Abstract
The Cystic Fibrosis Transmembrane Conductance Regulator (CFTR, ABCC7) is a plasma membrane chloride ion channel in the ABC transporter superfamily. CFTR is a key target for cystic fibrosis drug development, and its structural elucidation would advance those efforts. However, the limited in vivo and in vitro stability of the protein, particularly its nucleotide binding domains, has made structural studies challenging. Here we demonstrate that phosphatidylserine uniquely stimulates and thermally stabilizes the ATP hydrolysis function of purified human CFTR. Among several lipids tested, the greatest stabilization was observed with brain phosphatidylserine, which shifted the Tm for ATPase activity from 22.7 ± 0.8 °C to 35.0 ± 0.2 °C in wild-type CFTR, and from 26.6 ± 0.7 °C to 42.1 ± 0.2 °C in a more stable mutant CFTR having deleted regulatory insertion and S492P/A534P/I539T mutations. When ATPase activity was measured at 37 °C in the presence of brain phosphatidylserine, Vmax for wild-type CFTR was 240 ± 60 nmol/min/mg, a rate higher than previously reported and consistent with rates for other purified ABC transporters. The significant thermal stabilization of CFTR by phosphatidylserine may be advantageous in future structural and biophysical studies of CFTR.
Keywords: Cystic Fibrosis Transmembrane Conductance Regulator, phosphatidylserine, ATP hydrolysis, thermal stability, blind docking, ABC transporters
Graphical abstract
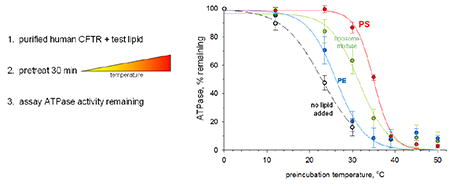
1 Introduction
Cystic fibrosis is a grave genetic disease caused by mutations in the Cystic Fibrosis Transmembrane Conductance Regulator, an ABC transporter that functions as a chloride ion channel [1]. CFTR is gated by ATP binding and hydrolysis, and this gating is regulated by phosphorylation [1-3]. Disease-causing CFTR mutations are numerous, with the most prevalent mutations leading to CFTR misfolding and degradation [4, 5]. CFTR-targeted drug development is being pursued aggressively, with the notable success of ivacaftor for patients with gating mutations [6]. On the other hand, the first drug (Orkambi) approved for patients with the misfolded ΔF508 form of CFTR has delivered only moderate improvement [7, 8], and patients with many other types of mutations still await new therapeutic strategies. Rational drug design for cystic fibrosis would be greatly advanced by an understanding of CFTR gating and regulatory mechanisms, interaction with drugs, and the impacts of clinical mutations. Solving the three-dimensional structure of CFTR would expedite these efforts.
Protein quality is key to solving structure at high resolution, and often hinges on protein stability [9-11]. A recent investigation of the temperature sensitivity of CFTR single channel conductance showed that even in the context of cellular membranes the wild-type protein ceases to gate above 40 °C [12]. CFTR becomes even more unstable upon solubilization, with the purified protein exhibiting both detergent sensitivity and thermal instability [13, 14], properties that have impeded progress toward its structural determination. Some improvement in thermal stability of CFTR has been achieved by introducing strategic mutations [12, 15]. The addition of specific lipids represents a standard approach to enhancing stability of membrane proteins for structural biology [9]. Yet in the case of CFTR, there remains a dearth of information regarding its interaction with specific phospholipids. A single earlier study reported quenching of tryptophan fluorescence by phospholipids at a site in NBD1, and loss of lipid head group selectivity in the clinically important, misfolded ΔF508 form of CFTR [16]. Here we utilize the readily quantifiable ATPase function of purified human CFTR to demonstrate its specific and significant thermal stabilization by phosphatidylserine.
2 Methods
We previously described the cell line D165 for expression of human CFTR modified with His10-SUMO* and 901Flag affinity purification tags and C-terminally fused green fluorescent protein (EGFP) [17]. The recombinant CFTR protein, with a molecular mass of 212 kDa, is referred to herein as wild-type. We generated an equivalent CFTR construct with stabilizing mutations ΔRI/2PT [12] as follows. CFTR DNA sequence with 901FLAG epitope, with RI residues 404-435 deleted, and with NBD1 mutations S492P A534P and I539T (2PT), was ligated in-frame into the previously described lentiviral vector between SUMO* and EGFP sequences [17], to give the His10-SUMO*-ΔRI/2PT-CFTRFLAG-EGFP open reading frame under transcriptional control of the tetracycline response element. The vector was packaged, pseudotyped with vesicular stomatitis virus G protein, and used to transduce CHO-S cells (Invitrogen) that constitutively express the reverse Tet transactivator [18, 19]. The suspension culture-adapted transduced cell line is designated D727. To produce ΔRI/2PT CFTR, D727 cells were treated with 1 μg/ml of doxycycline in CD4CHO medium (Thermo Fisher), and harvested 2 days after induction.
