Abstract
BACKGROUND
Recent attention to adverse effects of intensive care unit (ICU) sedation has led to the use of strategies that target a “lighter” depth of sedation. Among these strategies are “analgosedation” protocols, which prioritize pain management and preferentially use IV opioids before administration of continuously infused sedatives such as propofol or midazolam. We hypothesized that using an analgosedation protocol would result in a shorter duration of mechanical ventilation than a protocol with greater emphasis on IV sedatives
METHODS
We conducted a retrospective study comparing the duration of mechanical ventilation before and after implementation of an analgosedation protocol in a 24-bed medical ICU. Patients were aged 18 years or older and required mechanical ventilation where a light level of sedation was clinically appropriate. Exclusion criteria included a clinical need for deeper levels of sedation or tracheal intubation confined to the perioperative period.
RESULTS
Seventy-nine patients were included in the postimplementation group and 65 in the preimplementation group. After adjustment for baseline covariates, introduction of the 2013 analgosedation protocol was associated with a decreased duration of mechanical ventilation (−26.62 hours; 95% confidence interval, − 44.98 to −8.26, P = 0.005). Patients managed with the analgosedation protocol experienced a lighter level of sedation (median Richmond Agitation-Sedation Scale, −2.57 vs −1.25, P = 0.001) and improved pain management (median Critical-Care Pain Observation Tool score, 2.0 vs 1.5, P = 0.03). The use of continuously infused sedatives was reduced by 54.3% (92.3% vs 38.0%, P < 0.001).
CONCLUSIONS
Our findings suggest that implementation of an analgosedation protocol was associated with an overall lighter level of sedation, shorter mean ventilator duration, and a reduced use of continuous infusion sedatives. Further studies are needed to assess the impact of such protocols on ICU delirium.
Provision of analgesia and sedation is a core pharmacotherapeutic aspect of the care of critically ill patients. Patients in the intensive care unit (ICU) commonly require analgesia and sedation for treatment of pain, facilitation of mechanical ventilation, or management of acute agitation and psychoses. Pain is also a common experience in the ICU, with up to 77% of patients reporting moderate to severe pain during their ICU stay.1 Untreated pain can have short- and long-term consequences on patients, ranging from agitation and delirium, and may be associated with the development of acute stress disorders and posttraumatic stress disorder.2,3 This pain-related agitation or hyperactive delirium may in turn be treated with sedatives or antipsychotics. 3 Although symptomatic treatment of agitation may facilitate medical treatment, it does not address the underlying pain and delirium that is often the root cause of agitated behavior. The most recent iteration of the Society of Critical Care Medicine guidelines on pain, agitation, and delirium indicate that pain should be treated before addressing agitation or delirium.1
In addition to relieving discomfort, early treatment of pain with IV opioid agents may allow patients to reach a desired light level of sedation without the additional need for IV sedatives. This practice has been termed analgosedation and is a new paradigm in ICU sedation management.4 Using IV opioids to treat pain and reduce pain-related agitation before administration of IV sedatives seems intuitively reasonable and has been proposed in several published protocols and guidelines.1–6 However, the overuse of IV opioids may lead to prolonged respiratory depression, bowel dysmotility, and immunosuppression.7 Few studies have evaluated the benefit of a fentanyl-based protocol on outcomes in mechanically ventilated critically ill patients.8 The objective of this study was to evaluate the impact of an analgosedation protocol on duration of mechanical ventilation, ICU length of stay (LOS), sedation levels, and medication costs.
