Abstract
The objective of this study was to determine the comparative pharmacodynamics of four different carbapenems in combination with polymyxin B (PMB) against carbapenem-resistant Acinetobacter baumannii isolates using time–kill experiments at two different inocula. Two A. baumannii strains (03-149-1 and N16870) with carbapenem minimum inhibitory concentrations (MICs) ranging from 8 to 64 mg/L were investigated in 48-h time–kill experiments using starting inocula of 106 CFU/mL and 108 CFU/mL. Concentration arrays of ertapenem, doripenem, meropenem and imipenem at 0.25×, 0.5×, 1×, 1.5× and 2× published maximum serum concentration (Cmax) values (Cmax concentrations of 12, 21, 48 and 60 mg/L, respectively) were investigated in the presence of 1.5 mg/L PMB. Use of carbapenems without PMB resulted in drastic re-growth. All carbapenem combinations were able to achieve a ≥3 log10 CFU/mL reduction by 4 h against both strains at 106 CFU/mL, whereas maximum reductions against strain 03-149-1 at 108 CFU/mL were 1.0, 3.2, 2.2 and 3.3 log10 CFU/mL for ertapenem, doripenem, meropenem and imipenem, respectively. None of the combinations were capable of reducing 108 CFU/mL of N16870 by ≥2 log10 CFU/mL. Ertapenem combinations consistently displayed the least activity, whereas doripenem, meropenem and imipenem combinations had similar activities that were poorly predicted by carbapenem MICs. As doripenem, meropenem, or imipenem displayed similar pharmacodyanmics in combination, the decision of which carbapenem to use in combination with PMB may be based on toxicodynamic profiles if drastic discordance in MICs is not present.
Keywords: Acinetobacter baumannii, Carbapenem resistance, Polymyxins
1. Introduction
Acinetobacter baumannii is an invasive, opportunistic, Gram-negative pathogen responsible for an alarming rate of morbidity and mortality in the intensive care population [1]. Historically, carbapenems have been the most reliable treatment option for serious nosocomial A. baumannii infections. However, identification of the ideal carbapenem for combatting multidrug-resistant A. baumannii has been confounded by conflicting reports of minimum inhibitory concentration (MIC) testing and in vitro kill assessments. Whilst ertapenem reportedly possesses the least intrinsic activity [2], opposing studies have suggested that either doripenem [2], meropenem [3] or imipenem [4] is the most active against multidrug-resistant A. baumannii. Unfortunately, acquisition of carbapenem resistance mechanisms has further obscured the ideal treatment of A. baumannii.
To counter the increasing prevalence of carbapenem-resistant A. baumannii, clinicians are now forced to utilise a polymyxin [colistin or polymyxin B (PMB)] as a drug of last resort [5]. However, the emergence of colistin heteroresistance and the increasing frequency of polymyxin resistance have precipitated the search for polymyxin combinations that elicit greater bacterial killing than is possible with a polymyxin alone [6]. Enhanced activity against A. baumannii has been observed when a polymyxin is paired with a carbapenem in vitro [7], and successful use of carbapenem/polymyxin combinations has been reported clinically [8]. Although a meta-analysis of in vitro carbapenem/polymyxin killing identified meropenem and doripenem as the most likely candidates for enhancing polymyxin activity, the study results were based on rates of synergy and did not examine the rate and extent of killing for each combination [9]. It is also unknown whether the density of the A. baumannii inoculum influences selection of the optimal carbapenem. In the present study, we sought to characterise the comparative pharmacodynamics of each carbapenem in combination with PMB against carbapenem-resistant A. baumannii utilising time–kill experiments conducted at two different starting inocula.
2. Materials and methods
Two polymyxin-susceptible A. baumannii strains (N16870 and 03-149-1) were utilised in this study. Time–kill experiments were conducted over 48 h at starting inocula of 106 CFU/mL and 108 CFU/mL in cation-adjusted Mueller–Hinton broth as detailed previously [10]. Solutions of ertapenem, doripenem, meropenem and imipenem (Sigma Chemical Co., St Louis, MO) were freshly prepared on the day of each experiment. An array of five antibiotic concentrations was prepared for each carbapenem. The highest unbound maximum serum concentration (Cmax) resulting from the largest clinical dose reported in the package insert of each carbapenem was used to standardise the concentration of each agent to clinically achievable levels [11–14]. The other four concentrations used in the arrays corresponded to 0.25×, 0.5×, 1.5× and 2× the chosen Cmax value. The following carbapenem concentrations were investigated: ertapenem at 3, 6, 12, 18 and 24 mg/L; doripenem at 5.25, 10.5, 21, 31.5 and 42 mg/L; meropenem at 12, 24, 48, 72 and 96 mg/L; and imipenem at 15, 30, 60, 90 and 120 mg/L. Bactericidal activity was defined as a ≥3 log10 CFU/mL reduction within 24 h.