Recombinant wild-type or mutant CFTR was phosphorylated with protein kinase A catalytic subunit and purified to homogeneity in the presence of 0.05% decyl maltose neopentyl glycol as described [13]. Purified protein concentrations were quantitated by densitometry after Coomassie Blue G250 staining [20, 21], with external standardization using aldolase (GE Life Sciences) which was calibrated spectrophotometrically.
Hydrolysis of 0.3 mM α-[32P]-ATP was measured in incubations containing 1.5 mM MgCl2, pH 7.5, at 33°C for 2 h as described [13]. Background measured with reagent blanks containing all components except CFTR was subtracted. Vmax values were determined at pH 7.5 by varying ATP concentration (0.3-3.0 mM, with 3.6 mM MgCl2) in 1.5 h incubations at 37 °C. The Michaelis-Menten equation was fit to the data using Excel with the Solver add-in.
Lipids (Avanti Polar Lipids) were peroxide tested [22] and stored at -80 °C. Sonicated liposomes (POPE/brain PS/egg PC/cholesterol, weight ratio 5:3:1:1, or as otherwise specified) were mixed 4:1 (w/w) with C12E8. Titrations with monitoring of turbidity (data not shown) demonstrated this ratio to be near Rsat, the point at which the bilayer is maximally perturbed [23] and favorable for membrane protein reconstitution [24]. We previously showed that this destabilization protocol enhanced lipid stimulation of ATP hydrolysis by CFTR [13]. A C12E8:lipid ratio of 1:4 (w/w) was used for all lipid preparations, giving stable emulsions in every case.
To determine lipid effects on functional stability, aliquots of CFTR (9 μl) were premixed on ice with the indicated lipid (6 μg in 2 μl). After 30 min at varying temperatures and return to ice, POPS (6 μg in 2 μl) was added, unless PS was already present. Substrate α-[32P]-ATP (2 μl) was then added for measurement of ATPase activity as described above. Results for enzymatic activity remaining vs. pretreatment temperature were fit to three parameter sigmoidal equations in SigmaPlot. The derived inflection points, described here as functional Tm, were compared using Student’s two-tailed t-test.
Methods for blind docking simulations are presented in Supplemental Data.
3 Results
We previously showed that ATP-hydrolysis by detergent-solubilized and purified CFTR requires addition of lipid [13]. In that study we used a PE/PS/PC/cholesterol mixture that is typical for studies of reconstituted CFTR [12, 25, 26]. Here we examine the specificity of CFTR’s lipid requirement. We performed preliminary molecular docking studies, which suggested preferential interaction with PS, especially with the NBDs (Table S1 in Supplemental Data). To test this prediction experimentally, we compared ATPase activities after titrating in the standard lipid mixture vs. a variety of other lipids prior to substrate addition (Figure 1). The standard PE/PS/PC/cholesterol liposome formulation (5:3:1:1) stimulated maximally at 0.2-0.5 mg/ml (Figure 1A), and omission of PS from the mixture greatly reduced the extent of stimulation. Brain polar extract containing 18% PS and other phospholipids stimulated activity to a similar extent as the defined liposome mixture, though it was somewhat less potent, requiring 1 mg/ml (Figure 1A). For synthetic lipids (Figure 1B), maximum stimulation was attained at 0.2 mg/ml lipid (0.24-0.28 mM), close to equimolar with the amount of detergent present (0.3 mM). The comparison of different head groups indicated a clear preference for PS over other lipids (Figure 1B), a result compatible with predictions of the docking analysis (Table S1). Stimulation by PS was acyl chain-dependent: brain PS containing predominantly C18 chains produced higher ATPase activity than POPS, while soluble short-chain forms (diC8- or diC10-PS) were completely ineffective (Figure 1C). PS is an anionic lipid, so we compared two other anionic species, PG and PI (Figure 1C). Both reproducibly demonstrated a spike of stimulation at low concentration, but reduced activity at higher concentration, suggesting a combination of stimulatory and inhibitory actions. Indeed, mixing experiments showed that even in the presence of 0.4 mg/ml PS, the addition of 0.4 mg/ml PG or PI inhibited ATPase activity by 100% and 63%, respectively, whereas neither PE nor PC inhibited (data not shown). Docking analysis had indeed suggested similar binding sites for POPS and POPG (Figure S2), but would not distinguish between stimulatory sites of interaction and inhibitory ones. In sum, the lipid addition experiments demonstrated that robust CFTR ATPase activity required the presence of long chain PS.