METHODS
Patient Selection
This retrospective cohort study was conducted among patients admitted to the medical ICU (MICU) at Texas Health Presbyterian Hospital of Dallas. This study was approved by the Texas Health Resources IRB. The hospital is a large, teaching, community hospital with a 24-bed MICU. The MICU has 24 hour per day, 7 days per week coverage by intensivists and mid-level practitioners and a patient-to-nurse ratio of 2:1. The study included adult MICU patients receiving mechanical ventilation between June 1, 2011, and December 1, 2011 (preimplementation group) and compared them with mechanically ventilated patients in the MICU between June 1, 2013, and December 1, 2013 (postimplementation group). These time frames were selected to allow for adequate education and uptake of the new analgosedation protocol and to minimize the influence of seasonal disease patterns (eg, influenza). Patients were identified from a patient log kept at the MICU and screened for inclusion criteria using an electronic medical record. Exclusion criteria included a diagnosis requiring a deeper level of sedation (eg, status epilepticus, therapeutic hypothermia, prone positioning, pressure control ventilation, or neuromuscular blockade use), death within 48 hours of hospital admission or moribund on admission, requirement of mechanical ventilation at an outside facility and were transferred on the ventilator, existing tracheostomy, or intubation during the immediate perioperative period (ie, intubated before the operating room and extubated within 24 hours postoperatively).
Intervention
Patients in the preimplementation group (2011 group) were managed using the MICU’s 2009 sedation policy and protocol for care of mechanically ventilated patients. This protocol emphasized evaluation of the Richmond Agitation-Sedation Scale (RASS) at least every 2 hours with a goal of RASS 0 to −2.9 The preferred sedation agent in this protocol was propofol, with analgesia management based on the Critical-Care Pain Observation Tool (CPOT) score.10 Patients remaining agitated (RASS +1 to +4) despite propofol and intermittent boluses of narcotic medication were started on infusions of IV narcotics (typically morphine) or a secondary sedative (eg, midazolam). Confusion Assessment Method for ICU monitoring and therapy were not routinely performed during the preimplementation period. In late 2012, a revised sedation protocol was implemented that focused on treating pain before sedative or antipsychotic use. This protocol targeted the same RASS score (−2 to 0) as during the preimplementation period (RASS between 0 and −2). The preferred agent in this algorithm was fentanyl administered as intermittent, IV boluses and, if needed, continuous infusion. For acute agitation, patients were managed first with an IV bolus of fentanyl and then intermittent midazolam, if needed. If patients remained agitated (RASS + 1 to + 4) despite as needed and continuous administration of narcotic analgesics, continuous infusions of propofol or dexmedetomidine were begun. Although RASS was the primary tool to determine administration of analgesia or sedation, the CPOT was also performed, and patients were provided analgesia as needed. In both preimplementation and postimplementation groups, daily awakening trials were performed once per shift, unless contraindicated by clinical status (eg, receiving neuromuscular blocking drugs, hemodynamically unstable, required positive end-expiratory pressure >10 cm H2O, or clinical requirement for deep sedation). Eligible patients were weaned from sedation and analgesia to achieve a RASS score of 0 to +1. Patients then underwent a spontaneous breathing trial and were liberated from mechanical ventilation when possible. If a patient could not be liberated from the ventilator, analgesia and sedative infusions were restarted at half the rate before the sedation interruptions.
Data Collected and Outcomes
Baseline data collected included age, sex, underlying pulmonary disease, pneumonia, or chronic obstructive pulmonary disease exacerbation as the initial diagnosis, an aspiration event preceding respiratory failure, chronic opioid use per medication history, and alcohol and illicit drug use. In addition, a Simplified Acute Physiology score (SAPS II) was calculated on all patients during the first 24 hours of mechanical ventilation to analyze the severity of illness.11 The primary outcome of the study was the duration of mechanical ventilation. Secondary outcomes included MICU LOS, RASS scores during mechanical ventilation, CPOT scores, sedative and analgesic medication use and costs, incidence of self-extubation, and mortality during hospital stay. Ventilator-free days were determined by counting the number of days off the ventilator in the first 28 days after ICU admission. This count was then compared for the overall group and censored for patients who died while on mechanical ventilation. Total analgesic and sedative doses were also recorded. Analgesic doses were converted to fentanyl equivalents (in micrograms) using the conversion 100 μg fentanyl = 1.5 mg IV hydromorphone = 10 mg IV morphine. Benzodiazepine doses were converted to midazolam equivalents (in milligrams) using the conversion 1 mg lorazepam = 2 mg midazolam.
