Abstract
The proliferation of extensively drug-resistant Gram-negative pathogens has necessitated the therapeutic use of colistin and polymyxin B. However, treatment failures with polymyxin monotherapies and the emergence of polymyxin resistance have catalysed the search for polymyxin combinations that synergistically kill polymyxin-susceptible and -resistant organisms. This mini-review examines recent (2011–2016) in vitro and in vivo studies that have attempted to identify synergistic polymyxin combinations against Pseudomonas aeruginosa, Klebsiella pneumoniae and Acinetobacter baumannii. Clinical evidence for the use of combination regimens is also discussed.
Keywords: Polymyxins, Synergy, Combinations, Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa
1. Introduction
After decades of antimicrobial exposure, multidrug-resistant (MDR) pathogens are now emerging with resistance to three or more antibiotic classes [1,2]. Even more troubling are extensively drug-resistant (XDR) Gram-negative pathogens that are non-susceptible to all but one or two antibiotic classes [3]. In the face of such extensive levels of antibiotic resistance, clinicians have been forced to utilise colistin and polymyxin B (PMB) as last-line agents against XDR Pseudomonas aeruginosa, Klebsiella pneumoniae and Acinetobacter baumannii that are capable of resisting carbapenems and most other agents [4,5]. However, the emergence of polymyxin heteroresistance and polymyxin-resistant strains has brought the utility of polymyxin monotherapies into question [6,7].
In response to the global decline in polymyxin susceptibilities, clinicians may be tempted to simply increase the dose of a polymyxin to maximise bacterial killing. Unfortunately, polymyxins are highly nephrotoxic agents and the likeliness of renal impairment has been associated with the daily dose of a polymyxin [8,9]. Given the narrow therapeutic indices of polymyxins, a strategy for overcoming attenuated polymyxin susceptibility without increasing polymyxin exposure is the use of polymyxins in combination with other agents. Polymyxins have a unique mechanism of action that involves disruption of the outer membrane integrity of Gram-negative bacteria, which may enhance the activity of other antibiotic classes [1,10]. Despite promising in vitro results, the usefulness of synergistic polymyxin combinations in the clinical setting remains controversial [11,12].
2. Methodology
This review covers recent studies that examined the in vitro and in vivo synergy of polymyxin combinations and evaluates whether any clinical evidence exists to validate the translation of preclinical work into human patients. Studies were retrieved using the search terms ‘colistin combination’ or ‘polymyxin combination’ in PubMed, with an emphasis on manuscripts published after 2010. In vitro studies were restricted to more advanced measures of bacterial killing such as time–killing investigations and dynamic models, whereas studies involving minimum inhibitory concentration (MIC) testing, chequerboard synergy and Etest methods were not examined. After consolidating all of the literature, manuscripts were chosen that epitomised recent in vitro, in vivo and clinical studies. To maintain consistency, synergy will be defined as a ≥2 log reduction in bacterial counts compared with reductions achieved by individual agents at any time during an experiment unless a time point is specified.
3. Pseudomonas aeruginosa
Several in vitro studies that utilised static antibiotic concentrations of polymyxins in combination with an aminoglycoside have recently been published. An investigation of P. aeruginosa biofilms found that colistin and tobramycin at 2× their respective MICs (MICcolistin = 2 mg/L; MICtobramycin = 1 mg/L) separately reduced bacterial counts of a single P. aeruginosa strain to 4.59 log10 CFU/mL and 4.85 log10 CFU/mL after 24 h from a 7.95 log10 CFU/mL starting inoculum, whereas the combination of both agents resulted in a 24-h count of 2.60 log10 CFU/mL [13]. Another study investigated the activity of PMB (2 mg/L), rifampicin (2 mg/L), meropenem (64 mg/L) and amikacin (80 mg/L), alone and in combination, against 22 clinical XDR P. aeruginosa isolates collected in Singapore [14]. After 24 h of antibiotic exposure, none of the single agents achieved a 3 log reduction in bacterial counts, whereas the combination of meropenem + PMB resulted in ≥3 log reduction in 8/22 strains, and the triple combination of PMB + amikacin + rifampicin (or meropenem) achieved ≥3 log reduction in an additional 7 strains (6 strains for meropenem).
Another static time–killing experiment evaluated the potential synergy between fosfomycin (30, 150 or 300 mg/L) and PMB (0.5, 1 or 2 mg/L) against four clinical P. aeruginosa isolates and one reference strain at 106 CFU/mL [15]. In 24-h experiments, synergistic killing was achieved at fosfomycin concentrations ≥30 mg/L and at PMB concentrations ≥1 mg/L against two isolates susceptible to both antibiotics. In contrast, synergistic killing was most evident at fosfomycin concentrations ≥150 mg/L for the polymyxin-resistant strain, and synergy was only detected in one of two strains that were resistant to both antimicrobials.
Other static time–killing experiments investigated the killing of a polymyxin with a carbapenem. A study examining PMB in combination with doripenem against wild-type P. aeruginosa and hypermutator strains (MICpolymyxin = 1–2 mg/L) found that PMB concentrations up to 64 mg/L resulted in re-growth of all six stains at a 108 CFU/mL inoculum, whereas 4 mg/L PMB + 8 mg/L doripenem resulted in sustained killing up to 48 h [16]. Similarly, a study investigating colistin + imipenem in clinically relevant concentration arrays observed that several combinations of colistin + imipenem achieved synergy against colistin-resistant and imipenem-resistant P. aeruginosa isolates over 48 h both at 106 CFU/mL and 108 CFU/ mL inocula [17].