Figure 1. CFTR requires phosphatidylserine for maximum ATPase activity.

Aliquots of wild-type CFTR were preincubated on ice with the indicated lipids for 15 min prior to substrate addition, which was 1/5th of the final assay volume. Extent of ATP hydrolysis was then determined. Final lipid concentrations in assay are given. Representative titration data are shown, and have been duplicated for each lipid with similar results. The data are displayed in three panels for clarity.
Brain polar lipid extract contained 33.1% PE, 18.5% PS, 12.6% PC, 4.1% PI, 0.8% phosphatidic acid, and 30.9% unknown, w/w.
Precedent in the CFTR literature is for ATP hydrolysis to be assayed at 33 °C or higher [25, 27], and for the above comparisons ATPase was assayed at 33 °C. Further investigation demonstrated that CFTR, in the absence of lipid or nucleotide, was unstable at room temperature, losing approximately half its ATP hydrolysis activity over a 30 minute period (data not shown). This suggested the possibility that lipid supported ATPase activity of purified CFTR by stabilizing the protein. To test this hypothesis, we performed 30 min preincubations of CFTR/lipid mixtures at varying temperatures, then measured ATPase activity remaining. As illustrated by the examples in Figure 2, the resulting curves were sigmoidal, and curve fitting allowed determination of an inflection temperature that we will refer to as the functional Tm. Functional Tm was shifted to higher temperature in the presence of lipids, and the magnitude of the shift depended strongly on lipid composition, with PS producing the largest shift. These results indicated that CFTR would be significantly more stable at assay temperature in the presence of certain lipids, likely accounting for the apparent stimulation of ATP hydrolysis seen in Figure 1.
Figure 2. Phospholipids differ in protecting CFTR ATPase from thermal inactivation.
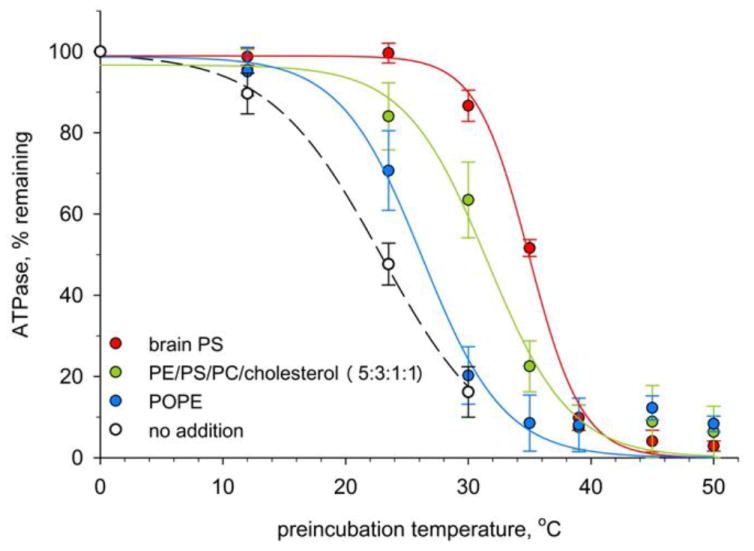
Aliquots of wild-type CFTR were mixed with 0.4 mg/ml of the indicated lipid, then treated at varying temperatures for 30 min. The remaining ATPase activity was then assayed in the presence of POPS, as detailed in section 2, Methods. Data points represent mean ± standard deviation for combined results of three experiments. Inflection points of fitted sigmoidal curves were taken as functional Tm values.
Compiled results of independent functional Tm determinations for various lipid additions are presented in Table 1. PE addition produced a modest 3 °C shift of Tm to higher temperature. Similarly, liposomes containing PE but not PS (i.e. 5:0:1:1) shifted the Tm by just 2 °C . In contrast, addition of POPS or brain PS produced much larger 10 and 12 °C stabilizations of CFTR. Liposomes of the conventional 5:3:1:1 formula (i.e. 30% PS) were also strongly stabilizing, and were significantly more effective than the formula without PS (P< 0.001). For comparison, we chose the open channel blocker glibenclamide whose CFTR affinity is known (Kd = 13 μM) [28]. Glibenclamide is one of relatively few available reagents whose CFTR binding site has been well characterized; this site lies within the ion channel pore in the inner vestibule [28]. In our assays, glibenclamide partially inhibited ATP hydrolysis (by 40%, not shown), but had no effect on CFTR thermal stability (Table 1).
