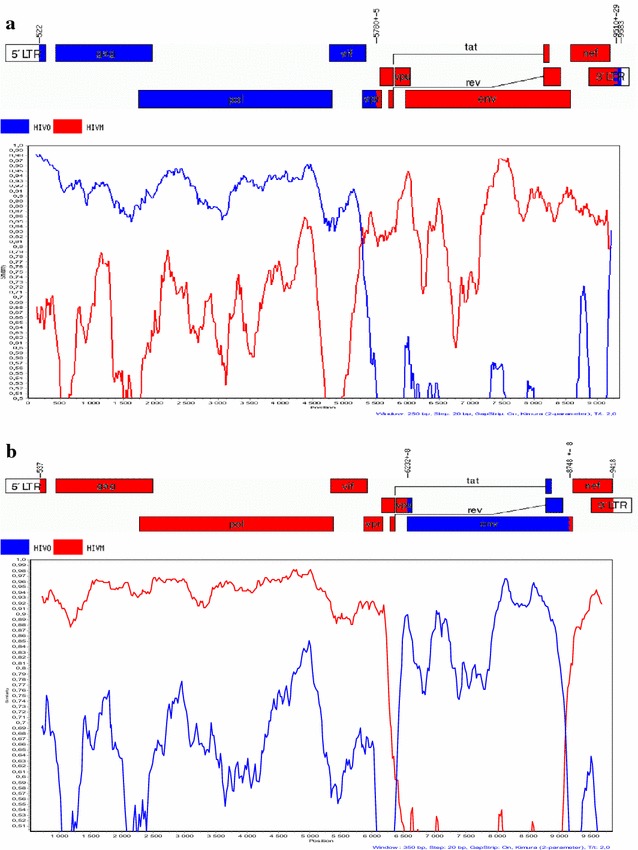
a Recombination pattern of the full-length genome of YBF274. Analysis was performed using SimPlot software with 250 nucleotide (nt) windows, 20 nt increments, and the Kimura 2-parameter method with a transition-transversion (Ts/Tv) ratio of 2.0. Sequences representative of a strain of HIV-1/M (D.CD.1983.ELI_patent.A07108 in red) and O (O.CM.98.98CMA104.AY169802 in blue) were used. The genome map was obtained using the Recombinant HIV Drawing Tool (http://www.hiv.lanl.gov/content/sequence/DRAW_CRF/recom_mapper.html). b Recombination pattern of the full-length genome of YBF282. Analysis was performed using SimPlot software with 350 nucleotide (nt) windows, 20 nt increments, and the Kimura 2-parameter method with a transition-transversion (Ts/Tv) ratio of 2.0. Sequences representative of a strain of HIV-1/M (02_AG.NG.IBNG-patent DD409979 in red) and O (O.CM.98.98CMA104.AY169802 in blue) were used. The genome map was obtained using the Recombinant HIV Drawing Tool (http://www.hiv.lanl.gov/content/sequence/DRAW_CRF/recom_mapper.html)
For the YBF282 sample, group specific PCRs were discordant in pol and env, suggesting the presence of a [MO] recombinant virus (Table 1). However, only the presence of an HIV-1/M fragment was detected in the targeted vpr gene. These results suggest the existence of a recombinant virus with the breakpoint situated downstream of vpr. Sequencing of the complete genome confirmed the pattern pol M (subtype CRF02_AG) and env O (subgroup H), with two breakpoints, in vpu and at the end of gp41 (Fig. 4b).
Characterization of the ARV resistance profiles of the Protease and Reverse Transcriptase regions of the HIV-1/O and HIV-1/M fragments
Partial amplification of pol gave us the opportunity to analyze the ARV resistance profiles of these new strains involved in multiple infections associated or not with recombinant viruses. We performed interpretation of resistance of the HIV-1/M and HIV-1/O species according to v25 of the ANRS algorithm for interpretation of resistance (http://www.hivfrenchresistance.org/). The HIV-1/M PR sequences harbored mutations conferring resistance or possible resistance to nelfinavir and/or saquinavir, for three of the samples (YBF301, YBF205, and YBF298) (Table 2). On the RT sequences, resistance mutations were observed only for YBF212, leading to resistance to rilpivirine and possible resistance to etravirine. Many more mutations for PR and RT of HIV-1/O species were observed, leading to: (1) resistance or possible resistance to atazanavir, indinavir, lopinavir and saquinavir; (2) resistance to all NNRTI classes for 6 of 8 viruses (YBF221, YBF301, YBF320, YBF211, YBF212, YBF274) due to the presence of Y181C residue; and (3) resistance to lamivudine/emtricitabine and possible resistance to abacavir for two of the samples (YBF221 and YBF301) due to the presence of M184V residue.