The combination of a polymyxin and a carbapenem has also been investigated using dynamic in vitro models. In a dynamic biofilm model that utilised three colistin-susceptible P. aeruginosa isolates with varying doripenem susceptibilities (MICdoripenem = 0.125–128 mg/L), the combination of doripenem [peak concentration (Cmax) = 25 mg/L] + colistin (constant at 3.5 mg/L) achieved ≥1–2 log of additional killing by 4 h compared with either agent alone, and the enhanced killing was sustained up to 72 h in all three strains [18]. A separate investigation used a one-compartment model to investigate doripenem (Cmax = 2.5 mg/L or 25 mg/L) + colistin (constant at 0.5 mg/L or 2 mg/L) against a colistin-heteroresistant strain and a colistin-resistant MDR P. aeruginosa strain and observed that combinations of doripenem + colistin were capable of synergistic killing over 96 h against both stains at 106 CFU/mL and 108 CFU/ mL [19]. Lastly, a 10-day hollow-fibre infection model was used to examine the activity of colistin (constant at 2 mg/L or 5 mg/L) + doripenem (Cmax = 25 mg/L) against two colistin-heteroresistant strains and one colistin-resistant isolate with an initial inoculum of 109 CFU/mL (Fig. 1) [20]. Both colistin-heteroresistant strains were eradicated by colistin + doripenem by 72 h (monotherapies re-grew), and although combination treatment was unable to eradicate the colistin-resistant strain, colistin + doripenem achieved >4 log reduction compared with either agent alone by 72 h.
Fig. 1.
Viable counts from a 240-h hollow-fibre infection model that examined the killing of colistin-heteroresistant Pseudomonas aeruginosa strain FADDI PA033 [colistin minimum inhibitory concentration (MIC) = 1 mg/L; doripenem MIC = 0.5 mg/L] at 109 CFU/mL [20]. Investigated antibiotic regimens included: (a) growth control; (b) colistin as a continuous infusion that achieved a constant free steady-state concentration (fCss) of 2 mg/L; (c) colistin given as a continuous infusion with a fCss of 5 mg/L; (d) doripenem given as a bolus dose with a free peak concentration (fCmax) of 25 mg/L every 8 h (q8h) with a simulated half-life of 1.5 h; (e) colistin given as a continuous infusion with a fCss of 2 mg/L and doripenem given as a bolus dose with fCmax of 25 mg/L q8h; and (f) colistin given as a continuous infusion with a fCss of 5 mg/L and doripenem given as a bolus dose with a fCmax of 25 mg/L q8h. In the key, the total population represents viable counts on antibiotic-free agar plates, and colistin concentrations represent the viable counts on colistin-containing agar plates. Symbols represent the observed counts and lines are the fits based on mathematical modelling. Reproduced with permission from Tsuji et al [20].
In summary, the use of an aminoglycoside, fosfomycin or a carbapenem in conjunction with a polymyxin was able to confer additive or synergistic killing against several P. aeruginosa strains in static or dynamic in vitro models. As expected, strains that were susceptible to one or both of the study antimicrobials generally experienced the most rapid killing; however, the presence of aminoglycoside resistance, fosfomycin resistance or carbapenem resistance did not prevent synergistic killing during combination exposure. Encouragingly, the combination of a carbapenem and a polymyxin was able to achieve synergistic killing against polymyxin-resistant P. aeruginosa at 106 CFU/mL and 108 CFU/mL inocula.
4. Klebsiella pneumoniae
Several static time–killing studies have recently investigated the activities of polymyxin combinations against metallo-β-lactamase (MBL)-producing K. pneumoniae. In a study that examined 12 antibiotics alone and in dual combinations against six NDM-1-producing K. pneumoniae strains at 105 CFU/mL, the double combinations of meropenem (64 mg/L) + tigecycline (2 mg/L) and of PMB (2 mg/ L) + either meropenem or imipenem (32 mg/L) achieved a ≥3 log reduction in four and three NDM-1-producing strains, respectively, and PMB + meropenem resulted in ≥2 log reduction in an additional two strains (individual agents resulted in re-growth) [21]. Another investigation analysed the killing of PMB and chloramphenicol against four NDM-producing K. pneumoniae isolates at 106 CFU/mL with varying degrees of chloramphenicol susceptibility (MICchloramphenicol = 4–256 mg/L) and found that 1 mg/L PMB + 8 mg/L chloramphenicol achieved synergy against all four strains (at the 6 h and 24 h time points) [22]. Lastly, a study that investigated various antibiotic combinations against two VIM-1- and two NDM-1-producing K. pneumoniae isolates at 5 × 106 CFU/mL identified the combination of colistin (4.0 mg/L) + meropenem (6.8 mg/L) + rifampicin (1.7 mg/L) as the most effective, with synergistic killing and a ≥3 log reduction by 24 h in all four isolates [23]. The study also demonstrated synergy between fosfomycin (83 mg/L) and colistin (4.0 mg/L) against one VIM-producing strain (fosfomycin-susceptible) and both NDM-producing strains (fosfomycin-resistant).