Statistical Analyses
We hypothesized that introduction of an analgosedation protocol would result in a shorter duration of mechanical ventilation without an increase in self-extubation rates. Because this study enrolled all eligible patients (per inclusion/exclusion criteria) during the prespecified time period, an a priori sample size calculation was not performed. Categorical data were analyzed with the Fisher exact test using a 2 × 2 contingency table. RASS and CPOT scores were analyzed using the Shapiro-Wilk W test for normality. Nonnormal data were reported as medians with interquartile ranges and analyzed using Mann-Whitney U tests. Other continuous data were reported as means and SDs and were analyzed with a Student t test. Ninety-five percent confidence intervals (CIs) were constructed for these outcomes. All tests were 2 sided, and a P < 0.05 was considered statistically significant. Linear regression analysis with robust SE was conducted using ventilator hours as the variable of interest. Only single, discrete values were included in the regression model, and there were no multiple measurements over time. Results of the linear regression analysis are presented as regression coefficients with 95% CIs and P values. For continuous variables (age and SAPS II score), the regression coefficients represent the incremental change in ventilator hours with each incremental change in the covariate. Assumptions were assessed for the linear regression analysis and presented in the Supplemental Digital Content (http://links.lww.com/AA/B426). Statistics were analyzed with JMP® software (SAS Institute, Inc, Cary, NC) and STATA 14 (StataCorp LP, College Station, TX).
RESULTS
Patients
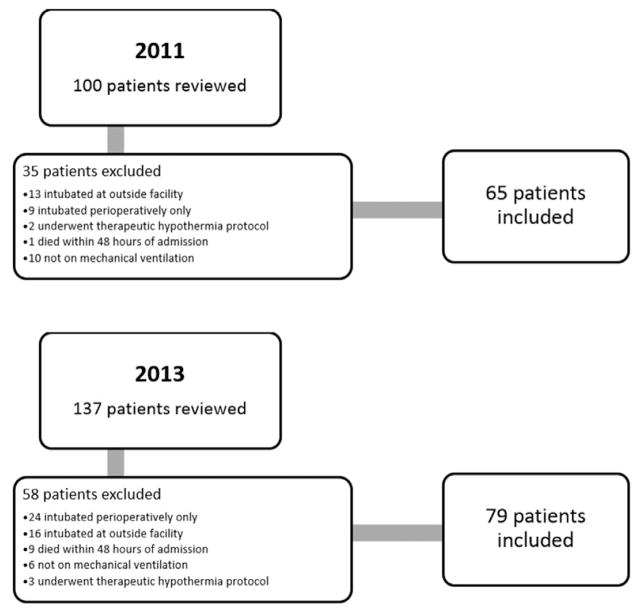
Two hundred thirty-seven patients were identified during the 2 study periods: 100 patients in the preimplementation group and 137 patients in the postimplementation group. After applying exclusion criteria, 65 patients in the preimplementation group (year 2011) and 79 patients in the postimplementation group (year 2013) were included (Figure 1). Aside from sex, baseline characteristics of the 2 groups were similar (Table 1). Median SAPS II scores were 62 in the 2011 group and 59 in the 2013 group (P = 0.28).
Figure 1.
Patient flow diagram.
Table 1.
Baseline Characteristics
| 2011 | 2013 | P Value | |
|---|---|---|---|
| Male patients, n (%) | 46 (70.7) | 40 (50.6) | 0.017 |
| Age (y) | |||
| Median | 65 | 63 | 0.89 |
| IQR | 55–73 | 54.5–75 | |
| Underlying pulmonary conditions, n (%) | 30 (46.1) | 37 (46.8) | 1.00 |
| Alcohol withdrawal, n (%) | 13 (20) | 11 (13.7) | 0.37 |
| Chronic opioid use, n (%) | 9 (13.8) | 5 (6.3) | 0.16 |
| Illicit drug use, n (%) | 6 (9.2) | 7 (8.9) | 0.77 |
| Pneumonia, n (%) | 11 (17.0) | 19 (24.0) | 0.31 |
| COPD exacerbation, n (%) | 5 (7.7) | 5 (6.4) | 0.75 |
| Aspiration event, n (%) | 7 (10.8) | 4 (5.1) | 0.22 |
| SAPSII score | |||
| Median | 62 | 59 | 0.28 |
| IQR | 51–72 | 50–69 | |
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SAPS, Simplified Acute Physiology score.