Table 2.
Resistance profiles of the 10 samples presenting a dual infection HIV-1/M+O and/or a HIV-1/MO recombinant form
| Samples | Profile | Protease | RT | Resistance associated mutations and resistance interpretationa | |||||
|---|---|---|---|---|---|---|---|---|---|
| HIV-1/M species | HIV-1/O species | ||||||||
| Protease | RT | Resistance profileb | Protease | RT | Resistance profile | ||||
| YBF221 | Dual | M + O | M + O | K20I M36I L63P/L L89M | V90I | L10V L33I M36I M46L/M I62V A71V K20C L63T L89T | T69S M184V A98G V179E Y181C L210Y | ATV, IDV, 3TC/ FTC, NNRTI LPV, SQV, ABC | |
| YBF280 | Dual | M + O | M + O | K20I M36I V77I L89M | None | L10V V11I I15V M36I I62V A71V K20C D60N/S L63I L89I | A98G K103R V179E L210Y | SQV ATV | |
| YBF301 | Dual | M + O | M + O | L10V K20I L24L/I M36I L89M | None | SQV | L10V I15V M36I I62V A71V K20C L63T/A L89M | M184V A98G K103R V179E Y181C L210Y | SQV, 3TC/FTC, NNRTI ATV, ABC |
| YBF320 | Dual | M + O | M + O | K20I M36I L89M | K101Q | L10I/V I15V M36I I62V A71V K20C L63T L89I | A98G V179E Y181C L210Y | SQV, NNRTI ATV | |
| YBF211 | Dual + recombinant | M + O | M + O | I15L K20I M36I L63P L89M | None | L10V I15V M36I I62V A71V K20C L63T L89I | A98G V179E Y181C L210Y | SQV, NNRTI ATV | |
| YBF212 | Dual + recombinant | M + O | M + O | K20I M36I L89M | E138A V179I K101Q | RPV ETR | L10I I15V L33V M36I I62V A71V/I K20C F53Y/C D60S L63A L89I | A98G V179E Y181C L210Y | ATV, SQV, NNRTI |
| YBF205 | Dual + recombinant | M + O | O | K20I L24L/I M36I L89M | SQV | L10I I15V M36I I62V A71V K20C D60N/S L63T L89I | A98G K103R V179E L210Y | SQV ATV | |
| YBF274 | Single + Recombinant | O | O | – | – | L10V/I I15V M36I D60E I62V A71V K20C L63T L89I | A98G V179E Y181C L210Y | ATV, SQV, NNRTI | |
| YBF298 | Single + recombinant | M | M | K20I D30N M36I D60N G73S L89M | None | NFV SQV | – | – | – |
| YBF282 | Recombinant alone | M | M | G16E K20I M36I L63P/L L89M | None | - | - | ||
aAccording to the ANRS resistance interpretation’s algorithm v25
bIn underline = resistance; italic = possible resistance
Discussion
The aim of this work was to detect HIV-1/M + O dual infections and inter-group recombinant forms in Cameroon. We designed a sero-molecular strategy based on three hypotheses: (1) M + O dual infections are the pre-requisite for the genesis of HIV-1/MO recombinant forms (as the three recombinants described by 2004 were in patients with parental strains [10–12]; detecting dual infections may facilitate detection of recombinants), (2) vpr is a hotspot of recombination between HIV-1/M and HIV-1/O (two of the three MO recombinants forms exhibited a vpr breakpoint [10–12], and (3) since a breakpoint was found two times in vpr and once in the Integrase, the mosaic pattern could be as simple as pol O–env M or pol M–env O, so that performing molecular analyses in these regions could be sufficient to detect putative recombinant forms.