In contrast to the synergistic killing observed between fosfomycin and colistin against MBL-producing K. pneumoniae, a study evaluating the killing of fosfomycin combinations against KPC-2 producing K. pneumoniae observed a relatively low rate of synergy [24]. Using a 106 CFU/mL inoculum, fosfomycin (100 mg/L) and colistin (5 mg/L) were evaluated alone and in combination against 17 KPC-2-producing K. pneumoniae isolates in static time–killing experiments. Despite fosfomycin susceptibility in 13 strains and colistin susceptibility in 9 strains, synergistic killing was only observed in 2/17 K. pneumoniae isolates (11.8%); however, bactericidal activity was observed against 11/17 isolates. The rate of synergy observed in this study (11.8%) may be lower than the MBL investigation owing to rapid bactericidal activity achieved by colistin or fosfomycin in this study, whereas colistin or fosfomycin alone were unable to achieve substantial killing against the VIM- or NDM-producing K. pneumoniae potentially due to unknown phenotypic differences between the bacterial strains [23].
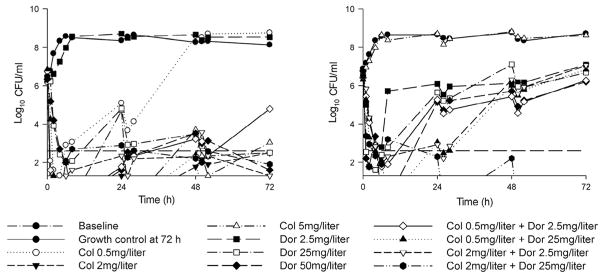
Several in vitro studies have explored polymyxin and carbapenem combinations against K. pneumoniae carbapenemase (KPC)-producing organisms. A static time–killing study of doripenem (6 mg/L) in combination with colistin or PMB (2 × MIC; polymyxin MICs ranged from 0.0625 to 0.125 mg/L) against 105.5 CFU/mL of polymyxin-susceptible KPC-3-producing K. pneumoniae found that polymyxins alone resulted in re-growth by 48 h, whereas a polymyxin + doripenem achieved synergistic reductions compared with a polymyxin alone by 48 h [25]. Another static time–killing investigation utilising doripenem (8 mg/L), colistin (1 mg/L), gentamicin (2 mg/L) and doxy-cycline (2 mg/L) combinations against 12 KPC-producing K. pneumoniae strains at 106 CFU/mL identified the combination of doripenem + colistin as superior to other combinations [26]. After 24 h of incubation, doripenem + colistin achieved a >3 log reduction in 9/12 strains and synergistic killing in 6/12 strains (4/6 pan-resistant isolates). The utility of colistin + doripenem against K. pneumoniae was further validated in a dynamic one-compartment model that examined doripenem (Cmax = 2.5 mg/L or 25 mg/L) + colistin (constant at 0.5 mg/L or 2 mg/L) against four isolates with varying degrees of colistin and doripenem resistance (MICdoripenem <0.125–8 mg/L; MICcolistin = 1 mg/L to >128 mg/L with two heteroresistant isolates) at 106 CFU/mL and 108 CFU/mL [27]. At the 106 CFU/mL inoculum, co-listin + doripenem achieved synergy in all four strains over 72 h (Fig. 2), whereas the combination resulted in synergistic killing of every isolate except the colistin-resistant strain at the 108 CFU/mL inoculum.
Fig. 2.
Time–killing experiments utilising a one-compartment model over 72 h that investigated the killing of a colistin-susceptible doripenem-resistant Klebsiella pneumoniae strain (left) or a colistin-resistant doripenem-susceptible K. pneumoniae strain (right) at 106 CFU/mL [27]. Simulated antibiotic regimens included constant colistin (Col) concentrations of 0.5, 2 or 5 mg/L, dynamic doripenem (Dor) regimens with a peak concentration (Cmax) of 2.5, 25 or 50 mg/L (bolus dosing every 8 h; half-life = 1.5 h) and combinations of doripenem and colistin. Figure adapted with permission from Nation et al [27].
Experiments have also been conducted against KPC-producing organisms using polymyxin combinations with tigecycline or rifampicin. A static time–killing investigation of tigecycline (MICtigecycline = 0.5–2 mg/L) dual combinations with colistin (MICcolistin = 0.5–1 mg/L) or meropenem (MICmeropenem = 4–16 mg/L) against four KPC-producing K. pneumoniae at 5 × 105 CFU/mL found that tigecycline + colistin (4× their respective MICs) resulted in synergistic killing and a ≥3 log reduction of all four strains by 24 h [28]. In contrast, a study examining dual and triple combinations of co-listin, tigecycline, rifampicin and other agents against eight colistin-resistant KPC-3-producing K. pneumoniae (MICtigecycline = 2 mg/L for 7/8 strains; MICrifampicin > 32 mg/L) at 5 × 105 CFU/mL found that colistin (2 mg/L) + rifampicin (2 mg/L) achieved synergy against all eight strains (other dual combinations did not achieve synergy), and the addition of tigecycline (0.125 mg/L) did not substantially improve killing for the majority of the strains [29]. Unfortunately, recent in vivo analyses of colistin + tigecycline in murine models have failed to demonstrate synergy [30] and the combination may even be antagonistic against some KPC-producing K. pneumoniae strains [31].
In short, in vitro experiments utilising carbapenems, rifampicin and chloramphenicol were able to demonstrate synergy with polymyxins against K. pneumoniae that produced KPC or MBL enzymes and displayed a range of polymyxin susceptibilities. The potential synergy between colistin + fosfomycin or tigecycline varied between studies and may suggest that phenotypic differences between K. pneumoniae isolates greatly influence the pharmacodynamics of such regimens. In particular, the poor killing achieved by tigecycline in vivo merits additional investigations as multiple studies were unable to demonstrate synergy.