Outcomes
Results are summarized in Table 2. We found that patients in the 2013 group had an overall lighter level of sedation than those in the 2011 group. The median RASS during mechanical ventilation was 1.32 points higher in the 2013 group, suggesting that implementation of the analgosedation protocol correlated with lighter overall levels of sedation (median RASS, −2.57 vs −1.25, P = 0.001). The 2013 group also had a decrease in the mean percentage of RASS scores between −3 to −5 in the first 24 hours (46.8% ± 46.9% vs 27.3% ± 37.3%; P = 0.006; 95% CI, −33.3 to −5.6), and higher median CPOT scores while intubated were improved with the use of the analgosedation protocol (median, 2.0 vs 1.50, P = 0.03). The rates of self-extubation, tracheostomy creation, and mortality did not differ between the 2 groups (Table 2).
Table 2.
Results
| Preintervention (2011 Group) | Postintervention (2013 Group) | 95% CI | P Value | |
|---|---|---|---|---|
| Duration of mechanical ventilation (h), mean ± SD | 138.3 ± 132.6 | 92.9 ± 73.3 | −10.96 to −80.04 | 0.01 |
| ICU length of stay (h), mean ± SD | 211.5 ± 164.3 | 160.7 ± 125.7 | −98.62 to −3.02 | 0.038 |
| 28-d ventilator-free days, mean ± SD | 23.6 ± 4.9 | 24.1 ± 3.1 | −0.8 to 1.8 | 0.47 |
| 28-d ventilator-free days (mortalities censored), mean ± SD | 23.41 ± 5.51 | 24.57 ± 2.92 | −0.51 to 2.84 | 0.17 |
| RASS score, median (IQR) | −2.57 (−3.23 to −1.40) | −1.25 (−2.3 to −0.40) | 0.001 | |
| % of RASS scores −3 to −5 in first 24 h, mean ± SD | 46.8% ± 46.9% | 27.3% ± 37.3% | −33.3 to −5.6 | 0.006 |
| CPOT score, median (IQR) | 2 (1.1–2.75) | 1.5 (0.79–2.39) | 0.03 | |
| Self-extubation, n (%) | 2 (3.0) | 5 (5.9) | 0.46 | |
| Reintubation within 24 h of self-extubation, n (%) | 1 (50) | 2 (40) | 1.00 | |
| Tracheostomy, n (%) | 11 (16.9) | 8 (10.1) | 0.32 | |
| Hospital mortality, n (%) | 22 (33.8) | 24 (30) | 0.72 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; CPOT, Critical-Care Pain Observation Tool; ICU, intensive care unit; IQR, interquartile range; RASS, Richmond Agitation-Sedation Scale; SAPS, Simplified Acute Physiology score.
In unadjusted comparisons, patients managed in 2013 with the analgosedation protocol required an average of 45.5 fewer hours of mechanical ventilation (92.9 ± 73.3 vs 138.3 ± 132.6 hours; P = 0.01; 95% CI, −10.96 to −80.04). Mean ICU LOS was 50.8 hours shorter in the 2013 cohort (160.7 ± 125.7 vs 211.5 ± 164.3 hours; P = 0.038; 95% CI, −98.62 to −3.02). The number of 28-day ventilator-free days between the groups was similar (24.1 ± 3.1 in 2013 vs 23.6 ± 4.9 in 2011; P = 0.47; 95% CI, −0.8 to 1.8). This finding did not change after excluding patients who died while on the ventilator (24.57 in 2013 ± 2.92 vs 23.41 in 2011 ± 5.51 days; P = 0.17; 95% CI, −0.51 to 2.84).