Table 1.
Phosphatidylserine improves thermal stability of wild-type CFTR ATPase
| functional Tm, °C | Tm shift, °C | P valuea | |
|---|---|---|---|
| no addition | 22.7 ± 0.8 (7) | ||
| PE/PS/PC/cholesterol 5:3:1:1 | 31.5 ± 1.1 (3) | ±8.8 | <0.0001 |
| PE/PS/PC/cholesterol 5:0:1:1 | 24.9 ± 0.6 (3) | ±2.2 | 0.003 |
| cholesterol | 23.0 ± 0.7 (3) | ns | |
| liver PI | 22.7 ± 1.4 (3) | ns | |
| POPE | 26.0 ± 1.1 (3) | ±3.3 | 0.0006 |
| POPC | 23.9 ± 0.6 (3) | ns | |
| POPS | 32.6 ± 0.9 (4) | ±9.9 | <0.0001 |
| porcine brain PS | 35.0 ± 0.2 (3) | ±12.3 | <0.0001 |
| porcine brain sphingomyelin | 21.9 ± 1.6 (3) | ns | |
| 0.3 mM glibenclamide | 22.6 ± 0.9 (3) | ns | |
| 1.5% dimethylsulfoxideb | 22.7 ± 0.4 (3) | ns |
Functional Tms were derived from thermal inactivation data as illustrated in Figure 2. Data represent mean ± standard deviation, with number of replicate experiments given in parentheses.
two-tailed Student’s t-test for difference from the no lipid control.
ns: not statistically significant (P >0.05)
vehicle control for glibenclamide
Certain mutations have been shown to thermally stabilize CFTR; these include deletion of the regulatory insertion (ΔRI) and/or the NBD1 mutations S492P/A534P/I539T (2PT) [12]. We introduced both ΔRI and 2PT mutations into the CFTR construct used in the above studies, and determined that the functional Tm for ATPase inactivation was shifted 3.9 °C to 26.6 ± 0.7 °C. Addition of brain PS significantly stabilized the more stable ΔRI/2PT mutant protein, giving a functional Tm of 42.1 ± 0.2 °C (figure 3).
Figure 3. Phosphatidylserine shifts the functional Tm of a stabilized CFTR mutant at least as much as for wild-type CFTR.
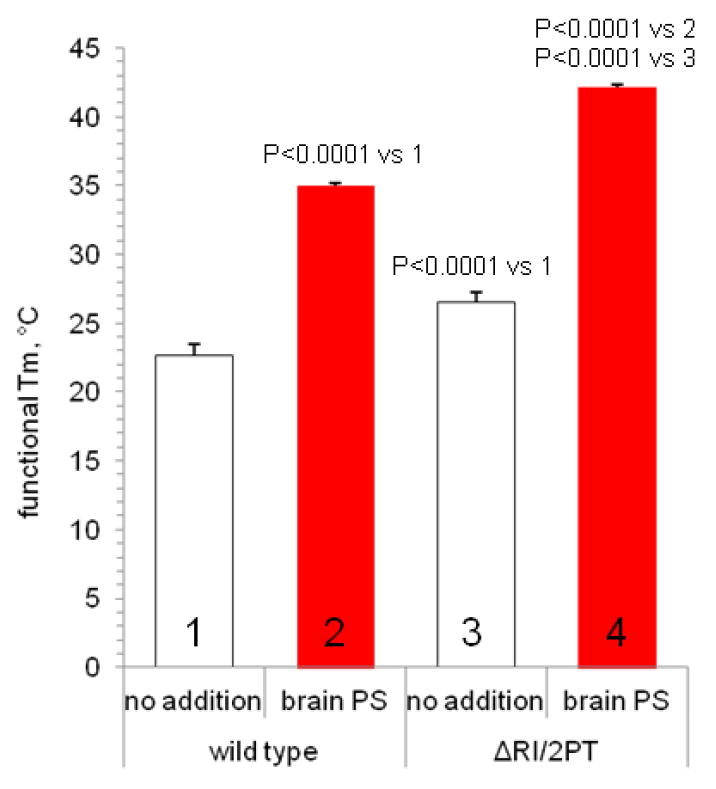
Functional Tms were determined in the presence or absence of 0.4 mg/ml brain PS. Data from replicate determinations are represented as mean ± standard deviation (n=7 for condition 1, n=3 for other conditions).