5. Acinetobacter baumannii
Despite concerns over an increased risk of nephrotoxicity, the simultaneous use of a polymyxin and a glycopeptide has received considerable attention as an unorthodox strategy to combat MDR and XDR A. baumannii. The combination of colistin (1 mg/ L) + teicoplanin (20 mg/L) achieved dramatic synergy (>8 log reduction by 24 h in comparison with colistin alone) against five MDR A. baumannii strains at >105 CFU/mL in static time–killing experiments [32]. Using the same methodology, the combination of colistin (1 mg/L) + vancomycin (20 mg/L) was synergistic by 24 h in all 5 strains (4/5 strains at 48 h) [10], and the dual combinations of colistin (2.5 mg/kg) + teicoplanin (10 mg/kg) and colistin + vancomycin (10 mg/kg) significantly improved survival of Galleria mellonella inoculated with 104 CFU/larvae of MDR A. baumannii (P < 0.05 and P < 0.001, respectively) [33]. In a static time–killing investigation that utilised four A. baumannii strains (MICcolistin = 1–8 mg/L; MICvancomycin = 128 mg/L to >128 mg/L) at 105–106 CFU/mL, the combination of colistin + vancomycin (0.25× and 0.5× their respective MICs) resulted in synergistic killing by 24 h in all four strains [34]. Similarly, synergy with a polymyxin + telavancin at clinically relevant concentrations has recently been observed in vitro [35] and in vivo [36], and there is in vitro evidence supporting the use of a polymyxin + daptomycin as well [37].
The combination of a polymyxin and a carbapenem remains an area of active research for the treatment of MDR and XDR A. baumannii. Building upon previous studies, investigators at a single institution noted that 10/11 transplant recipients with A. baumannii infections died during treatment with various tigecycline and colistin combinations [7]. In response, static time–killing experiments utilising colistin (0.25 mg/L) + doripenem (8 mg/L) against five XDR A. baumannii strains at 106 CFU/mL resulted in synergistic killing and eradication by 8 h in all five strains, which prompted the successful treatment of A. baumannii infections in four of five additional transplant recipients using colistin (5 mg/kg) + doripenem (500 mg every 8 h). A separate static–time killing investigation of imipenem dual combinations against 12 carbapenem-resistant A. baumannii isolates (MICcolistin = 0.125–4 mg/L; MICimipenem = 8–128 mg/L) at 5 × 105 CFU/mL identified the combination of colistin and imipenem (0.5 × their respective MICs) as the most synergistic combination (synergy achieved in 9/12 isolates vs. 6/12 for second best combination) [38]. Another study examined dual and triple combinations of colistin, doripenem and sulbactam against six colistin-resistant A. baumannii isolates collected from patients previously treated with colistin methanesulfonate and doripenem, and although colistin + doripenem was synergistic against five isolates, the addition of sulbactam to colistin + doripenem resulted in universal synergy and greater bacterial reductions (P = 0.04) [39]. In agreement with the previously discussed studies, a recent meta-analysis of polymyxin time–killing experiments against A. baumannii calculated that carbapenems had the highest rate of synergy (80.6%), followed by glycopeptides (70.8%), whereas rifampicin (57.2%) and tigecycline (41.6%) had less favourable rates of synergy [40].
In vitro and in vivo studies have also examined the potential synergy of polymyxins in combination with rifampicin. Using a dynamic one-compartment model, colistin (constant at 2 mg/L) + rifampicin (Cmax = 5 mg/L) achieved synergistic killing over 72 h against two MDR A. baumannii strains (colistin-susceptible and colistin-resistant) at 106 CFU/mL and 108 CFU/mL inocula [41]. Similarly, an in vivo murine thigh infection model was used to evaluate synergistic killing of colistin (20 mg/kg every 8 h) in combination with various antimicrobials against 12 XDR A. baumannii strains [42]. Despite rifampicin MICs of ≥8 mg/L, colistin + rifampicin (25 mg/ kg every 6 h) achieved synergy against all 12 strains at 24 h and 28 h time points, whereas colistin in combination with tigecycline, fosfomycin or sulbactam did not demonstrate synergistic killing.
Lastly, multiple in vitro investigations have evaluated the synergistic killing of a polymyxin with either tigecycline or minocycline. A study in a one-compartment model investigated the combination of PMB (Cmax = 1.16 mg/L) + tigecycline (Cmax = 1.2 mg/L) against four MDR A. baumannii isolates, which resulted in synergistic killing against one isolate and additive killing against two other isolates [43]. In static time–killing experiments, colistin + tigecycline (0.5× their respective MICs) achieved synergistic activity by 8 h against 106 CFU/mL of an XDR A. baumannii strain (MICcolistin = 0.25 mg/L; MICtigecycline = 1 mg/L); however, colistin (1.25 mg/kg every 6 h) + tigecycline (10 mg/kg every 12 h) did not result in significantly improved killing versus monotherapy in rats inoculated with 1.2 mL/kg of 5 × 108 CFU/mL of the XDR strain in a pneumonia model [44]. In a similar investigation, PMB (0.5 mg/L) + minocycline (8 mg/ L) was evaluated in static time–killing experiments against four A. baumannii strains with various minocycline susceptibilities (MICminocycline = 0.25–16 mg/L) at 106 CFU/mL [45]. Of the four A. baumannii strains, only two experienced a ca. 2 log reduction with combination exposure compared with either agent alone by 24 h, whereas one of three clinical strains investigated in a murine pneumonia model displayed a similar reduction in bacterial counts.