The first step was to perform serotyping screening based on V3-O and V3-M antigens, to discriminate between HIV-1/M or HIV-1/O mono- and dual- seroreactivities easily and rapidly among the 21 796 samples available. We detected 142 samples with reactivity at least against the group O antigen, among which 53 (37.3%) presented with M + O dual reactivities. As previously described, this serotyping can present cross-reactivity [35], since 29/39 samples were concluded as mono-infection by PCR (HIV-1/M: n = 6; HIV-1/O: n = 23). Therefore, dual seroreactivity must not be systematically concluded as dual infection. Despite these limitations, our results showed that our serotyping screening strategy was useful for selecting samples with possible dual infections with or without recombinant forms for further additional molecular exploration.
The second step was to perform molecular investigation using group-specific PCRs. Among the 10 samples with dual seroreactivities, we identified: (1) 4 HIV-1/M + O dual infections without evidence of a vpr recombinant; (2) 5 vpr recombinants associated with one or both parental strains; and (3) 1 vpu recombinant without parental strains. All these cases are new except for YBF298, which could correspond to the 97CA.MP645 strain previously described or to this strain transmitted to another patient with an epidemiological link.
The diversity patterns of the six recombinant forms were coherent with HIV molecular epidemiology in Cameroon, since they implied parental HIV-1/O subgroup H (6/6) and HIV-1/M subtype CRF02_AG (4/6, 67%), the most prevalent forms of each group [15, 36]. Thus, our work has shown that the co-circulation of HIV-1/M and HIV-1/O leads to dual infections and recombinant forms that could correspond to as many URFs_MO as described for HIV-1/M. These data, and our recent report of the transmission of an URF_MO in a couple, highlight the fact that recombinants are circulating in Cameroon, are more numerous than initially thought, and that a CRFs_MO or _OM could emerge in the next years. This hypothesis could be supported by the previous report that an MO recombinant form is more replicative than the parental strains [10].
The impact on public health of these dual infections and recombinants is difficult to predict. Paradoxically, even though we found a relatively small number of dual seroreactivities (53 among the 21 796 samples), these atypical infections accounted for 26% of dual seroreactive samples, highlighting that these phenomena are not as rare as previously thought. The negative impact of the genetic diversity of group O on serological diagnosis, resistance to NNRTI-based treatments and RNA plasmatic misquantification is now well-known. Similarly, the circulation of M + O dual infections and/or mosaic strains including group O fragments could have deleterious consequences. Thus, recombinant forms with a group O envelope (in absence of parental forms) will not be diagnosed by serological tests that are not adapted to this group due to antigenic variability [17, 18, 37], thus enabling silent spreading of these viruses. Moreover, if M + O dual infections and recombinants with group O fragments (particularly in RT) are unknown, unadapted therapeutic management could lead to rapid virological failure of the cART due to the natural resistance of group O to NNRTIs, and subsequently to the emergence of strains resistant to other partner drugs. We have shown here that some HIV-1/M species and many HIV-1/O species are resistant or possibly resistant to many drugs; some of these mutations are classically found in HIV-1/M non-B subtypes or correspond to the natural polymorphism of HIV-1/O [22]. But, some others are selected under pressure of cART, as M184V or D30N for example, indicating that even in absence of therapeutic data, patients may have had a cART. This natural polymorphism together with selected mutations as found in YBF221 and YBF301 dual infections (Table 2) is of concern if a recombinant form emerges with the virological properties of HIV-1/M associated with the multi-resistance pattern of the HIV-1/O species. Another concern is the virological follow-up, which if performed with a group specific technique, may underestimate the viral populations according to the amplified regions; paradoxically, the development of non-specific viral load kits (RealTime HIV-1, Abbott; Cobas TaqMan HIV-1 v.2, Roche for example) will quantify global M + O populations without distinguishing them, and will lead to the non-identification of recombinants. As a consequence, knowledge of these particular cases of multiple and recombinant infections is essential for accurate virological monitoring and therapeutic management with specific tools. We already observed an HIV-1 group M superinfection in an HIV-1 group O-infected patient in a pregnancy context [38].
At an individual level, these dual infections and recombinant forms also raise questions about their natural evolution, especially concerning pathogenesis compared to HIV-1/M or HIV-1/O mono-infections.
