Abstract
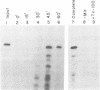
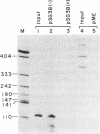
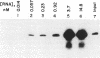
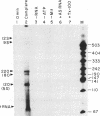
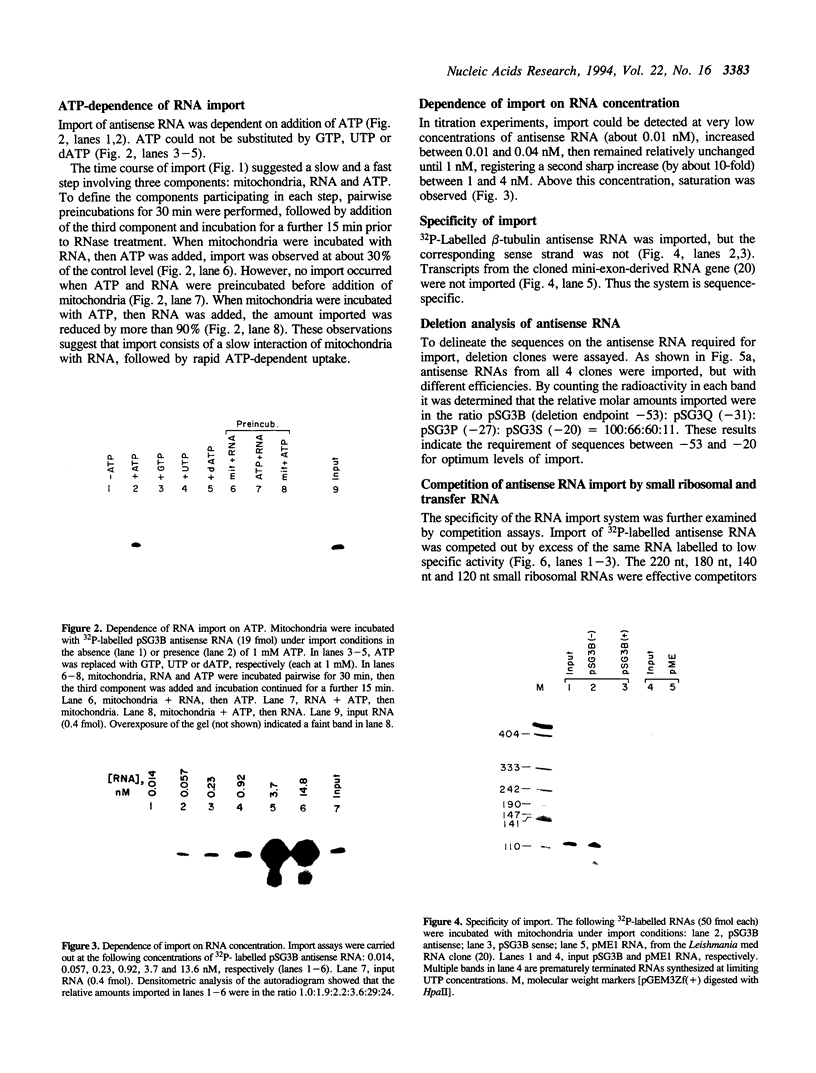
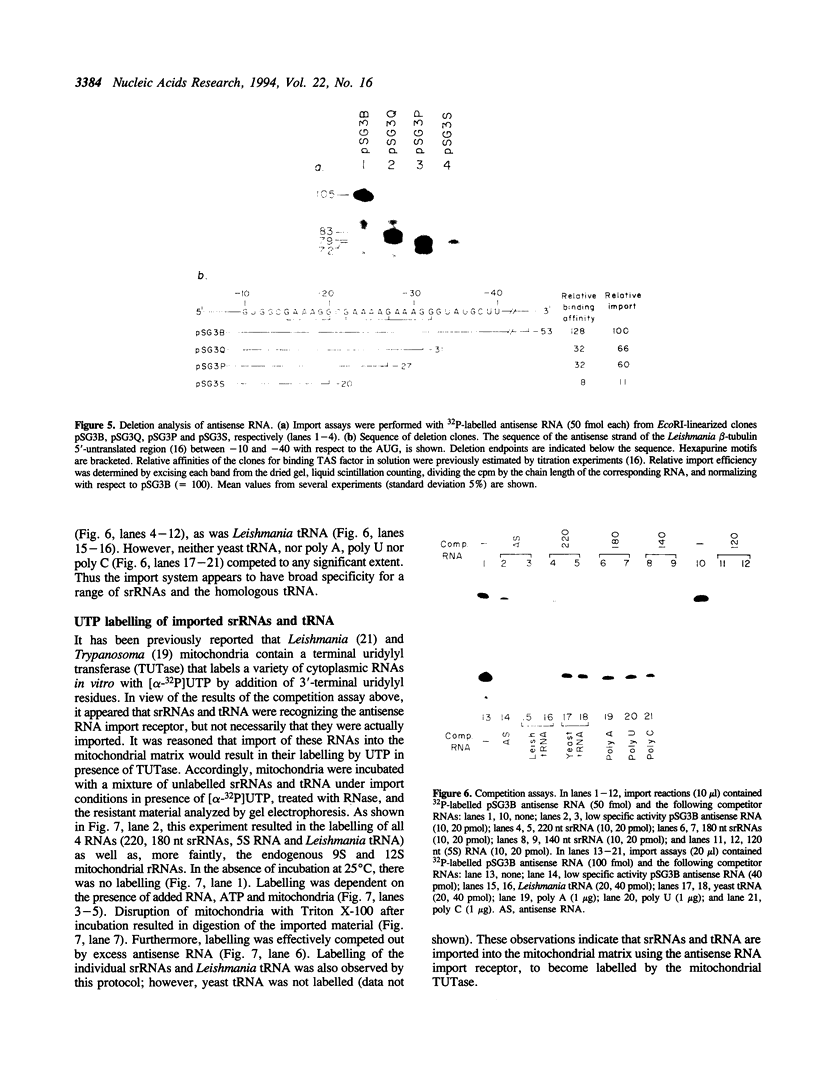
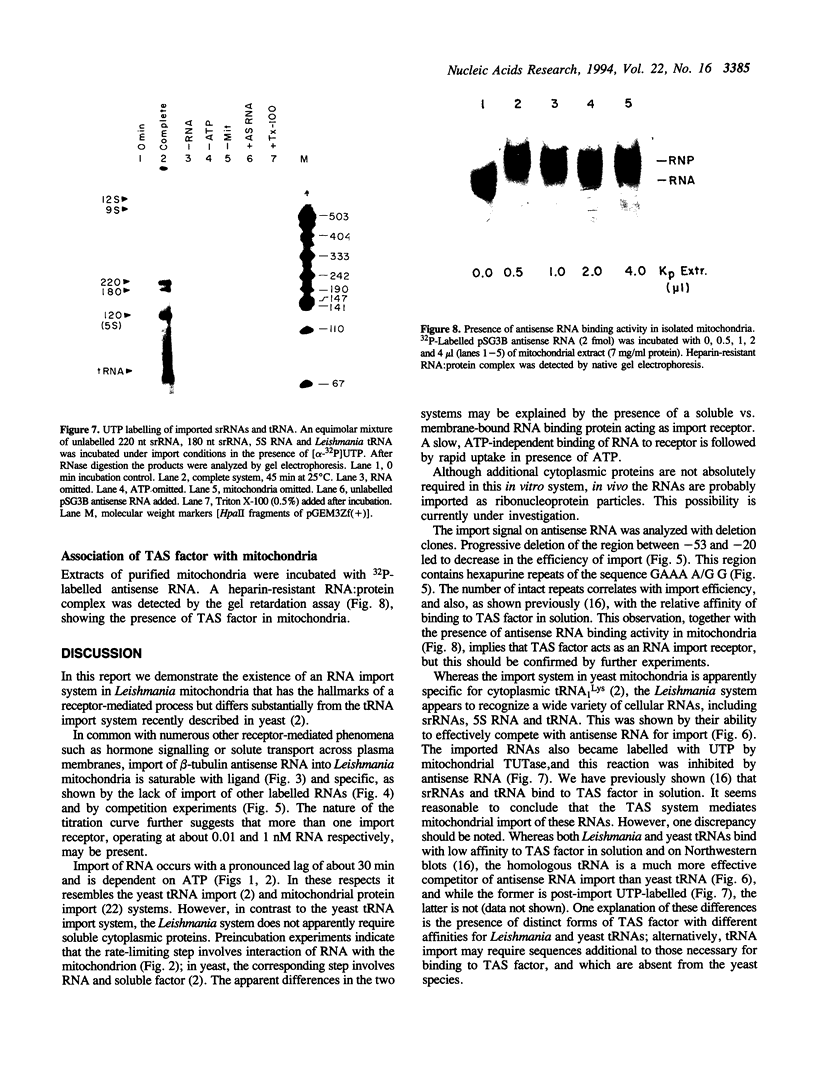
Using an in vitro ribonuclease protection assay, it was shown that synthetic antisense transcripts from the 5'-upstream region of the beta-tubulin gene are efficiently imported into isolated Leishmania mitochondria. Import occurred after a lag of about 30 min at 25 degrees C and was dependent on ATP. Preincubation experiments suggested that import consists of a slow interaction of mitochondria with RNA, followed by rapid ATP-dependent uptake. Import was saturable with antisense RNA at about 1 nM concentration, and sequence-specific, as shown by lack of import of other labelled transcripts. Deletion analysis demonstrated a correlation between efficiency of import and the number of oligopurine motifs on the antisense RNA. Several small ribosomal RNAs (srRNAs) and Leishmania tRNA competed with antisense RNA for import. Incubation of mitochondria with srRNAs and tRNA in the presence of radiolabelled UTP resulted in the ribonuclease-resistant labelling of these RNAs by the mitochondrial terminal uridylyl transferase. Extracts of isolated mitochondria contain a factor binding to antisense RNA, as shown by gel retardation assay. These observations indicate the presence of a receptor-mediated import pathway for srRNAs and tRNA in Leishmania mitochondria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakalara N., Simpson A. M., Simpson L. The Leishmania kinetoplast-mitochondrion contains terminal uridylyltransferase and RNA ligase activities. J Biol Chem. 1989 Nov 5;264(31):18679–18686. [PubMed] [Google Scholar]