The synergistic killing of A. baumannii has therefore recently been demonstrated in multiple in vitro studies when a polymyxin was paired with a glycopeptide, a carbapenem or rifampicin. Although the majority of the A. baumannii strains were susceptible to polymyxins, synergy was achieved against polymyxin-heteroresistant and -resistant strains as well, suggesting that polymyxins may have clinical utility against A. baumannii with elevated polymyxin MICs. Similar to the bacterial killing achieved against K. pneumoniae when a polymyxin was paired with tigecycline, the combinatorial pharmacodynamics of the combination varied during in vitro studies and failed to achieve synergy in an in vivo pneumonia model, which may potentially offer insight into reports of poor outcomes following use of the combination in the clinic [46].
6. Clinical studies
Although clinical evidence supporting the use of polymyxin combinations against MDR and XDR Gram-negative pathogens has been scarce, recent studies of K. pneumoniae treatment have been published that favour combination treatment. A large retrospective study evaluated the 14-day survival of 661 patients treated for KPC-producing K. pneumoniae and found that combination therapy was associated with favourable survival compared with monotherapy [odds ratio (OR) = 0.64, 95% confidence interval (CI) 0.45–0.90, P = 0.01; colistin = 39% of monotherapies] [47]. Similarly, a small retrospective cohort study evaluated 34 patients with bacteraemia caused by KPC-producing K. pneumoniae and found that 28-day mortality was lower in patients receiving combinations (47% of combinations used colistin or PMB) in comparison with patients receiving monotherapies (13.3% vs. 57.8%; P = 0.01) [48]. Another retrospective study of 205 patients treated for carbapenemase-producing K. pneumoniae bloodstream infections found that all-cause 28-day mortality was higher in patients who received monotherapy (72 patients total, 22 received colistin alone) than in patients who received combination therapy (44.4% vs. 27.2%; P = 0.018) [49]. A systematic review of 20 studies that included patients treated for carbapenemase-producing Enterobacteriaceae posited that a reliable meta-analysis was not possible given the heterogeneity of the studies, but a methodical review of each study led the authors to tentatively claim that combination therapy may benefit critically ill patients with severe K. pneumoniae infections [50].
There is also emerging evidence for the survival benefit of polymyxin combination therapy for the treatment of MDR and XDR A. baumannii. A retrospective cohort study evaluating 101 patients treated for A. baumannii and P. aeruginosa infections found that 30-day mortality was lower for patients treated with PMB combinations versus patients receiving PMB monotherapy (42.4% vs. 67.7%; P = 0.03) [51]. A smaller retrospective study that analysed the clinical treatments of 17 patients with colistin-resistant A. baumannii infections found that the combination of colistin + a carbapenem + ampicillin/ sulbactam resulted in a lower 30-day mortality in comparison with other antibiotic regimens (0/7 deaths vs. 6/10; P = 0.03) [6]. A multicentre retrospective study of hospitalised patients who received colistin for MDR Gram-negative bacteria (60% A. baumannii, 19% P. aeruginosa and 15% K. pneumoniae) evaluated the rate of mortality for patients receiving colistin alone or those receiving colistin with a concomitant glycopeptide [52]. Using a sample size of 166 patients with confirmed MDR Gram-negative infections, co-administration of a glycopeptide for ≥5 days was associated with a favourable 30-day morality for infections due to MDR Gram-negative bacteria [hazard ratio (HR) = 0.42, 95% CI 0.18–0.93] and due to A. baumannii specifically (HR = 0.41, 95% CI 0.17–0.98) in multivariate analyses. Another retrospective evaluation of 291 patients treated with colistin for XDR Gram-negative bacillus infections (78% of patients cultured A. baumannii, 21% P. aeruginosa and 5% K. pneumoniae) found that colistin monotherapy and colistin combinations without guidance from rapid time–killing experiments were eight times and six times more likely to result in infection-related mortality (monotherapy OR = 8.49, 95% CI 1.56–46.05; combinations without guidance OR = 5.75, 95% CI 1.25–25.73), respectively, in comparison with patients who received colistin combinations optimised by multiple-combination bactericidal testing [53]. Lastly, a prospective comparison of 55 patients treated for XDR A. baumannii found that the combination of colistin + tigecycline resulted in higher mortality than colistin + a carbapenem when the tigecycline MIC of the causative pathogen was >2 mg/L (HR = 6.93, 95% CI 1.61–29.78; P = 0.009), suggesting that caution is needed when developing polymyxin combination regimens [46].