Carbapenem arrays were investigated in the presence of 1.5 mg/L PMB (Sigma Chemical Co.) to approximate the average free steady-state plasma concentration (Css) of PMB achieved by a 1.5 mg/kg every 12 h regimen proposed by Sandri et al [15]. PMB alone and the highest investigational carbapenem concentration alone served to control for the independent activity of each agent. Reaction vessels were incubated in a 37 °C water-bath with constant shaking and samples were collected at 0, 1, 2, 4, 8, 24, 26, 28, 32 and 48 h, were serially diluted with saline and were plated onto Mueller–Hinton agar. MIC testing was performed on 03-149-1 and N16870 in quadruplicate following Clinical and Laboratory Standards Institute (CLSI) guidelines M07-A10. Although both strains were susceptible to PMB (MIC of 0.5 mg/L for both isolates), the respective carbapenem MICs for 03-149-1 and N16870 were both 64 mg/L for ertapenem, 32 mg/L and 16 mg/L for doripenem, 64 mg/L and 16 mg/L for meropenem, and 32 mg/L and 8 mg/L for imipenem.
The log ratio change (Eq. 1) was calculated for the ertapenem, doripenem, meropenem and imipenem combinations that achieved the greatest A. baumannii killing [16]. As the log ratio change is only sensitive to A. baumannii counts at a discrete time and does not reflect the bacterial burden throughout the 48-h experiments, the log ratio area (Eq. 2) was also calculated for the most active carbapenem combinations to provide insight into antimicrobial activity achieved over the course of the entire experiment. The log ratio change and the log ratio area of each carbapenem combination were compared with one another for both A. baumannii strains at both starting inocula to determine differences in the rate and extent of killing for each combination.
| (1) |
| (2) |
where AUCFU is the area under the CFU–time curve.
3. Results
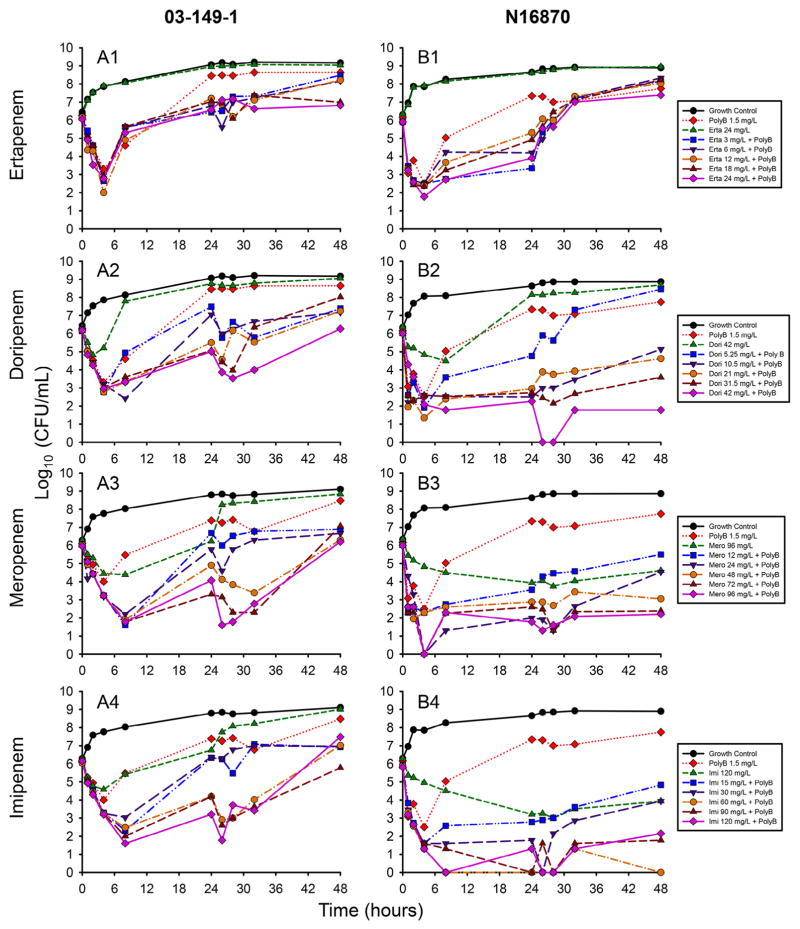
The results of the time–kill experiments at the 106 CFU/mL inoculum are displayed in Fig. 1. For strain 03-149-1, all four carbapenems were able to achieve a ≥3 log10 CFU/mL reduction by 6 h [Fig. 1(A1–A4)]. Ertapenem combinations displayed the least activity, with bacterial counts that began to recover by 8 h independent of the ertapenem concentration used in the combination (mean standard deviation 0.4 log10 CFU/mL). The majority of doripenem combinations also resulted in regrowth by 8 h, with a maximum reduction of 3.8 log10 CFU/mL by 8 h. Meropenem and imipenem combinations achieved sustained killing up to 8 h, with maximum reductions at 8 h of 4.5 log10 CFU/mL for both carbapenem combinations.