In the presence of the most stabilizing lipid, brain PS (0.4 mg/ml), we found the optimum assay temperature of wild-type CFTR to be 37 °C for a 1.5 h incubation at 0.3 mM ATP (data not shown). Using these same assay conditions, we then varied [ATP] to determine the Vmax for purified, PKA-phosphorylated human CFTR (Figure 4). For three independently purified CFTR preparations, the Vmax value was 240 ± 60 nmol/min/mg, a rate several fold greater than previous values of 30 nmol/min/mg reported by our own laboratory [13] or 13-77 nmol/min/mg by others [26, 27, 29, 30].
Figure 4. Maximum rate for CFTR ATPase at physiological temperature.
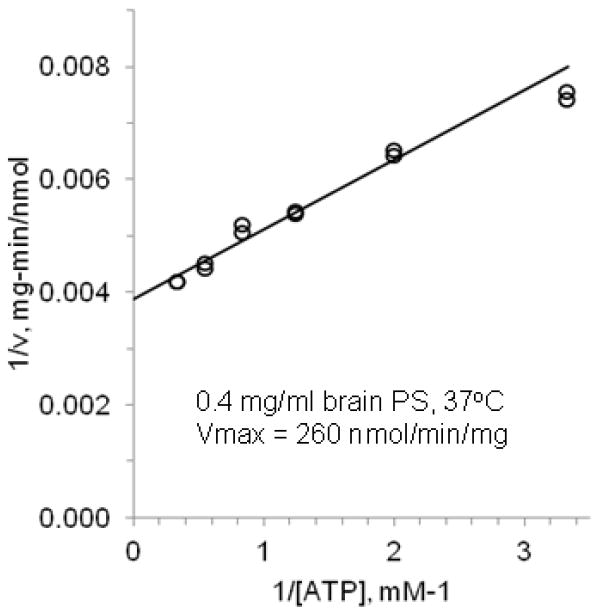
Double reciprocal plot showing duplicate data points in one representative assay, and the fitted Michaelis-Menten curve. The experiment was replicated for three independently purified wild-type CFTR preparations, giving similar Vmax values.
4 Discussion
Although purified CFTR in detergent solution loses activity rapidly at just 23 °C, we have shown that the transition can be shifted to higher temperature by addition of select phospholipids. Docking simulations successfully predicted preferential interaction of CFTR with PS over PG or PC. Experimentally, PS produced by far the largest stabilizing effect among the lipids tested (Table 1), and brain PS shifted the functional Tm to 35 °C. To determine functional Tms, CFTR was heated in the absence of ATP, but the optimum assay temperature with brain PS (determined in the presence of substrate ATP) was 37 °C. Observation of somewhat greater CFTR stability in the presence of ATP than in its absence is not surprising, because differential scanning calorimetry studies have firmly established nucleotide stabilization of CFTR isolated NBDs [31]. Tm shifts achieved by addition of PS were larger than any stabilizations yet achieved through selective mutation of CFTR NBD1 [15, 32, 33] or the full length protein [12]. Further, we demonstrated proof of principle for combining thermal stabilization of CFTR by PS with stabilizing effects of introduced mutations (Figure 3). It is therefore anticipated that thermal stabilization of CFTR through judicious combination of mutations, nucleotide, and PS will help to advance efforts to solve the structure of this difficult but medically important membrane protein.
A number of membrane proteins exhibit specifically bound lipid playing structural and physiological roles [34-37]. Indeed, a growing body of literature implicates PS or other anionic phospholipids in modulating the function of ion channels [38, 39]. Because the subcellular distribution of anionic phospholipid species is tightly controlled, such regulatory lipid interactions may provide a mechanism for differentially regulating ion channel function in different organellar compartments [40]. While PS is low in endoplasmic reticulum and Golgi compartments, it is particularly enriched in the cytoplasmic leaflet of plasma membrane and early endosomes [41], where it would be available to CFTR upon its maturation and delivery to the cell surface. We cannot predict on the basis of our in vitro study whether PS plays a chaperone-like role in CFTR processing in the living cell. A very recent report showed that ATP8B1 depletion in human lung or intestinal cell lines led to failure of fully glycosylated CFTR to traffic to the apical cell surface [42]. ATP8B1 is a P4 ATPase-type lipid flippase that maintains the asymmetric distribution of PS between leaflets of the cell membrane, and is thought to play a role in vesicular transport [43]. Thus it is difficult to know whether the influence of the flippase on CFTR processing should be ascribed to a perturbation in phospholipid distribution or to collapse of vesicular transport.