In contrast to the encouraging results previously discussed, many clinical studies have failed to demonstrate superiority of polymyxin combinations over monotherapy. A prospective study by Durante-Mangoni et al enrolled 210 patients to randomly receive colistin or colistin + rifampicin for the treatment of life-threatening XDR A. baumannii infections and found that the risk of death within 30 days was not significantly lower during combination treatment (OR = 0.88, 95% CI 0.46–1.69; P = 0.71) despite significantly better microbiological cure in patients receiving colistin + rifampicin (P = 0.034) [54]. A similar outcome was observed in a retrospective study that evaluated 250 patients treated for XDR A. baumannii bacteraemia with colistin combinations or colistin alone; 14-day survival was comparable in both groups (68.2% combination vs. 55.5% monotherapy; P > 0.05), whereas microbiological cure (79.9% vs. 55.6%; P = 0.001) and in-hospital mortality (52.3% vs. 72.2%; P = 0.03) were superior during combination treatment [55]. In a small prospective trial of 94 patients with carbapenem-resistant A. baumannii, subjects were randomised to receive colistin alone or colistin + fosfomycin and a statistical difference in the infection-related 28-day mortality was not observed (23.1% vs. 16.3%; P = 0.507); however, microbiological cure in the first 72 h (65.7% vs. 78.8%; P = 0.028) and at the end of treatment (84.5% vs. 100%; P = 0.023) did favour combination therapy [56]. A meta-analysis that evaluated the clinical response of patients with MDR A. baumannii treated with colistin alone or in combination found that colistin combinations did not improve clinical response (OR = 1.37, 95% CI 0.86–2.19; P = 0.18), but microbiological response did improve with combination therapy (OR = 2.14, 95% CI 1.48–3.07; P < 0.0001) [57]. Another meta-analysis evaluated all-cause mortality in patients treated with colistin or colistin combinations for carbapenem-resistant Gram-negative bacteria and found no mortality benefit for colistin paired with rifampicin (P = 0.81), carbapenems (P = 0.91), tigecycline (P = 0.77), sulbactam (P = 0.81) or aminoglycosides (P = 0.07) [58].
Despite the large amount of clinical literature surrounding the use of a polymyxin alone or in combination, severe limitations in many of the study designs have obscured the interpretation of such studies and may explain the apparent discordance in study results. For example, the investigation by Durante-Mangoni et al [54] has been criticised for not accounting for the addition of a carbapenem or tigecycline to the ‘colistin alone’ arm, and also failing to evaluate whether microbiological cure impacted treatment duration or recurrence [59,60]. Many of the clinical studies that evaluated polymyxin combinations to date utilised small sample sizes, and numerous retrospective analyses are confounded by biases such as sicker or unresponsive patients receiving combination therapy.
7. Conclusions
The proliferation of MDR and XDR Gram-negative pathogens has forced clinicians to revisit the use of the polymyxin drug class. In a desire to improve clinical outcomes with polymyxin therapy, the medical community has investigated the use of polymyxin combinations. An abundance of recent in vitro and preclinical in vivo studies has identified various polymyxin combinations that demonstrated synergistic killing against MDR and XDR P. aeruginosa, K. pneumoniae and A. baumannii. However, the utility of polymyxin combinations in the clinical setting has been obscured by conflicting studies that are typically limited by small sample sizes and retrospective study designs. To address the ambiguity surrounding the use of polymyxin combinations, two large, prospective, randomised clinical trials comparing colistin alone to colis-tin + meropenem are currently underway in Europe and the USA (ClinicalTrials.gov IDs NCT01732250 and NCT01597973). Both trials will provide valuable insight into the potential benefits of colistin in combination with a carbapenem for MDR and XDR Gram-negative pathogens, but additional prospective trials may be needed to assess synergy between polymyxins and other agents for specific pathogens.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [award no. R01AI111990].
Footnotes
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Karaiskos I, Giamarellou H. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: current and emerging therapeutic approaches. Expert Opin Pharmacother. 2014;15:1351–70. doi: 10.1517/14656566.2014.914172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 4.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis. 2015;15:225–34. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 5.Bergen PJ, Landersdorfer CB, Lee HJ, Li J, Nation RL. ‘Old’ antibiotics for emerging multidrug-resistant bacteria. Curr Opin Infect Dis. 2012;25:626–33. doi: 10.1097/QCO.0b013e328358afe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qureshi ZA, Hittle LE, O’Hara JA, Rivera JI, Syed A, Shields RK, et al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis. 2015;60:1295–303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields RK, Kwak EJ, Potoski BA, Doi Y, Adams-Haduch JM, Silviera FP, et al. High mortality rates among solid organ transplant recipients infected with extensively drug-resistant Acinetobacter baumannii: using in vitro antibiotic combination testing to identify the combination of a carbapenem and colistin as an effective treatment regimen. Diagn Microbiol Infect Dis. 2011;70:246–52. doi: 10.1016/j.diagmicrobio.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284–94. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubrovskaya Y, Prasad N, Lee Y, Esaian D, Figueroa DA, Tam VH. Risk factors for nephrotoxicity onset associated with polymyxin B therapy. J Antimicrob Chemother. 2015;70:1903–7. doi: 10.1093/jac/dkv014. [DOI] [PubMed] [Google Scholar]