- Cordingley J. S., Turner M. J. 6.5 S RNA; preliminary characterisation of unusual small RNAs in Trypanosoma brucei. Mol Biochem Parasitol. 1980 Apr;1(2):91–96. doi: 10.1016/0166-6851(80)90003-1. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Ghosh A., Ghosh T., Ghosh S., Das S., Adhya S. Interaction of small ribosomal and transfer RNAs with a protein from Leishmania donovani. Nucleic Acids Res. 1994 May 11;22(9):1663–1669. doi: 10.1093/nar/22.9.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B., Schatz G. Import of proteins into mitochondria. Annu Rev Genet. 1991;25:21–44. doi: 10.1146/annurev.ge.25.120191.000321. [DOI] [PubMed] [Google Scholar]
- Hancock K., Hajduk S. L. The mitochondrial tRNAs of Trypanosoma brucei are nuclear encoded. J Biol Chem. 1990 Nov 5;265(31):19208–19215. [PubMed] [Google Scholar]
- Harris M. E., Moore D. R., Hajduk S. L. Addition of uridines to edited RNAs in trypanosome mitochondria occurs independently of transcription. J Biol Chem. 1990 Jul 5;265(19):11368–11376. [PubMed] [Google Scholar]
- Lenardo M. J., Dorfman D. M., Reddy L. V., Donelson J. E. Characterization of the Trypanosoma brucei 5S ribosomal RNA gene and transcript: the 5S rRNA is a spliced-leader-independent species. Gene. 1985;35(1-2):131–141. doi: 10.1016/0378-1119(85)90165-9. [DOI] [PubMed] [Google Scholar]
- Lye L. F., Chen D. H., Suyama Y. Selective import of nuclear-encoded tRNAs into mitochondria of the protozoan Leishmania tarentolae. Mol Biochem Parasitol. 1993 Apr;58(2):233–245. doi: 10.1016/0166-6851(93)90045-y. [DOI] [PubMed] [Google Scholar]
- Mottram J. C., Bell S. D., Nelson R. G., Barry J. D. tRNAs of Trypanosoma brucei. Unusual gene organization and mitochondrial importation. J Biol Chem. 1991 Sep 25;266(27):18313–18317. [PubMed] [Google Scholar]