Previous studies of purified CFTR have likely underestimated ATPase activity due to suboptimal assay conditions. Using brain PS to maximally stabilize CFTR, we could assay activity at physiological temperature, and obtained a Vmax for the wild-type protein of 240 ± 60 nmol/min/mg (turnover rate 0.9 ± 0.2 sec-1). This rate is in better agreement with Vmax values measured for ATP hydrolysis by other solubilized and purified ABC transporters, e.g. 170, 287, 329, and 460 nmol/min/mg for ABCC3, ABCG5/G8, MsbA, and ABCC1, respectively [44-47]. We conclude that CFTR should no longer be regarded as a slow ATPase in comparison to other members of the ABC transporter family.
Supplementary Material
Highlights.
Phosphatidylserine specifically stabilizes ATPase activity of purified human CFTR
Select mutations together with phosphatidylserine further enhance thermal stability
With PS, turnover for ATP hydrolysis by CFTR is comparable to other ABC transporters
Acknowledgments
We thank Michael Blanton for guidance at the outset of the project, and Qinghai Zhang for his helpful critique of the manuscript. DNA comprising ΔRI/2PT CFTR was generously provided by John R. Riordan. This work was supported by Cystic Fibrosis Foundation Therapeutics grants URBATS13XX0, SENDER13XX0 and DELUCA03G0, by the National Institutes of Health including R01GM095639, by the Virology, Genetic Sequencing and Flow Cytometry Cores of the UAB Center for AIDS Research (P30 AI27767), and by The CH Foundation.
abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- EGFP
enhanced green fluorescent protein
- NBD
nucleotide binding domain
- PI
phosphatidylinositol
- POPC
1-palmitoyl-2-oleoyl-phosphatidylcholine
- POPE
1-palmitoyl-2-oleoyl-phosphatidylethanolamine
- POPG
1-palmitoyl-2-oleoyl-phosphatidylglycerol
- POPS
1-palmitoyl-2-oleoyl-phosphatidylserine
- RI
regulatory insertion
- 2PT
S492P/A534P/I539T
Footnotes
The authors declare that they have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 2.Hunt JF, Wang C, Ford RC. Cystic fibrosis transmembrane conductance regulator (ABCC7) structure. Cold Spring Harb Perspect Med. 2013;3:a009514. doi: 10.1101/cshperspect.a009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang TC, Kirk KL. The CFTR ion channel: gating, regulation, and anion permeation. Cold Spring Harb Perspect Med. 2013;3:a009498. doi: 10.1101/cshperspect.a009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lommatzsch ST, Aris R. Genetics of cystic fibrosis. Semin Respir Crit Care Med. 2009;30:531–538. doi: 10.1055/s-0029-1238911. [DOI] [PubMed] [Google Scholar]
- 5.Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, Skach WR, Cutting GR, Frizzell RA, Sheppard DN, Cyr DM, Sorscher EJ, Brodsky JL, Lukacs GL. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell. 2016;27:424–433. doi: 10.1091/mbc.E14-04-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quon BS, Rowe SM. New and emerging targeted therapies for cystic fibrosis. BMJ. 2016;352:i859. doi: 10.1136/bmj.i859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholon DM, Esther CR, Jr, Gentzsch M. Efficacy of lumacaftor-ivacaftor for the treatment of cystic fibrosis patients homozygous for the F508del-CFTR mutation. Expert Rev Precis Med Drug Dev. 2016;1:235–243. doi: 10.1080/23808993.2016.1175299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP T.S. Group, T.S. Group. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loll PJ. Membrane proteins, detergents and crystals: what is the state of the art? Acta Crystallogr F Struct Biol Commun. 2014;70:1576–1583. doi: 10.1107/S2053230X14025035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bill RM, Henderson PJ, Iwata S, Kunji ER, Michel H, Neutze R, Newstead S, Poolman B, Tate CG, Vogel H. Overcoming barriers to membrane protein structure determination. Nat Biotechnol. 2011;29:335–340. doi: 10.1038/nbt.1833. [DOI] [PubMed] [Google Scholar]