Our data reveal that the molecular epidemiology of HIV in Cameroon is becoming more complex; this is not surprising due to the wide genetic diversity of viruses circulating in this region, but the situation is maybe even more complex. Indeed, our work has several limitations that could underestimate the prevalence of M + O dual infections and recombinant forms. The sample collection, performed from 2006 to 2009 and not according to epidemiological methodology, may not be representative of the current situation and cannot define the prevalence or frequency of such forms. We only analyzed M + O dual seroreactivity, but reports of two cases with recombinants only [13, 14] and a third case here (YBF282) raise the question of the circulation of a single recombinant with a serotype profile of HIV-1/M or/O mono-infection.
We searched for vpr recombinants, because we considered this gene as a potential hot-spot of recombination; indeed, three of four MO recombinant forms previously reported exhibited a vpr breakpoint. This and our data show that this region could be essential for MO recombination, compared to intra-group HIV-1/M recombination. Among the HIV-1/M CRFs, reported in the Los Alamos Database, only 12 show a recombinant breakpoint in vpr. The only HIV-2 circulating recombinant form has no breakpoint in the vpr gene. But, we also found a vpr recombinant, because we focused on this gene; the description of two recombinant forms with a breakpoint outside vpr, in vpu here (as well as at the end of env) and in Integrase in a previous report [12], highlights the fact that some recombinants do not harbor such a breakpoint, and that the situation could be more complex as for HIV-1/M CRFs. Thus, we report here four cases of dual infection without vpr recombinant, even though they may have recombinant viruses with another breakpoint. There is a crucial need to sequence the whole genome of these partial forms to identify other breakpoints. But full-length sequencing of multiple distinct populations present in the samples is technically difficult, even by cloning; we need to optimize such tools or use next generation sequencing technology, more adapted to discriminating between numerous populations. Lastly, we analyzed RNA or DNA forms according to the material available and at a unique sampling point for each patient, but dual infections and recombination are dynamic processes, combining co-infection or superinfection, emergence and selection of recombinants, and potential disappearance of one or both parental strains. To better understand these dynamics and the emergence of more adapted forms, we need to analyze sequential samples, and both DNA and RNA to distinguish between archived and replicative forms.
In conclusion, our combined sero-molecular strategy allowed us to identify new inter-group HIV-1/MO recombinant forms, with characterization of two completely new genomes, associated or not with dual infections. This work highlights the dynamic co-evolution of these two groups of strains in Cameroon; a new study taking into account our limitations is in process to better estimate the prevalence and genetic pattern of the [MO]/[OM] recombinants.
Authors’ contributions
DR and JCP conceived and designed the study. AV and PAN performed enrolment and biological follow-up of patients. FDO and AV carried out experiments. FDO, TM, AV, EAG and JCP performed data analysis. FDO, TM, EAG and JCP drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank the technical staff of the virology laboratories of the two sites. We are also grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
All the nucleotide sequences resulting from this study have been submitted to the GenBank database with accession numbers KX398152 to KX398187.
Ethics approval and consent to participate
All data were obtained from routine diagnostic activity. No specific consent was obtained from patients.
Funding
We thank the Centre Pasteur in Cameroun and Rouen University Hospital for financial support.
Footnotes
Fabienne De Oliveira and Thomas Mourez contributed equally to this article
Contributor Information
Fabienne De Oliveira, Email: fabienne.de-oliveira@chu-rouen.fr.
Thomas Mourez, Email: thomas.mourez@chu-rouen.fr.
Aurélia Vessiere, Email: aurelia.vessiere@hotmail.fr.
Paul-Alain Ngoupo, Email: ngoupo@yahoo.fr.
Elodie Alessandri-Gradt, Email: elodie.alessandri@chu-rouen.fr.
François Simon, Email: francois.simon@aphp.fr.
Dominique Rousset, Email: dominique.rousset@pasteur.fr.
Jean-Christophe Plantier, Email: jean-christophe.plantier@univ-rouen.fr.