Fig. 1.
Time–kill experiments of (A) strain 03-149-1 and (B) strain N16870 at a starting inoculum of 106 CFU/mL. Five concentrations of ertapenem (A1/B1), doripenem (A2/B2), meropenem (A3/B3) and imipenem (A4/B4) were investigated with 1.5 mg/L polymyxin B (PolyB). The highest concentrations of each carbapenem alone and PolyB alone were investigated separately to account for the independent activity of each agent.
Despite relatively similar MICs to strain 03-149-1, carbapenem combinations achieved more drastic killing against 106 CFU/mL of N16870 [Fig. 1(B1–B4)]. Ertapenem combinations again displayed the least activity, with all five combinations ascending above 107 CFU/mL by 32 h. Both meropenem and imipenem alone were capable of sustained killing against N16870, albeit more slowly than in combination treatments, whilst doripenem alone regrew by 24 h. Doripenem, meropenem and imipenem combinations all displayed sustained killing that was dependent on the carbapenem concentration, with maximum reductions at the limit of detection (100 CFU/mL) for all three combinations. The lowest doripenem concentration of 5.25 mg/L was also the only concentration that resulted in complete regrowth by 48 h when in combination with PMB.
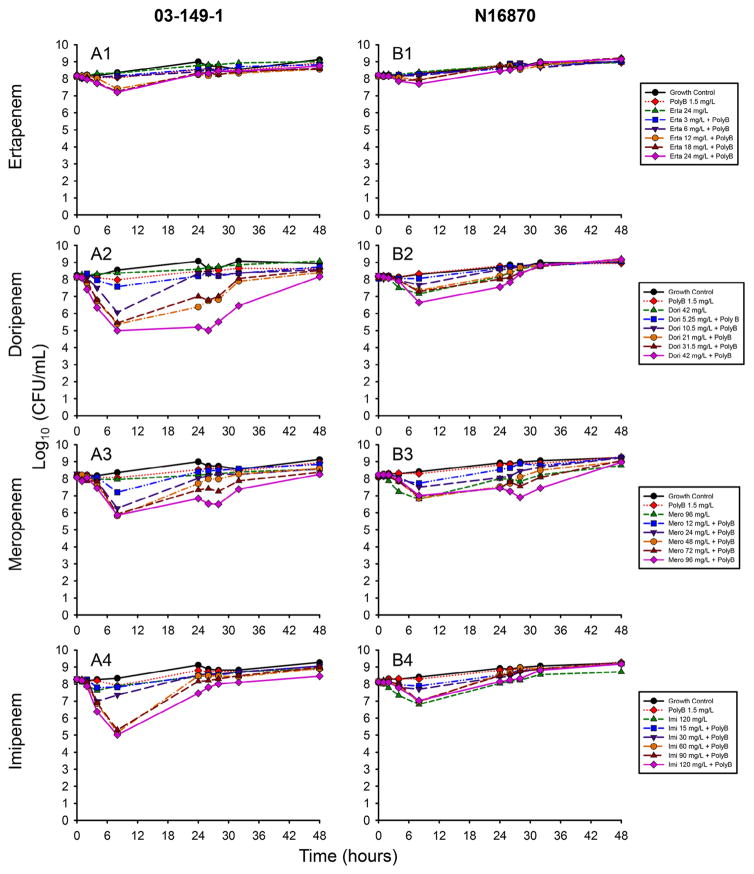
In contrast to the bactericidal activity observed at 106 CFU/mL, the majority of carbapenem combinations were unable to substantially reduce the 108 CFU/mL A. baumannii burden by 24 h (Fig. 2). The reductions in 03-149-1 counts at 8 h achieved by the highest concentration combinations were 1.0, 3.2, 2.2 and 3.3 log10 CFU/mL for ertapenem, doripenem, meropenem and imipenem, respectively [Fig. 2(A1–A4)]. The highest concentration of doripenem (42 mg/L) in combination with PMB resulted in the most sustained killing, with a 3.2 log10 CFU/mL reduction of 03-149-1 at 8 h that was maintained until 26 h.