In linear regression analysis, the use of the 2013 analgosedation protocol remained associated with a reduction in duration of mechanical ventilation (−26.62; 95% CI, −44.98 to −8.26, P = 0.005, Table 3). The type of admission was also correlated with the length of mechanical ventilation, although planned surgery was omitted for collinearity. Other covariables were not associated with duration of mechanical ventilation.
Table 3.
Linear Regression Analysis on Ventilator Hours
| Regression Coefficient | 95% CI | P Value | |
|---|---|---|---|
| Use of analgosedation protocol (year 2011 vs 2013) | −26.62 | −44.98 to −8.26 | 0.005 |
| Prior lung disease | |||
| °COPD | −0.23 | −58.74 to 58.27 | 0.994 |
| °Asthma | 60.71 | −30.72 to 152.14 | 0.191 |
| °Other underlying lung disease | 31.54 | −12.31 to 75.40 | 0.157 |
| Type of admission | |||
| °Medical | 44.17 | 0.20 to 88.14 | 0.049 |
| °Emergency surgery | 105.06 | 11.54 to 198.59 | 0.028 |
| Reason for intubation | |||
| °Congestive heart failure | 13.10 | −59.11 to 85.32 | 0.720 |
| °Pneumonia | 37.36 | −10.20 to 84.92 | 0.123 |
| °Aspiration event | −19.49 | −81.99 to 46.01 | 0.538 |
| °Acute exacerbation of COPD | −7.15 | −67.87 to 53.57 | 0.816 |
| Baseline characteristics | |||
| °Age | −0.822 | −2.33 to 0.69 | 0.284 |
| °SAPS II score | 0.54 | −0.55 to 1.62 | 0.328 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; SAPS, Simplified Acute Physiology score.
Doses of sedative and analgesic medication used during mechanical ventilation are summarized in Table 4. In general, use of the analgosedation protocol resulted in more fentanyl and fentanyl-equivalents used and less propofol. There were more patients given dexmedetomidine as a sedative in the 2013 cohort (3.08% vs 13.92%; P = 0.037), although the amount of dexmedetomidine used per patient during the 2 time periods was not different (58.46 vs 214.56 μg; P = 0.23). The use of other sedative agents did not differ between groups, although most benzodiazepines in the 2013 period were given by intermittent IV push rather than IV infusion.
Table 4.
Total Analgesic and Sedative Medication Use During Mechanical Ventilation
| Medication | Preintervention (2011)
|
Postintervention (2013)
|
P Value (per Patient Average) | ||
|---|---|---|---|---|---|
| Total Use (μg of Fentanyl Equivalents) | Per Patient Use (μg of Fentanyl Equivalents) | Total Use (μg of Fentanyl Equivalents) | Per Patient Use (μg of Fentanyl Equivalents) | ||
| Analgesics | |||||
| Fentanyl | 15,950 | 245.38 | 586,650 | 7425.95 | |
| Hydromorphone | 1933.33 | 29.74 | 7000 | 88.61 | |
| Morphine | 75,470 | 1161.1 | 8080 | 102.28 | |
| Total fentanyl equivalents | 93,353.33 | 1436.21 | 601,730 | 7516.84 | <0.001 |
| Total Use (mg of Midazolam Equivalents) | Per Patient Use (mg of Midazolam Equivalents) | Total Use (mg of Midazolam Equivalents) | Per Patient Use (mg of Midazolam Equivalents) | ||
| Benzodiazepines | |||||
| Lorazepam | 573.5 | 8.82 | 785.75 | 9.95 | |
| Midazolam | 9379 | 144.29 | 13,378 | 169.34 | |
| Total midazolam equivalents | 9952.5 | 153.12 | 14,163.75 | 179.29 | 0.80 |
| Total Use | Per Patient Use | Total Use | Per Patient Use | ||
| Nonbenzodiazepine sedatives | |||||
| Dexmedetomidine (μg) | 3800 | 58.46 | 16950 | 214.56 | 0.23 |
| Propofol (mg) | 922,500 | 14,192.31 | 118,750 | 1503.17 | <0.001 |
| Ketamine (mg) | 15,500 | 238.46 | 9000 | 113.92 | 0.54 |
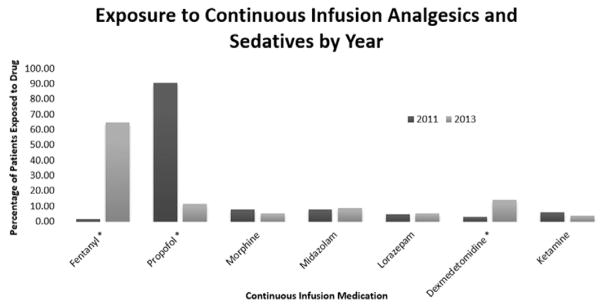