- 11.Kang HJ, Lee C, Drew D. Breaking the barriers in membrane protein crystallography. Int J Biochem Cell Biol. 2013;45:636–644. doi: 10.1016/j.biocel.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Aleksandrov LA, Jensen TJ, Cui L, Kousouros JN, He L, Aleksandrov AA, Riordan JR. Thermal stability of purified and reconstituted CFTR in a locked open channel conformation. Protein Expr Purif. 2015;116:159–166. doi: 10.1016/j.pep.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildebrandt E, Zhang Q, Cant N, Ding H, Dai Q, Peng L, Fu Y, DeLucas LJ, Ford R, Kappes JC, Urbatsch IL. A survey of detergents for the purification of stable, active human cystic fibrosis transmembrane conductance regulator (CFTR) Biochimica et Biophysica Acta (BBA) - Biomembranes. 2014;1838:2825–2837. doi: 10.1016/j.bbamem.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Wang C, Zhou Q, An J, Hildebrandt E, Aleksandrov LA, Riordan JR, Urbatsch IL, Hunt JF, Brouillette CG. Membrane protein stability can be compromised by detergent interactions with the extramembranous soluble domains. Protein Science. 2014;23:769–789. doi: 10.1002/pro.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabeh WM, Bossard F, Xu H, Okiyoneda T, Bagdany M, Mulvihill CM, Du K, di Bernardo S, Liu Y, Konermann L, Roldan A, Lukacs GL. Correction of both NBD1 energetics and domain interface is required to restore DeltaF508 CFTR folding and function. Cell. 2012;148:150–163. doi: 10.1016/j.cell.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eidelman O, BarNoy S, Razin M, Zhang J, McPhie P, Lee G, Huang Z, Sorscher EJ, Pollard HB. Role for phospholipid interactions in the trafficking defect of Delta F508-CFTR. Biochemistry. 2002;41:11161–11170. doi: 10.1021/bi020289s. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrandt E, Ding H, Mulky A, Dai Q, Aleksandrov AA, Bajrami B, Diego PA, Wu X, Ray M, Naren AP, Riordan JR, Yao X, DeLucas LJ, Urbatsch IL, Kappes JC. A Stable Human-Cell System Overexpressing Cystic Fibrosis Transmembrane Conductance Regulator Recombinant Protein at the Cell Surface. Mol Biotechnol. 2015;57:391–405. doi: 10.1007/s12033-014-9830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go EP, Herschhorn A, Gu C, Castillo-Menendez L, Zhang S, Mao Y, Chen H, Ding H, Wakefield JK, Hua D, Liao HX, Kappes JC, Sodroski J, Desaire H. Comparative Analysis of the Glycosylation Profiles of Membrane-Anchored HIV-1 Envelope Glycoprotein Trimers and Soluble gp140. J Virol. 2015;89:8245–8257. doi: 10.1128/JVI.00628-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 21.Hesse D, Jahn O. [07/13/2016];Colloidal Coomassie Staining. 2008 http://www1.em.mpg.de/proteomics/fileadmin/Downloads/080108_Coomassie.pdf.
- 22.Gay CA, Gebicki JM. Perchloric acid enhances sensitivity and reproducibility of the ferric-xylenol orange peroxide assay. Anal Biochem. 2002;304:42–46. doi: 10.1006/abio.2001.5566. [DOI] [PubMed] [Google Scholar]
- 23.Poolman B, Doeven MK, Geertsma ER, Biemans-Oldehinkel E, Konings WN, Rees DC. Functional analysis of detergent-solubilized and membrane-reconstituted ATP-binding cassette transporters. Methods Enzymol. 2005;400:429–459. doi: 10.1016/S0076-6879(05)00025-X. [DOI] [PubMed] [Google Scholar]
- 24.Geertsma ER, Nik Mahmood NA, Schuurman-Wolters GK, Poolman B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat Protoc. 2008;3:256–266. doi: 10.1038/nprot.2007.519. [DOI] [PubMed] [Google Scholar]
- 25.Kogan I, Ramjeesingh M, Li C, Bear CE. Studies of the molecular basis for cystic fibrosis using purified reconstituted CFTR protein. Methods Mol Med. 2002;70:143–157. doi: 10.1385/1-59259-187-6:143. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens JM, Galley K, Bear CE. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- 27.Ketchum CJ, Rajendrakumar GV, Maloney PC. Characterization of the adenosinetriphosphatase and transport activities of purified cystic fibrosis transmembrane conductance regulator. Biochemistry. 2004;43:1045–1053. doi: 10.1021/bi035382a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linsdell P. Location of a common inhibitor binding site in the cytoplasmic vestibule of the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem. 2005;280:8945–8950. doi: 10.1074/jbc.M414354200. [DOI] [PubMed] [Google Scholar]
- 29.Pasyk S, Li C, Ramjeesingh M, Bear CE. Direct interaction of a small-molecule modulator with G551D-CFTR, a cystic fibrosis-causing mutation associated with severe disease. Biochem J. 2009;418:185–190. doi: 10.1042/BJ20081424. [DOI] [PubMed] [Google Scholar]