- Myler P. J., Glick D., Feagin J. E., Morales T. H., Stuart K. D. Structural organization of the maxicircle variable region of Trypanosoma brucei: identification of potential replication origins and topoisomerase II binding sites. Nucleic Acids Res. 1993 Feb 11;21(3):687–694. doi: 10.1093/nar/21.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D., Darlix J. L. Composite structure of the chloroplast 23 S ribosomal RNA genes of Chlamydomonas reinhardii. Evolutionary and functional implications. J Mol Biol. 1982 Aug 15;159(3):383–395. doi: 10.1016/0022-2836(82)90290-x. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Heinonen T. Y., Young P. G., Gray M. W. A discontinuous small subunit ribosomal RNA in Tetrahymena pyriformis mitochondria. J Biol Chem. 1986 Apr 15;261(11):5187–5193. [PubMed] [Google Scholar]
- Schnare M. N., Spencer D. F., Gray M. W. Primary structures of four novel small ribosomal RNAs from Crithidia fasciculata. Can J Biochem Cell Biol. 1983 Jan;61(1):38–45. doi: 10.1139/o83-006. [DOI] [PubMed] [Google Scholar]
- Schneider A., Martin J., Agabian N. A nuclear encoded tRNA of Trypanosoma brucei is imported into mitochondria. Mol Cell Biol. 1994 Apr;14(4):2317–2322. doi: 10.1128/mcb.14.4.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilhamer J. J., Gutell R. R., Cummings D. J. Paramecium mitochondrial genes. II. Large subunit rRNA gene sequence and microevolution. J Biol Chem. 1984 Apr 25;259(8):5173–5181. [PubMed] [Google Scholar]
- Simpson A. M., Suyama Y., Dewes H., Campbell D. A., Simpson L. Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucleic Acids Res. 1989 Jul 25;17(14):5427–5445. doi: 10.1093/nar/17.14.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Sloof P., Van den Burg J., Voogd A., Benne R., Agostinelli M., Borst P., Gutell R., Noller H. Further characterization of the extremely small mitochondrial ribosomal RNAs from trypanosomes: a detailed comparison of the 9S and 12S RNAs from Crithidia fasciculata and Trypanosoma brucei with rRNAs from other organisms. Nucleic Acids Res. 1985 Jun 11;13(11):4171–4190. doi: 10.1093/nar/13.11.