Figure 2 shows the differences in medication use for continuous sedative and analgesic infusions used in the ICU. Fewer patients were managed with continuous propofol infusions in 2013 than 2011 (92.3% vs 13.9%, P < 0.0001), and more patients were managed with continuous fentanyl infusions in 2013 than 2011 (20.0% vs 65.8%, P < 0.0001). Although 92.3% of patients in the preimplementation group were exposed to continuous infusions of sedatives (ie, propofol, dexmedetomidine, midazolam, lorazepam, or ketamine), only 38.0% of patients postimplementation required these medications (P < 0.001). The percentage of patients exposed to both sedative and analgesic infusions did not differ between groups (92.3% preimplementation compared with 83.5% postimplementation, P = 0.13). Use of the analgosedation protocol resulted in an average per patient medication cost of $293.98 ± $641.37 compared with $519.05 ± $880.53 with the 2011 sedation protocol (difference, 225.07; P = 0.0786; 95% CI, −476.18 to 26.04).
Figure 2.
Percentage of patients exposed to continuous infusion medications. Medications marked with an asterisk were statistically significant (P < 0.05).
DISCUSSION
Analgosedation and targeting light levels of sedation are new paradigms in ICU management of patients on mechanical ventilation and form the basis for our current sedation practice.4 Our study demonstrated that an analgosedation protocol was associated with a lighter overall level of sedation than a previous sedative-based strategy. In addition, we found a shorter duration of mechanical ventilation time and ICU LOS. The study also demonstrated a significant decrease in the use of continuous infusion sedative agents, a significant increase in analgesics, and a potential reduction in associated medication costs.
Because blinding caregivers to specific sedation regimens is difficult, studies of analgosedation are challenging to perform. A 2012 review and critique of analgosedation studies found that analgosedation appears to be well tolerated and may improve patient outcomes.4 However, analgosedation studies have also been criticized for study design, analgesic selection, or feasibility. A 2010 trial5 assessing the impact of a “no sedation” scheme randomized patients to either continuous infusions of propofol or midazolam or to an analgesia-first method of morphine boluses only. Although the study demonstrated that patients in the “no sedation” arm experienced a shorter duration of mechanical ventilation and shorter ICU LOS, this study had several limitations. The choice of morphine for analgosedation has been associated with more frequent incidences of delirium, hypotension, anticholinergic effects, and renal impairment,12 which may have contributed to the higher incidence of delirium in the “no sedation” arm (20% vs 7%). This increase in delirium may also have been because of difficulty in detecting hypoactive delirium in more sedated patients. Finally, the use of 1:1 nursing during this study raises the question of whether such a strategy is feasible in other critical care settings.
A more recent 2014 retrospective study evaluated patients who required either continuous infusion fentanyl or continuous infusion propofol for management of agitation and sedation while on mechanical ventilation.8 Fentanyl infusion use reduced the need for rescue analgesia compared with propofol. Both duration of mechanical ventilation and frequency of gastrointestinal side effects were similar between groups. Our study differs from this one in several ways. We did not mandate patients be managed with continuous infusions. With implementation of our analgesia-first protocol, we successfully managed 16.5% of patients with only intermittent, IV boluses of fentanyl and midazolam.
