Abstract
Zika virus (ZIKV) is responsible for a major ongoing epidemic in the Americas and has been causally associated with fetal microcephaly. The development of a safe and effective ZIKV vaccine is therefore an urgent global health priority. Here we demonstrate that three different vaccine platforms protect against ZIKV challenge in rhesus monkeys. A purified inactivated virus vaccine induced ZIKV-specific neutralizing antibodies and completely protected monkeys against ZIKV strains from both Brazil and Puerto Rico. Purified immunoglobulin from vaccinated monkeys conferred passive protection in adoptive transfer studies. A plasmid DNA vaccine and a single-shot recombinant rhesus adenovirus serotype 52 vector expressing ZIKV prM-Env also elicited neutralizing antibodies and completely protected monkeys against ZIKV challenge. These data support the rapid clinical development of ZIKV vaccines for humans.
The explosive and unprecedented ZIKV outbreak in the Americas (1, 2) prompted the World Health Organization to declare this epidemic a public health emergency of international concern. ZIKV has been causally associated with fetal microcephaly, intrauterine growth retardation, and other congenital malformations in both humans (3–6) and mice (7–9), and has also been linked with neurologic disorders such as Guillain-Barre syndrome (10). Several reports have shown that ZIKV can infect placental and fetal tissues, leading to prolonged viremia in pregnant women (11) and nonhuman primates (12). ZIKV also appears to target cortical neural progenitor cells (7–9, 13, 14), which likely contributes to neuropathology.
We recently reported the protective efficacy of a ZIKV purified inactivated virus (PIV) vaccine from strain PRVABC59 and a DNA vaccine expressing an optimized pre-membrane and envelope (prM-Env) immunogen from strain BeH815744 against ZIKV challenges in mice (15). These studies utilized ZIKV challenge strains from both Brazil (ZIKV-BR; Brazil/ZKV2015) (9) and Puerto Rico (ZIKV-PR; PRVABC59). ZIKV replication in mice was dependent on the mouse strain (15) and may be less extensive than in nonhuman primates (12). We therefore evaluated the immunogenicity and protective efficacy of inactivated virus, gene-based, and vector-based vaccines in ZIKV challenge studies in rhesus monkeys.
ZIKV PIV Vaccine Study
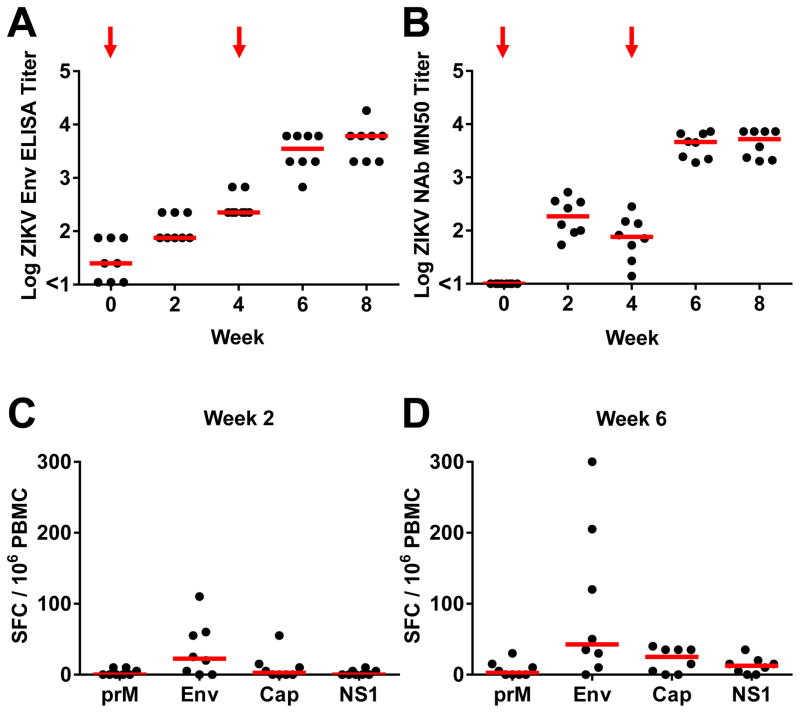
We first immunized 16 rhesus monkeys by the subcutaneous route with 5 μg ZIKV PIV vaccine with alum (N=8) or sham vaccine (alum only) (N=8) at weeks 0 and 4 (Fig. S1). All PIV vaccinated animals developed ZIKV Env-specific binding antibodies by ELISA as well as ZIKV-specific neutralizing antibodies by microneutralization (MN50) assays at week 2 following initial immunization. Median log antibody titers at week 2 were 1.87 by ELISA (Fig. 1A) and 2.27 by MN50 assays (Fig. 1B). Following the week 4 boost immunization, median log antibody titers increased substantially to 3.54 by ELISA (Fig. 1A) and 3.66 by MN50 assays (Fig. 1B) at week 6. In contrast, sham control monkeys did not develop detectable ZIKV-specific antibody responses (Fig. S2). Binding antibody titers correlated with neutralizing antibody titers in the PIV vaccinated animals (P<0.0001, R=0.88, Spearman rank correlation test; Fig. S3), although only minimal antibody-dependent cellular phagocytosis responses were observed. The majority of PIV vaccinated monkeys (Figs. 1C–D) but not sham control animals (Fig. S4) also developed modest cellular immune responses, primarily to Env, as measured by interferon (IFN)-γ ELISPOT assays.