Fig. 2.
Time–kill experiments of (A) strain 03-149-1 and (B) strain N16870 at a starting inoculum of 108 CFU/mL. Five concentrations of ertapenem (A1/B1), doripenem (A2/B2), meropenem (A3/B3) and imipenem (A4/B4) were investigated with 1.5 mg/L polymyxin B (PolyB). The highest concentrations of each carbapenem alone and PolyB alone were investigated separately to account for the independent activity of each agent.
Although killing of N16870 was more drastic compared with 03-149-1 at 106 CFU/mL, at the 108 CFU/mL inoculum N16870 was more tolerant to the carbapenem and PMB combinations [Fig. 2(B1–B4)]. At 8 h, maximum reductions achieved by carbapenem combinations were 0.5, 1.6, 1.2 and 1.1 log10 CFU/mL for ertapenem, doripenem, meropenem and imipenem, respectively. The only combination capable of sustained killing was 96 mg/L meropenem in combination with PMB, which resulted in a nadir of 6.5 log10 CFU/mL at 32 h followed by subsequent regrowth. PMB alone was unable to achieve sustained killing against either strain at either inoculum.
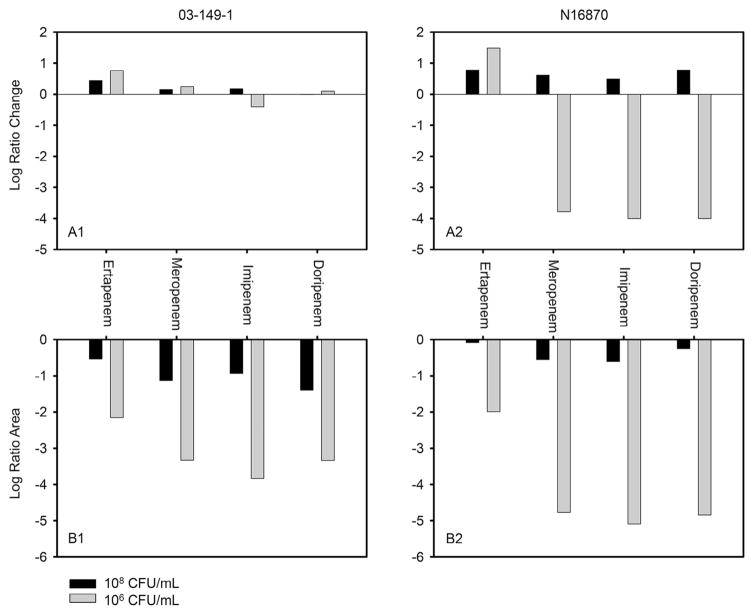
A comparison of the maximum activities displayed by all four carbapenem combinations expressed as either the log ratio area or log ratio change at 48 h is summarised in Fig. 3. After 48 h of antimicrobial exposure, none of the carbapenem combinations achieved substantial reductions in bacterial counts against strain 03-149-1 at either inoculum [Fig. 3(A1)]; however, the log ratio areas of doripenem, meropenem and imipenem combinations were ≥1.1 lower than the ertapenem combination at the 106 CFU/mL inoculum [Fig. 3(A2)]. Against the N16870 strain, both the log ratio change and the log ratio areas of all four carbapenem combinations were comparable at the 108 CFU/mL inoculum, whereas the log ratio change of the most active ertapenem combination was ≥5.2 higher than the other carbapenem combinations at 106 CFU/mL [Fig. 3(B1)]. Similarly, ertapenem with PMB achieved a log ratio area of −2.0 that was ≥2.7 higher than the other carbapenem combinations at 106 CFU/mL [Fig. 3(B2)].
Fig. 3.