In contrast to our study, many other analgosedation studies have used remifentanil, a short-acting opioid agonist.4,6,13,14 Trials comparing remifentanil with a sedative-hypnotic have demonstrated acceptable safety and a shortened duration of mechanical ventilation.4,6 However, remifentanil must be administered through continuous infusion, is associated with rebound hyperalgesia, and is more costly than other agents.13 A single study has compared remifentanil with fentanyl and found comparable outcomes between the agents.14
Another criticism of analgosedation studies has been the inconsistent use of daily sedation interruption. Although early studies found benefit to this strategy,15 a 2012 multicenter trial of 430 mechanically ventilated adults demonstrated that when compared with light levels of sedation, daily sedative interruption did not affect the time to successful extubation, duration of ICU or hospital LOS, or rates of delirium.16 Sedation interruption was an element of our sedation strategy in both 2011 and 2013 groups, possibly explaining why we did not observe reductions in LOS or duration of mechanical ventilation. With our protocol, we achieved a significantly lighter level of sedation and a significant reduction in oversedation during the first day of ICU care, which has been associated with improved outcomes in mechanically ventilated patients.17,18
Our study has several strengths. To maximize generalizability, our retrospective study was conducted in the MICU of a community, teaching hospital and did not interfere with clinical care. Our protocol was a multidisciplinary product of collaboration between clinical pharmacy, intensive care physicians, and ICU nursing staff. We did not employ specific “sedation” nurses or research staff. Before new protocol implementation, nurses, physicians, and other critical care providers were reeducated on the utility of RASS levels, titration of continuous infusion medications, and appropriate documentation. The study was conducted 6 months after implementation to ensure uniformity of practices among different health care providers. The institution used both RASS and CPOT, 2 of the validated, recommended tools for assessing pain and agitation in the ICU.1 We did not change the management of ventilator patients between time periods, including spontaneous awakening and spontaneous breathing trials. In addition, multiple baseline characteristics were similar between the 2 groups, limiting the potential effect of confounding factors such as severity of illness, underlying pulmonary diseases, and previous exposure to opioids and benzodiazepines.
Our study also had several limitations. Because it was performed in the MICU, our findings may not be generalizable to patients in other settings such as the surgical ICU, trauma ICU, or long-term ventilator care units. The inherent noncontrolled nature of a retrospective study also raises the possibility of confounding variables, which may have influenced study outcomes. It is possible that changes to practice from 2011 to 2013 may have affected our results, although we found that only the analgosedation protocol and type of admission were associated with duration of mechanical ventilation in the linear regression model. Although our protocol allowed for lighter levels of sedation with the use of an analgesia-first strategy, other factors including improved pain control may have affected the duration of mechanical ventilation. Thus, our findings should be considered hypothesis generating only, and randomized, controlled studies are needed to validate our results. We also did not assess the incidence of delirium between 2011 and 2013 because the Confusion Assessment Method for ICU was not fully implemented until after the 2011 study period. However, based on the guidelines and existing literature, managing pain and agitation with an analgosedation protocol is likely to reduce exposure to sedative-hypnotics, increase the rates of early mobilization of mechanically ventilated patients, and thus decrease the incidence of iatrogenic-induced delirium.
CONCLUSIONS
This study demonstrated that implementation of an analgesia-based sedation protocol, primarily with fentanyl, was correlated with lighter sedation levels, better pain management, and reduced both average duration of mechanical ventilation and ICU LOS. Further prospective, randomized studies are needed to fully elucidate the potential role of analgosedation in the critical care of ventilated patients.
Footnotes
Funding: None.
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES
Name: Andrew C. Faust, PharmD, BCPS.
Contribution: This author helped design the study, conduct the study, analyze the data, and prepare the manuscript, and is the archival author.