Figure 1. Immunogenicity of the ZIKV PIV vaccine.
(A) Env-specific ELISA titers and (B) ZIKV-specific microneutralization (MN50) titers following immunization of rhesus monkeys by the s.c route with 5 μg ZIKV PIV vaccine at weeks 0 and 4 (red arrows). The maximum measurable log MN50 titer in this assay was 3.86. Cellular immune responses by IFN-γ ELISPOT assays to prM, Env, Cap, and NS1 at (C) week 2 and (D) week 6. Red bars reflect medians.
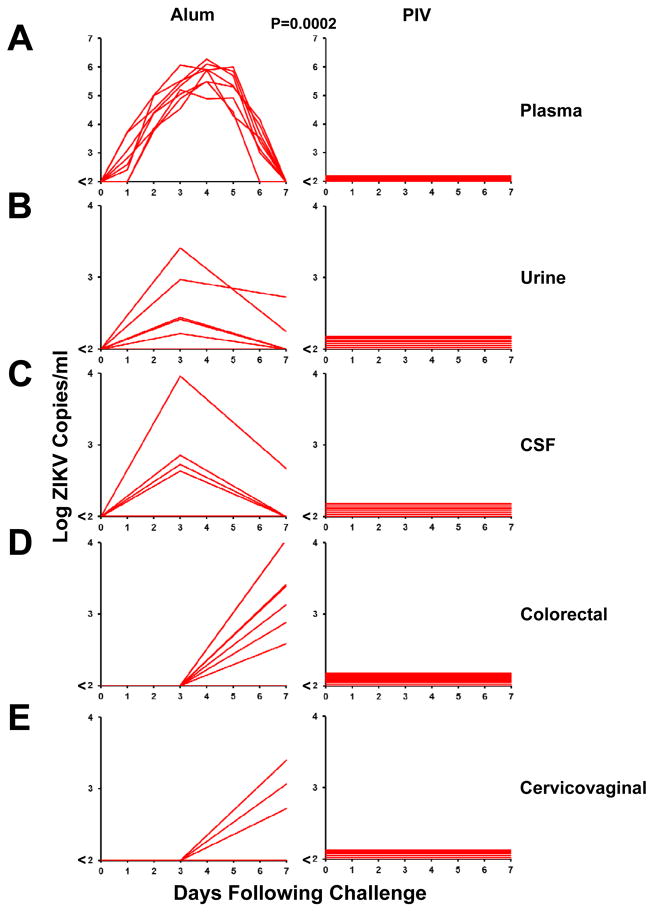
To assess the protective efficacy of the PIV vaccine against ZIKV challenge, we infected PIV immunized and sham control monkeys by the subcutaneous route with 106 viral particles (VP) [103 plaque-forming units (PFU)] of ZIKV-BR or ZIKV-PR (N=4/group) (15). Viral loads following ZIKV challenge were quantitated by RT-PCR (15), and viral infectivity was confirmed by growth in Vero cells. ZIKV-specific MN50 titers increased following challenge, particularly in the sham controls (Fig. S5). Sham control monkeys exhibited 6–7 days of detectable viremia with median peak viral loads of 5.82 log copies/ml (range 5.21–6.29 log copies/ml; N=8) on day 3–5 following challenge (Fig. 2A). Virus was also detected in the majority of sham control animals in urine and cerebrospinal fluid (CSF) on day 3, as well as in colorectal secretions and cervicovaginal secretions on day 7 (Fig. 2B–E). In contrast, PIV vaccinated monkeys showed complete protection against ZIKV challenge, as evidenced by no detectable virus (<100 copies/ml) in blood, urine, CSF, colorectal secretions, and cervicovaginal secretions in all animals following challenge (N=8; P=0.0002, Fisher’s exact test comparing PIV vaccinated animals vs. sham controls). We were unable to assess ZIKV in semen in the male animals in this study due to inadequate sample volumes. No major differences in plasma viral loads were observed between the sham controls that received ZIKV-BR vs. ZIKV-PR (Fig. S6).