References
- 1.De Leys R, Vanderborght B, Vanden Haesevelde M, Heyndrickx L, van Geel A, Wauters C, Bernaerts R, Saman E, Nijs P, Willems B, et al. Isolation and partial characterization of an unusual human immunodeficiency retrovirus from two persons of west-central African origin. J Virol. 1990;64:1207–1216. doi: 10.1128/jvi.64.3.1207-1216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon F, Mauclere P, Roques P, Loussert-Ajaka I, Muller-Trutwin MC, Saragosti S, Georges-Courbot MC, Barre-Sinoussi F, Brun-Vezinet F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat Med. 1998;4:1032–1037. doi: 10.1038/2017. [DOI] [PubMed] [Google Scholar]
- 3.Plantier JC, Leoz M, Dickerson JE, De Oliveira F, Cordonnier F, Lemee V, Damond F, Robertson DL, Simon F. A new human immunodeficiency virus derived from gorillas. Nat Med. 2009;15:871–872. doi: 10.1038/nm.2016. [DOI] [PubMed] [Google Scholar]
- 4.Leoz M, Feyertag F, Charpentier C, Delaugerre C, Wirden M, Lemee V, Plantier JC. Characterization of CRF56_cpx, a new circulating B/CRF02/G recombinant form identified in MSM in France. AIDS. 2013;27:2309–2312. doi: 10.1097/QAD.0b013e3283632e0c. [DOI] [PubMed] [Google Scholar]
- 5.Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS. 2011;25:679–689. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roques P, Robertson DL, Souquiere S, Damond F, Ayouba A, Farfara I, Depienne C, Nerrienet E, Dormont D, Brun-Vezinet F, Simon F, Mauclere P. Phylogenetic analysis of 49 newly derived HIV-1 group O strains: high viral diversity but no group M-like subtype structure. Virology. 2002;302:259–273. doi: 10.1006/viro.2002.1430. [DOI] [PubMed] [Google Scholar]
- 7.Ibe S, Yokomaku Y, Shiino T, Tanaka R, Hattori J, Fujisaki S, Iwatani Y, Mamiya N, Utsumi M, Kato S, Hamaguchi M, Sugiura W. HIV-2 CRF01_AB: first circulating recombinant form of HIV-2. J Acquir Immune Defic Syndr. 2010;54:241–247. doi: 10.1097/QAI.0b013e3181dc98c1. [DOI] [PubMed] [Google Scholar]
- 8.Simon-Loriere E, Rossolillo P, Negroni M. RNA structures, genomic organization and selection of recombinant HIV. RNA Biol. 2011;8:280–286. doi: 10.4161/rna.8.2.15193. [DOI] [PubMed] [Google Scholar]
- 9.Vessiere A, Rousset D, Kfutwah A, Leoz M, Depatureaux A, Simon F, Plantier JC. Diagnosis and monitoring of HIV-1 group O-infected patients in Cameroun. J Acquir Immune Defic Syndr. 2010;53:107–110. doi: 10.1097/QAI.0b013e3181b97ec1. [DOI] [PubMed] [Google Scholar]
- 10.Peeters M, Liegeois F, Torimiro N, Bourgeois A, Mpoudi E, Vergne L, Saman E, Delaporte E, Saragosti S. Characterization of a highly replicative intergroup M/O human immunodeficiency virus type 1 recombinant isolated from a Cameroonian patient. J Virol. 1999;73:7368–7375. doi: 10.1128/jvi.73.9.7368-7375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takehisa J, Zekeng L, Ido E, Yamaguchi-Kabata Y, Mboudjeka I, Harada Y, Miura T, Kaptu L, Hayami M. Human immunodeficiency virus type 1 intergroup (M/O) recombination in cameroon. J Virol. 1999;73:6810–6820. doi: 10.1128/jvi.73.8.6810-6820.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi J, Bodelle P, Vallari AS, Coffey R, McArthur CP, Schochetman G, Devare SG, Brennan CA. HIV infections in northwestern Cameroon: identification of HIV type 1 group O and dual HIV type 1 group M and group O infections. AIDS Res Hum Retroviruses. 2004;20:944–957. doi: 10.1089/aid.2004.20.944. [DOI] [PubMed] [Google Scholar]
- 13.Vessiere A, Leoz M, Brodard V, Strady C, Lemee V, Depatureaux A, Simon F, Plantier JC. First evidence of a HIV-1 M/O recombinant form circulating outside Cameroon. AIDS. 2010;24:1079–1082. doi: 10.1097/QAD.0b013e3283355659. [DOI] [PubMed] [Google Scholar]