- 30.Pollock N, Cant N, Rimington T, Ford RC. Purification of the cystic fibrosis transmembrane conductance regulator protein expressed in Saccharomyces cerevisiae. J Vis Exp. 2014 doi: 10.3791/51447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Protasevich I, Yang Z, Wang C, Atwell S, Zhao X, Emtage S, Wetmore D, Hunt JF, Brouillette CG. Thermal unfolding studies show the disease causing F508del mutation in CFTR thermodynamically destabilizes nucleotide-binding domain 1. Protein Sci. 2010;19:1917–1931. doi: 10.1002/pro.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Aleksandrov AA, An J, Cui L, Yang Z, Brouillette CG, Riordan JR. Restoration of NBD1 thermal stability is necessary and sufficient to correct F508 CFTR folding and assembly. J Mol Biol. 2015;427:106–120. doi: 10.1016/j.jmb.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendoza JL, Schmidt A, Li Q, Nuvaga E, Barrett T, Bridges RJ, Feranchak AP, Brautigam CA, Thomas PJ. Requirements for efficient correction of DeltaF508 CFTR revealed by analyses of evolved sequences. Cell. 2012;148:164–174. doi: 10.1016/j.cell.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510:172–175. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koshy C, Ziegler C. Structural insights into functional lipid-protein interactions in secondary transporters. Biochim Biophys Acta. 2015;1850:476–487. doi: 10.1016/j.bbagen.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Reichow SL, Gonen T. Lipid-protein interactions probed by electron crystallography. Curr Opin Struct Biol. 2009;19:560–565. doi: 10.1016/j.sbi.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornelius F, Habeck M, Kanai R, Toyoshima C, Karlish SJ. General and specific lipid-protein interactions in Na,K-ATPase. Biochim Biophys Acta. 2015;1848:1729–1743. doi: 10.1016/j.bbamem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Poveda JA, Giudici AM, Renart ML, Molina ML, Montoya E, Fernandez-Carvajal A, Fernandez-Ballester G, Encinar JA, Gonzalez-Ros JM. Lipid modulation of ion channels through specific binding sites. Biochim Biophys Acta. 2014;1838:1560–1567. doi: 10.1016/j.bbamem.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Weingarth M, Prokofyev A, van der Cruijsen EA, Nand D, Bonvin AM, Pongs O, Baldus M. Structural determinants of specific lipid binding to potassium channels. J Am Chem Soc. 2013;135:3983–3988. doi: 10.1021/ja3119114. [DOI] [PubMed] [Google Scholar]
- 40.Zhang XL, Li XR, Xu HX. Phosphoinositide isoforms determine compartment-specific ion channel activity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11384–11389. doi: 10.1073/pnas.1202194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 42.van der Mark VA, de Jonge HR, Chang JC, Ho-Mok KS, Duijst S, Vidovic D, Carlon MS, Oude Elferink RP, Paulusma CC. The phospholipid flippase ATP8B1 mediates apical localization of the cystic fibrosis transmembrane regulator. Biochim Biophys Acta. 2016;1863:2280–2288. doi: 10.1016/j.bbamcr.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Folmer DE, Elferink RP, Paulusma CC. P4 ATPases - lipid flippases and their role in disease. Biochim Biophys Acta. 2009;1791:628–635. doi: 10.1016/j.bbalip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Johnson BJ, Lee JY, Pickert A, Urbatsch IL. Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry. 2010;49:3403–3411. doi: 10.1021/bi902064g. [DOI] [PubMed] [Google Scholar]
- 45.Doshi R, Ali A, Shi W, Freeman EV, Fagg LA, van Veen HW. Molecular disruption of the power stroke in the ATP-binding cassette transport protein MsbA. J Biol Chem. 2013;288:6801–6813. doi: 10.1074/jbc.M112.430074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang XB, Hou YX, Riordan JR. ATPase activity of purified multidrug resistance-associated protein. J Biol Chem. 1997;272:30962–30968. doi: 10.1074/jbc.272.49.30962. [DOI] [PubMed] [Google Scholar]
- 47.Chloupkova M, Pickert A, Lee JY, Souza S, Trinh YT, Connelly SM, Dumont ME, Dean M, Urbatsch IL. Expression of 25 human ABC transporters in the yeast Pichia pastoris and characterization of the purified ABCC3 ATPase activity. Biochemistry. 2007;46:7992–8003. doi: 10.1021/bi700020m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