Name: Pearl Rajan, PharmD, BCPS.
Contribution: This author helped design the study, conduct the study, analyze the data, and prepare the manuscript.
Name: Lyndsay A. Sheperd, PharmD, BCPS.
Contribution: This author helped design the study, analyze the data, and prepare the manuscript.
Name: Carlos A. Alvarez, PharmD, MSc, MSCS, BCPS.
Contribution: This author helped analyze the data and prepare the manuscript.
Name: Phyllis McCorstin, APRN, CNS.
Contribution: This author helped design the study, conduct the study, and prepare the manuscript.
Name: Rebecca L. Doebele, MD.
Contribution: This author helped design the study and prepare the manuscript.
This manuscript was handled by: Avery Tung, MD.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
References
- 1.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 2.Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111:451–63. doi: 10.1213/ANE.0b013e3181d7e1b8. [DOI] [PubMed] [Google Scholar]
- 3.Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370:444–54. doi: 10.1056/NEJMra1208705. [DOI] [PubMed] [Google Scholar]
- 4.Devabhakthuni S, Armahizer MJ, Dasta JF, Kane-Gill SL. Analgosedation: a paradigm shift in intensive care unit sedation practice. Ann Pharmacother. 2012;46:530–40. doi: 10.1345/aph.1Q525. [DOI] [PubMed] [Google Scholar]
- 5.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475–80. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 6.Rozendaal FW, Spronk PE, Snellen FF, Schoen A, van Zanten AR, Foudraine NA, Mulder PG, Bakker J UltiSAFE Investigators. Remifentanil-propofol analgo-sedation shortens duration of ventilation and length of ICU stay compared to a conventional regimen: a centre randomised, cross-over, open-label study in the Netherlands. Intensive Care Med. 2009;35:291–8. doi: 10.1007/s00134-008-1328-9. [DOI] [PubMed] [Google Scholar]
- 7.Afsharimani B, Cabot P, Parat MO. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011;30:225–38. doi: 10.1007/s10555-011-9285-0. [DOI] [PubMed] [Google Scholar]
- 8.Tedders KM, McNorton KN, Edwin SB. Efficacy and safety of analgosedation with fentanyl compared with traditional sedation with propofol. Pharmacotherapy. 2014;34:643–7. doi: 10.1002/phar.1429. [DOI] [PubMed] [Google Scholar]
- 9.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 10.Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15:420–7. [PubMed] [Google Scholar]
- 11.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 12.Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. 2012;185:486–97. doi: 10.1164/rccm.201102-0273CI. [DOI] [PubMed] [Google Scholar]
- 13.Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, Chauvin M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–55. doi: 10.1097/00000542-200507000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Muellejans B, López A, Cross MH, Bonome C, Morrison L, Kirkham AJ. Remifentanil versus fentanyl for analgesia based sedation to provide patient comfort in the intensive care unit: a randomized, double-blind controlled trial [ISRCTN43755713] Crit Care. 2004;8:R1–R11. doi: 10.1186/cc2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, Jackson JC, Canonico AE, Light RW, Shintani AK, Thompson JL, Gordon SM, Hall JB, Dittus RS, Bernard GR, Ely EW. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–34. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 16.Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton J, Herridge M, Ferguson N, Devlin J, Tanios M, Dodek P, Fowler R, Burns K, Jacka M, Olafson K, Skrobik Y, Hébert P, Sabri E, Meade M SLEAP Investigators; Canadian Critical Care Trials Group. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308:1985–92. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 17.Shehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, Tan MA, Khoo TM, Ali SB, Saman MA, Shaltut A, Tan CC, Yong CY, Bailey M Sedation Practice in Intensive Care Evaluation (SPICE) Study Group investigators. Sedation depth and longterm mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39:910–8. doi: 10.1007/s00134-013-2830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, McArthur C, Seppelt IM, Webb S, Weisbrodt L Sedation Practice in Intensive Care Evaluation (SPICE) Study Investigators; ANZICS Clinical Trials Group. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–31. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]