- 14.Ngoupo PA, Sadeuh-Mba SA, De Oliveira F, Ngono V, Ngono L, Tchendjou P, Penlap V, Mourez T, Njouom R, Kfutwah A, Plantier JC. First evidence of transmission of an HIV-1 M/O intergroup recombinant virus. AIDS. 2016;30:1–8. doi: 10.1097/QAD.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 15.Leoz M, Feyertag F, Kfutwah A, Mauclere P, Lachenal G, Damond F, De Oliveira F, Lemee V, Simon F, Robertson DL, Plantier JC. The two-phase emergence of non pandemic HIV-1 group O in Cameroon. PLOS Pathog. 2015;11:e1005029. doi: 10.1371/journal.ppat.1005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zouhair S, Roussin-Bretagne S, Moreau A, Brunet S, Laperche S, Maniez M, Barin F, Harzic M. Group O human immunodeficiency virus type 1 infection that escaped detection in two immmunoassays. J Clin Microbiol. 2006;44:662–665. doi: 10.1128/JCM.44.2.662-665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plantier JC, Djemai M, Lemee V, Reggiani A, Leoz M, Burc L, Vessiere A, Rousset D, Poveda JD, Henquell C, Gautheret-Dejean A, Barin F. Census and analysis of persistent false-negative results in serological diagnosis of human immunodeficiency virus type 1 group O infections. J Clin Microbiol. 2009;47:2906–2911. doi: 10.1128/JCM.00602-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghokeng AF, Mpoudi-Ngole E, Dimodi H, Atem-Tambe A, Tongo M, Butel C, Delaporte E, Peeters M. Inaccurate diagnosis of HIV-1 group M and O is a key challenge for ongoing universal access to antiretroviral treatment and HIV prevention in Cameroon. PLOS ONE. 2009;4:e7702. doi: 10.1371/journal.pone.0007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mourez T, Simon F, Plantier JC. Non-m variants of human immunodeficiency virus type 1. Clin Microbiol Rev. 2013;26:448–461. doi: 10.1128/CMR.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourez T, Delaugerre C, Vray M, Lemee V, Simon F, Plantier JC. Comparison of the bioMerieux NucliSENS EasyQ HIV-1 v2.0-HIV-1 RNA quantification assay versus Abbott RealTime HIV-1 and Roche Cobas TaqMan HIV-1 v2.0 on current epidemic HIV-1 variants. J Clin Virol. 2015;71:76–81. doi: 10.1016/j.jcv.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Rouet F, Liegeois F, Mouinga-Ondeme A, Kania D, Viljoen J, Wambua S, Ngo-Giang-Huong N, Menan H, Peeters M, Nerrienet E. Current challenges to viral load testing in the context of emerging genetic diversity of HIV-1. Expert Opin Med Diagn. 2011;5:183–202. doi: 10.1517/17530059.2011.566860. [DOI] [PubMed] [Google Scholar]
- 22.Depatureaux A, Charpentier C, Leoz M, Unal G, Damond F, Kfutwah A, Vessiere A, Simon F, Plantier JC. Impact of HIV-1 group O genetic diversity on genotypic resistance interpretation by algorithms designed for HIV-1 group M. J Acquir Immune Defic Syndr. 2011;56:139–145. doi: 10.1097/QAI.0b013e318201a904. [DOI] [PubMed] [Google Scholar]
- 23.Tuaillon E, Gueudin M, Lemee V, Gueit I, Roques P, Corrigan GE, Plantier JC, Simon F, Braun J. Phenotypic susceptibility to nonnucleoside inhibitors of virion-associated reverse transcriptase from different HIV types and groups. J Acquir Immune Defic Syndr. 2004;37:1543–1549. doi: 10.1097/00126334-200412150-00001. [DOI] [PubMed] [Google Scholar]