(A) Log ratio change and (B) log ratio area after 48 h of exposure to the most active combinations of polymyxin B with either ertapenem, meropenem, imipenem or doripenem for strain 03-149-1 (A1/B1) and strain N16870 (A2/B2) at two different initial inocula.
4. Discussion
The ability of A. baumannii to acquire resistance mechanisms to commonly used antibiotics has made the pathogen particularly troubling in the nosocomial environment [1]. Here we sought to characterise the pharmacodynamics of four different carbapenems in combination with PMB at two different inocula to better understand the killing of antimicrobial combinations against carbapenem-resistant A. baumannii. Regardless of the inoculum, a PMB concentration of 1.5 mg/L was unable to achieve sustained killing for up to 48 h despite PMB MICs of 0.5 mg/L. When carbapenems were added to PMB, killing drastically improved, but was more pronounced at the 106 CFU/mL inoculum. The utility of colistin in combination with meropenem is currently being investigated in two clinical trials (NCT01732250 and NCT01597973) that will help illuminate the clinical utility of polymyxin combinations.
A previous hollow-fibre infection analysis of PMB alone against A. baumannii with susceptible PMB MICs also failed to eradicate the pathogen in vitro [17]. Despite rapid initial killing, polymyxin-resistant subpopulations amplified by over 5 log10 CFU/mL within 24 h of PMB exposure to allow for continued growth in the presence of PMB. In the present study, the rapid initial killing by PMB alone at the 106 CFU/mL inoculum was likely followed by a similar amplification of polymyxin-resistant subpopulations. When the A. baumannii was exposed to PMB and a carbapenem, PMB likely permeabilised the outer membrane of the bacterial cells and increased the accessibility of penicillin-binding proteins for the β-lactams [18].
Using the measures of log ratio area and log ratio change, doripenem, meropenem and imipenem combinations resulted in similar killing profiles against both A. baumannii strains. However, the use of only two A. baumannii isolates limits our ability to generalise the findings to other A. baumannii clinical strains. Similar to how ertapenem was previously identified as the least active agent against carbapenem-susceptible A. baumannii [2], ertapenem in combination with PMB displayed the least killing against both strains regardless of the inoculum. Interest in the utility of ertapenem against carbapenem-resistant Gram-negative organisms has risen following the reported use of ertapenem combination therapies targeted at carbapenem-resistant Klebsiella pneumoniae [19]. Although the results of the current investigation suggest that ertapenem may offer unfavourable pharmacodynamics in combination with PMB against carbapenem-resistant A. baumannii, dynamic in vitro models are needed to fully define the combinatorial pharmacodynamics of such a combination.
Not only were similar killing profiles observed for doripenem, meropenem and imipenem, but a large inoculum effect was consistently observed among the three carbapenem combinations as well. Killing was the most drastic against N16870 in both the log ratio area and log ratio change analyses at a 106 CFU/mL inoculum, yet the most substantial killing at a 108 CFU/mL inoculum was achieved against strain 03-149-2. The magnitude of the inoculum effect in carbapenem-resistant A. baumannii is therefore difficult to predict, as the attenuation in antimicrobial activity varied markedly between both strains. Given the similar declines in bacterial killing as the inoculum increased from 106 CFU/mL to 108 CFU/mL, the bacterial burden of A. baumannii may not influence whether doripenem, meropenem or imipenem is the ideal agent for polymyxin combination therapy.
Although both A. baumannii strains possessed different carbapenem MICs that varied by multiple dilutions, the MIC of each carbapenem was not able to fully predict the activity of each combination. When used in monotherapy, the prevailing dogma for β-lactams asserts that obtaining a sufficient %T>MIC (percentage of the dosing interval that the plasma level exceeds the MIC of the causative pathogen) is necessary for bactericidal activity [20]. However, the ability of PMB to perturb the integrity of both the outer and inner membranes of A. baumannii and increase carbapenem permeability is not accounted for by simply using the MIC of a carbapenem to predict the activity of combination treatment [18]. At a 106 CFU/mL inoculum, doripenem, meropenem and imipenem combinations utilising carbapenem concentrations below the MIC achieved bactericidal activity (0%T>MIC), suggesting that a concentration dependence exists during combination therapy that is not entirely accounted for by the %T>MIC metric.
A significant limitation of the current study is the use of static time–kill experiments that do not simulate the dynamic pharmacokinetics of carbapenems and PMB. Although doripenem, meropenem and imipenem all share a similar half-life of ca. 1 h in healthy volunteers [11,13,14], ertapenem possesses a half-life of 4 h that may confer additional bacterial killing in comparison with the other carbapenems [12]. Further work with dynamic in vitro models or in vivo animal models is needed to fully define the combinatorial pharmacodynamics of carbapenems in combination with PMB.