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloof P., de Haan A., Eier W., van Iersel M., Boel E., van Steeg H., Benne R. The nucleotide sequence of the variable region in Trypanosoma brucei completes the sequence analysis of the maxicircle component of mitochondrial kinetoplast DNA. Mol Biochem Parasitol. 1992 Dec;56(2):289–299. doi: 10.1016/0166-6851(92)90178-m. [DOI] [PubMed] [Google Scholar]
- Small I., Maréchal-Drouard L., Masson J., Pelletier G., Cosset A., Weil J. H., Dietrich A. In vivo import of a normal or mutagenized heterologous transfer RNA into the mitochondria of transgenic plants: towards novel ways of influencing mitochondrial gene expression? EMBO J. 1992 Apr;11(4):1291–1296. doi: 10.1002/j.1460-2075.1992.tb05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K. RNA editing in mitochondrial mRNA of trypanosomatids. Trends Biochem Sci. 1991 Feb;16(2):68–72. doi: 10.1016/0968-0004(91)90027-s. [DOI] [PubMed] [Google Scholar]
- Tarassov I. A., Entelis N. S. Mitochondrially-imported cytoplasmic tRNA(Lys)(CUU) of Saccharomyces cerevisiae: in vivo and in vitro targetting systems. Nucleic Acids Res. 1992 Mar 25;20(6):1277–1281. doi: 10.1093/nar/20.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz V. F., Lake J. A., Simpson A. M., Simpson L. A minimal ribosomal RNA: sequence and secondary structure of the 9S kinetoplast ribosomal RNA from Leishmania tarentolae. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1401–1405. doi: 10.1073/pnas.82.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz V. F., Simpson A. M., Lake J. A., Simpson L. Primary sequence and partial secondary structure of the 12S kinetoplast (mitochondrial) ribosomal RNA from Leishmania tarentolae: conservation of peptidyl-transferase structural elements. Nucleic Acids Res. 1985 Apr 11;13(7):2337–2356. doi: 10.1093/nar/13.7.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]