- 24.Tebit DM, Lobritz M, Lalonde M, Immonen T, Singh K, Sarafianos S, Herchenroder O, Krausslich HG, Arts EJ. Divergent evolution in reverse transcriptase (RT) of HIV-1 group O and M lineages: impact on structure, fitness, and sensitivity to nonnucleoside RT inhibitors. J Virol. 2010;84:9817–9830. doi: 10.1128/JVI.00991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Descamps D, Collin G, Letourneur F, Apetrei C, Damond F, Loussert-Ajaka I, Simon F, Saragosti S, Brun-Vezinet F. Susceptibility of human immunodeficiency virus type 1 group O isolates to antiretroviral agents: in vitro phenotypic and genotypic analyses. J Virol. 1997;71:8893–8898. doi: 10.1128/jvi.71.11.8893-8898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayouba A, Mauclere P, Martin PM, Cunin P, Mfoupouendoun J, Njinku B, Souquieres S, Simon F. HIV-1 group O infection in Cameroon, 1986 to 1998. Emerg Infect Dis. 2001;7:466–467. doi: 10.3201/eid0703.017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vergne L, Bourgeois A, Mpoudi-Ngole E, Mougnutou R, Mbuagbaw J, Liegeois F, Laurent C, Butel C, Zekeng L, Delaporte E, Peeters M. Biological and genetic characteristics of HIV infections in Cameroon reveals dual group M and O infections and a correlation between SI-inducing phenotype of the predominant CRF02_AG variant and disease stage. Virology. 2003;310:254–266. doi: 10.1016/S0042-6822(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 28.Kfutwah A, Lemee V, Ngono HV, De Oliveira F, Njouom R, Plantier JC. Field evaluation of the Abbott ARCHITECT HIV Ag/Ab Combo immunoassay. J Clin Virol. 2013;58(Suppl 1):e70–e75. doi: 10.1016/j.jcv.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Heyndrickx L, Janssens W, Gurtler L, Zekeng L, Loussert-Ajaka I, Vereecken K, Willems B, Coppens S, Ndumbe P, Fransen K, Saman E, Alary M, van der Groen G. Differential diagnosis of HIV type 1 group O and M infection by polymerase chain reaction and PstI restriction analysis of the pol gene fragment. AIDS Res Hum Retroviruses. 1998;14:973–977. doi: 10.1089/aid.1998.14.973. [DOI] [PubMed] [Google Scholar]
- 30.Dachraoui R, Brand D, Brunet S, Barin F, Plantier JC. RNA amplification of the HIV-1 Pol and env regions on dried serum and plasma spots. HIV Med. 2008;9:557–561. doi: 10.1111/j.1468-1293.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 31.Plantier JC, Dachraoui R, Lemee V, Gueudin M, Borsa-Lebas F, Caron F, Simon F. HIV-1 resistance genotyping on dried serum spots. AIDS. 2005;19:391–397. doi: 10.1097/01.aids.0000161768.98534.e7. [DOI] [PubMed] [Google Scholar]
- 32.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siepel AC, Halpern AL, Macken C, Korber BT. A computer program designed to screen rapidly for HIV type 1 intersubtype recombinant sequences. AIDS Res Hum Retroviruses. 1995;11:1413–1416. doi: 10.1089/aid.1995.11.1413. [DOI] [PubMed] [Google Scholar]
- 35.Simon F, Souquiere S, Damond F, Kfutwah A, Makuwa M, Leroy E, Rouquet P, Berthier JL, Rigoulet J, Lecu A, Telfer PT, Pandrea I, Plantier JC, Barre-Sinoussi F, Roques P, Muller-Trutwin MC, Apetrei C. Synthetic peptide strategy for the detection of and discrimination among highly divergent primate lentiviruses. AIDS Res Hum Retroviruses. 2001;17:937–952. doi: 10.1089/088922201750290050. [DOI] [PubMed] [Google Scholar]
- 36.Brennan CA, Bodelle P, Coffey R, Devare SG, Golden A, Hackett J, Jr, Harris B, Holzmayer V, Luk KC, Schochetman G, Swanson P, Yamaguchi J, Vallari A, Ndembi N, Ngansop C, Makamche F, Mbanya D, Gurtler LG, Zekeng L, Kaptue L. The prevalence of diverse HIV-1 strains was stable in Cameroonian blood donors from 1996 to 2004. J Acquir Immune Defic Syndr. 2008;49:432–439. doi: 10.1097/QAI.0b013e31818a6561. [DOI] [PubMed] [Google Scholar]
- 37.Laperche S, Leballais L, Ly TD, Plantier JC. Failures in the detection of HIV p24 antigen with the determine HIV-1/2 Ag/Ab Combo rapid test. J Infect Dis. 2012;206:1946–1947. doi: 10.1093/infdis/jis616. [DOI] [PubMed] [Google Scholar]
- 38.Plantier JC, Lemee V, Dorval I, Gueudin M, Braun J, Hutin P, Ruffault A, Simon F. HIV-1 group M superinfection in an HIV-1 group O-infected patient. AIDS. 2004;18:2444–2446. [PubMed] [Google Scholar]