Figure 2. Protective efficacy of the ZIKV PIV vaccine.
PIV vaccinated and sham control rhesus monkeys (N=8/group) were challenged by the s.c route with 106 VP (103 PFU) ZIKV-BR or ZIKV-PR. Each group contained 6 female and 2 male animals. Viral loads are shown in (A) plasma, (B) urine, (C) CSF, (D) colorectal secretions, and (E) cervicovaginal secretions. Viral loads were determined on days 0, 1, 2, 3, 4, 5, 6, 7 for the plasma samples (A) and on days 0, 3, 7 for the other samples (B–E). Data is shown for all 8 animals in each panel, except for the 6 females for cervicovaginal secretions in (E). P-value reflects Fisher’s exact test.
Adoptive Transfer Studies
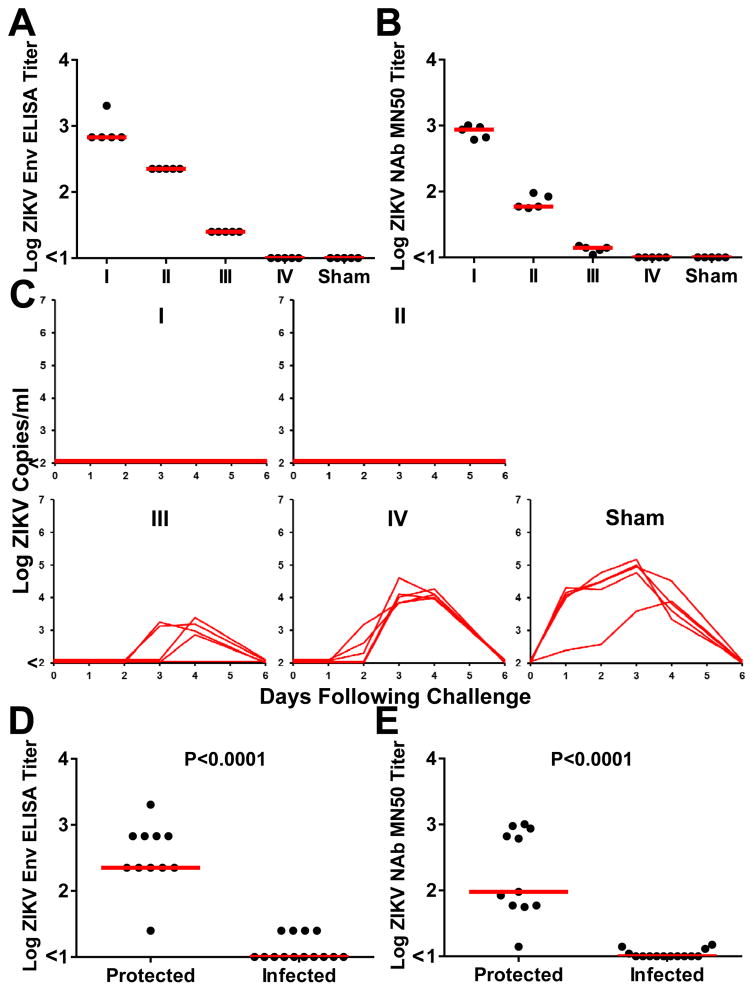
We next explored the mechanism of the observed protection by adoptive transfer studies. We purified IgG from plasma from ZIKV PIV vaccinated monkeys at week 8 by protein G affinity chromatography. Vaccine-elicited, ZIKV-specific IgG was then infused into four groups of naïve Balb/c mice (N=5/group) by 5-fold serial dilutions of the purified IgG preparation, which had a log ELISA titer of 3.30 and a log MN50 titer of 3.30. Following infusion, these groups of recipient mice (designated I, II, III, IV) had median log ELISA titers of 2.83, 2.35, 1.40, and <1.00 (Fig. 3A) and median log MN50 titers of 2.93, 1.77, 1.14, and <1.00 (Fig. 3B). Mice were then challenged by the intravenous route with 105 VP (102 PFU) of ZIKV-BR, as we previously described (15). The higher two doses of purified IgG provided complete protection following ZIKV challenge, whereas the lower two doses of purified IgG resulted in reduced viremia as compared with sham infused control mice (Fig. 3C–E).
Figure 3. Adoptive transfer studies in mice.
(A) Env-specific serum ELISA titers and (B) ZIKV-specific microneutralization (MN50) titers in serum from recipient Balb/c mice (N=5/group) 1 hour following adoptive transfer of 5-fold serial dilutions (Groups I, II, III, IV) of IgG purified from PIV vaccinated rhesus monkeys or sham controls. (C) Plasma viral loads in mice following challenge with 105 VP (102 PFU) ZIKV-BR. (D, E) Immune correlates of protection. Red bars reflect medians. P-values reflect t-tests.