- 10.Gordon NC, Png K, Wareham DW. Potent synergy and sustained bactericidal activity of a vancomycin–colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:5316–22. doi: 10.1128/AAC.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tängdén T. Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Ups J Med Sci. 2014;119:149–53. doi: 10.3109/03009734.2014.899279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev. 2012;25:450–70. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann G, Yang L, Wu H, Song Z, Wang H, Høiby N, et al. Colistin–tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J Infect Dis. 2010;202:1585–92. doi: 10.1086/656788. [DOI] [PubMed] [Google Scholar]
- 14.Lim TP, Lee W, Tan TY, Sasikala S, Teo J, Hsu LY, et al. Effective antibiotics in combination against extreme drug-resistant Pseudomonas aeruginosa with decreased susceptibility to polymyxin B. PLoS ONE. 2011;6:e28177. doi: 10.1371/journal.pone.0028177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh CC, Landersdorfer CB, McIntosh MP, Peleg AY, Hirsch EB, Kirkpatrick CM, et al. Clinically relevant concentrations of fosfomycin combined with polymyxin B, tobramycin or ciprofloxacin enhance bacterial killing of Pseudomonas aeruginosa, but do not suppress the emergence of fosfomycin resistance. J Antimicrob Chemother. 2016;71:2218–29. doi: 10.1093/jac/dkw115. [DOI] [PubMed] [Google Scholar]
- 16.Ly NS, Bulman ZP, Bulitta JB, Baron C, Rao GG, Holden PN, et al. Optimization of polymyxin B in combination with doripenem to combat mutator Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60:2870–80. doi: 10.1128/AAC.02377-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, et al. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother. 2011;55:5134–42. doi: 10.1128/AAC.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lora-Tamayo J, Murillo O, Bergen PJ, Nation RL, Poudyal A, Luo X, et al. Activity of colistin combined with doripenem at clinically relevant concentrations against multidrug-resistant Pseudomonas aeruginosa in an in vitro dynamic biofilm model. J Antimicrob Chemother. 2014;69:2434–42. doi: 10.1093/jac/dku151. [DOI] [PubMed] [Google Scholar]
- 19.Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Jacob J, Sidjabat HE, et al. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/ pharmacodynamic model. Antimicrob Agents Chemother. 2011;55:5685–95. doi: 10.1128/AAC.05298-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ly NS, Bulitta JB, Rao GG, Landersdorfer CB, Holden PN, Forrest A, et al. Colistin and doripenem combinations against Pseudomonas aeruginosa: profiling the time course of synergistic killing and prevention of resistance. J Antimicrob Chemother. 2015;70:1434–42. doi: 10.1093/jac/dku567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim TP, Cai Y, Hong Y, Chan EC, Suranthran S, Teo JQ, et al. In vitro pharmacodynamics of various antibiotics in combination against extensively drug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2015;59:2515–24. doi: 10.1128/AAC.03639-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdul Rahim N, Cheah SE, Johnson MD, Yu H, Sidjabat HE, Boyce J, et al. Synergistic killing of NDM-producing MDR Klebsiella pneumoniae by two ‘old’ antibiotics—polymyxin B and chloramphenicol. J Antimicrob Chemother. 2015;70:2589–97. doi: 10.1093/jac/dkv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tängdén T, Hickman RA, Forsberg P, Lagerbäck P, Giske CG, Cars O. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time–kill experiments. Antimicrob Agents Chemother. 2014;58:1757–62. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souli M, Galani I, Boukovalas S, Gourgoulis MG, Chryssouli Z, Kanellakopoulou K, et al. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob Agents Chemother. 2011;55:2395–7. doi: 10.1128/AAC.01086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee GC, Burgess DS. Polymyxins and doripenem combination against KPC-producing Klebsiella pneumoniae. J Clin Med Res. 2013;5:97–100. doi: 10.4021/jocmr1220w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56:3395–8. doi: 10.1128/AAC.06364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deris ZZ, Yu HH, Davis K, Soon RL, Jacob J, Ku CK, et al. The combination of colistin and doripenem is synergistic against Klebsiella pneumoniae at multiple inocula and suppresses colistin resistance in an in vitro pharmacokinetic/ pharmacodynamic model. Antimicrob Agents Chemother. 2012;56:5103–12. doi: 10.1128/AAC.01064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pournaras S, Vrioni G, Neou E, Dendrinos J, Dimitroulia E, Poulou A, et al. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time–kill assay. Int J Antimicrob Agents. 2011;37:244–7. doi: 10.1016/j.ijantimicag.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Gaibani P, Lombardo D, Lewis RE, Mercuri M, Bonora S, Landini MP, et al. In vitro activity and post-antibiotic effects of colistin in combination with other antimicrobials against colistin-resistant KPC-producing Klebsiella pneumoniae bloodstream isolates. J Antimicrob Chemother. 2014;69:1856–65. doi: 10.1093/jac/dku065. [DOI] [PubMed] [Google Scholar]
- 30.Demiraslan H, Dinc G, Ahmed SS, Elmali F, Metan G, Alp E, et al. Carbapenem-resistant Klebsiella pneumoniae sepsis in corticosteroid receipt mice: tigecycline or colistin monotherapy versus tigecycline/colistin combination. J Chemother. 2014;26:276–81. doi: 10.1179/1973947813Y.0000000143. [DOI] [PubMed] [Google Scholar]
- 31.Michail G, Labrou M, Pitiriga V, Manousaka S, Sakellaridis N, Tsakris A, et al. Activity of tigecycline in combination with colistin, meropenem, rifampin, or gentamicin against KPC-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother. 2013;57:6028–33. doi: 10.1128/AAC.00891-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wareham DW, Gordon NC, Hornsey M. In vitro activity of teicoplanin combined with colistin versus multidrug-resistant strains of Acinetobacter baumannii. J Antimicrob Chemother. 2011;66:1047–51. doi: 10.1093/jac/dkr069. [DOI] [PubMed] [Google Scholar]