5. Conclusions
During A. baumannii time–kill experiments, ertapenem was the least active carbapenem in combination with PMB, whilst doripenem, meropenem and imipenem combinations displayed slight differences in their activities that were influenced by the A. baumannii strain and bacterial burden. Individual carbapenem MICs were also poorly predictive of how each carbapenem performed when in combination with PMB. Given the similar activities of doripenem, meropenem and imipenem when paired with PMB, the decision of which carbapenem to use for combination treatment may be driven by the toxicity profiles of each carbapenem if MICs are similar. Further investigations that use dynamic models to completely simulate the pharmacokinetics of carbapenems in combination with PMB are needed to better translate the findings into the clinical setting.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) [award no. R01AI111990]. CBL is an Australian National Health and Medical Research Council (NHMRC) Career Development Fellow. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RN, Sader HS, Fritsche TR. Comparative activity of doripenem and three other carbapenems tested against Gram-negative bacilli with various β-lactamase resistance mechanisms. Diagn Microbiol Infect Dis. 2005;52:71–4. doi: 10.1016/j.diagmicrobio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Ikonomidis A, Pournaras S, Maniatis AN, Legakis NJ, Tsakris A. Discordance of meropenem versus imipenem activity against Acinetobacter baumannii. Int J Antimicrob Agents. 2006;28:376–7. doi: 10.1016/j.ijantimicag.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Lesho E, Wortmann G, Moran K, Craft D. Fatal Acinetobacter baumannii infection with discordant carbapenem susceptibility. Clin Infect Dis. 2005;41:758–9. doi: 10.1086/432623. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Li W, Feng Y, Tao C. Efficacy and safety of polymyxins for the treatment of Acinetobacter baumannii infection: a systematic review and meta-analysis. PLoS ONE. 2014;9:e98091. doi: 10.1371/journal.pone.0098091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946–50. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pankey GA, Ashcraft DS. The detection of synergy between meropenem and polymyxin B against meropenem-resistant Acinetobacter baumannii using Etest and time–kill assay. Diagn Microbiol Infect Dis. 2009;63:228–32. doi: 10.1016/j.diagmicrobio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Shields RK, Kwak EJ, Potoski BA, Doi Y, Adams-Haduch JM, Silviera FP, et al. High mortality rates among solid organ transplant recipients infected with extensively drug-resistant Acinetobacter baumannii: using in vitro antibiotic combination testing to identify the combination of a carbapenem and colistin as an effective treatment regimen. Diagn Microbiol Infect Dis. 2011;70:246–52. doi: 10.1016/j.diagmicrobio.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57:5104–11. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenhard JR, von Eiff C, Hong IS, Holden PN, Bear MD, Suen A, et al. Evolution of Staphylococcus aureus under vancomycin selective pressure: the role of the small-colony variant phenotype. Antimicrob Agents Chemother. 2015;59:1347–51. doi: 10.1128/AAC.04508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangden T, Hickman RA, Forsberg P, Lagerback P, Giske CG, Cars O. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time–kill experiments. Antimicrob Agents Chemother. 2014;58:1757–62. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58:654–63. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souli M, Galani I, Boukovalas S, Gourgoulis MG, Chryssouli Z, Kanellakopoulou K, et al. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob Agents Chemother. 2011;55:2395–7. doi: 10.1128/AAC.01086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–13. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis. 2013;57:524–31. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji BT, von Eiff C, Kelchlin PA, Forrest A, Smith PF. Attenuated vancomycin bactericidal activity against Staphylococcus aureus hemB mutants expressing the small-colony-variant phenotype. Antimicrob Agents Chemother. 2008;52:1533–7. doi: 10.1128/AAC.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji BT, Landersdorfer CB, Lenhard JR, Cheah SE, Thamlikitkul V, Rao GG, et al. Paradoxical effect of polymyxin B: high drug exposure amplifies resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60:3913–20. doi: 10.1128/AAC.02831-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol. 2013;8:711–24. doi: 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cprek JB, Gallagher JC. Ertapenem-containing double-carbapenem therapy for treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2015;60:669–73. doi: 10.1128/AAC.01569-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am. 2003;17:479–501. doi: 10.1016/s0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]