- 33.Hornsey M, Wareham DW. In vivo efficacy of glycopeptide–colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2011;55:3534–7. doi: 10.1128/AAC.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidaillac C, Benichou L, Duval RE. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob Agents Chemother. 2012;56:4856–61. doi: 10.1128/AAC.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornsey M, Longshaw C, Phee L, Wareham DW. In vitro activity of telavancin in combination with colistin versus Gram-negative bacterial pathogens. Antimicrob Agents Chemother. 2012;56:3080–5. doi: 10.1128/AAC.05870-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornsey M, Phee L, Longshaw C, Wareham DW. In vivo efficacy of telavancin/ colistin combination therapy in a Galleria mellonella model of Acinetobacter baumannii infection. Int J Antimicrob Agents. 2013;41:285–7. doi: 10.1016/j.ijantimicag.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Galani I, Orlandou K, Moraitou H, Petrikkos G, Souli M. Colistin/daptomycin: an unconventional antimicrobial combination synergistic in vitro against multidrug-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2014;43:370–4. doi: 10.1016/j.ijantimicag.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Sheng WH, Wang JT, Li SY, Lin YC, Cheng A, Chen YC, et al. Comparative in vitro antimicrobial susceptibilities and synergistic activities of antimicrobial combinations against carbapenem-resistant Acinetobacter species: Acinetobacter baumannii versus Acinetobacter genospecies 3 and 13TU. Diagn Microbiol Infect Dis. 2011;70:380–6. doi: 10.1016/j.diagmicrobio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Oleksiuk LM, Nguyen MH, Press EG, Updike CL, O’Hara JA, Doi Y, et al. In vitro responses of Acinetobacter baumannii to two- and three-drug combinations following exposure to colistin and doripenem. Antimicrob Agents Chemother. 2014;58:1195–9. doi: 10.1128/AAC.01779-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni W, Shao X, Di X, Cui J, Wang R, Liu Y. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents. 2015;45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Bergen PJ, Bulitta JB, Tsuji B, Forrest A, Nation RL, et al. Synergistic activity of colistin and rifampin combination against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2013;57:3738–45. doi: 10.1128/AAC.00703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan B, Guan J, Wang X, Cong Y. Activity of colistin in combination with meropenem, tigecycline, fosfomycin, fusidic acid, rifampin or sulbactam against extensively drug-resistant Acinetobacter baumannii in a murine thigh-infection model. PLoS ONE. 2016;11:e0157757. doi: 10.1371/journal.pone.0157757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagihara M, Housman ST, Nicolau DP, Kuti JL. In vitro pharmacodynamics of polymyxin B and tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2014;58:874–9. doi: 10.1128/AAC.01624-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutlu Yilmaz E, Sunbul M, Aksoy A, Yilmaz H, Guney AK, Guvenc T. Efficacy of tigecycline/colistin combination in a pneumonia model caused by extensively drug-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2012;40:332–6. doi: 10.1016/j.ijantimicag.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Bowers DR, Cao H, Zhou J, Ledesma KR, Sun D, Lomovskaya O, et al. Assessment of minocycline and polymyxin B combination against Acinetobacter baumannii. Antimicrob Agents Chemother. 2015;59:2720–5. doi: 10.1128/AAC.04110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng A, Chuang YC, Sun HY, Sheng WH, Yang CJ, Liao CH, et al. Excess mortality associated with colistin–tigecycline compared with colistin–carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med. 2015;43:1194–204. doi: 10.1097/CCM.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 47.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70:2133–43. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 48.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–13. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58:2322–8. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58:654–63. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigatto MH, Vieira FJ, Antochevis LC, Behle TF, Lopes NT, Zavascki AP. Polymyxin B in combination with antimicrobials lacking in vitro activity versus polymyxin B in monotherapy in critically ill patients with Acinetobacter baumannii or Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 2015;59:6575–80. doi: 10.1128/AAC.00494-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrosillo N, Giannella M, Antonelli M, Antonini M, Barsic B, Belancic L, et al. Clinical experience of colistin–glycopeptide combination in critically ill patients infected with Gram-negative bacteria. Antimicrob Agents Chemother. 2014;58:851–8. doi: 10.1128/AAC.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai B, Cai Y, Liew YX, Chua NG, Teo JQ, Lim TP, et al. Clinical efficacy of polymyxin monotherapy versus nonvalidated polymyxin combination therapy versus validated polymyxin combination therapy in extensively drug-resistant Gram-negative bacillus infections. Antimicrob Agents Chemother. 2016;60:4013–22. doi: 10.1128/AAC.03064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57:349–58. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 55.Batirel A, Balkan II, Karabay O, Agalar C, Akalin S, Alici O, et al. Comparison of colistin–carbapenem, colistin–sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol Infect Dis. 2014;33:1311–22. doi: 10.1007/s10096-014-2070-6. [DOI] [PubMed] [Google Scholar]
- 56.Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2014;58:5598–601. doi: 10.1128/AAC.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z, Chen Y, Fang Y, Wang X, Chen Y, Qi Q, et al. Meta-analysis of colistin for the treatment of Acinetobacter baumannii infection. Sci Rep. 2015;5:17091. doi: 10.1038/srep17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paul M, Carmeli Y, Durante-Mangoni E, Mouton JW, Tacconelli E, Theuretzbacher U, et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother. 2014;69:2305–9. doi: 10.1093/jac/dku168. [DOI] [PubMed] [Google Scholar]
- 59.Huang DB, Pastagia M, Chiang T. Challenges to conducting a clinical trial of combination therapy of colistin and rifampicin for extensively drug-resistant Acinetobacter baumannii. Clin Infect Dis. 2014;58:141–2. doi: 10.1093/cid/cit630. [DOI] [PubMed] [Google Scholar]
- 60.Pogue JM, Kaye KS. Is there really no benefit to combination therapy with colistin? Expert Rev Anti Infect Ther. 2013;11:881–4. doi: 10.1586/14787210.2013.827881. [DOI] [PubMed] [Google Scholar]