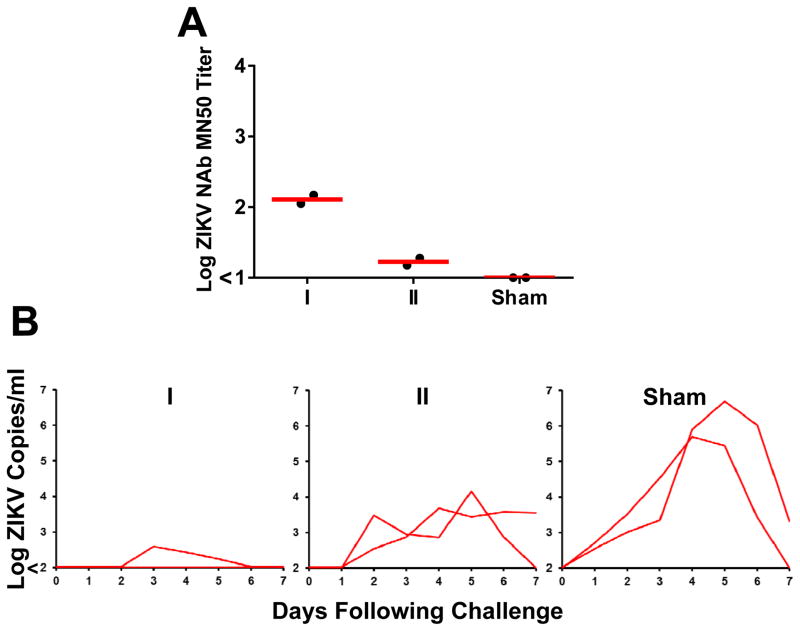
Vaccine-elicited, ZIKV-specific IgG was also infused into two groups of naïve rhesus monkeys (N=2/group). Following infusion, these groups of recipient monkeys (designated I, II) had median log MN50 titers of 2.11 and 1.22 (Fig. 4A). Monkeys were then challenged with 106 VP (103 PFU) of ZIKV-BR. In the animals that received the higher IgG dose, one animal was completely protected and the other showed a blip of viremia on days 3–5 (Fig. 4B). No enhancement of viral replication was observed at subtherapeutic IgG concentrations. Taken together, these data demonstrate that purified IgG from ZIKV PIV vaccinated rhesus monkeys provided passive protection following adoptive transfer in both rodents and primates.
Figure 4. Adoptive transfer studies in rhesus monkeys.
(A) ZIKV-specific microneutralization (MN50) titers in serum from recipient rhesus monkeys (N=2/group) 1 hour following adoptive transfer of 5-fold dilutions (Groups I, II) of IgG purified from PIV vaccinated rhesus monkeys or sham controls. (B) Plasma viral loads in rhesus monkeys following challenge with 106 VP (103 PFU) ZIKV-BR. Red bars reflect medians.
ZIKV DNA and RhAd52 Vaccine Study
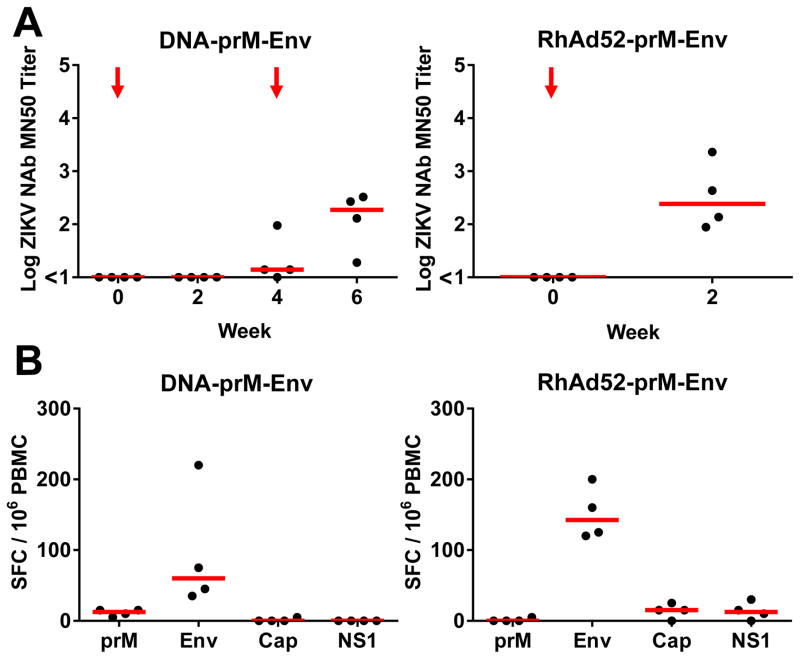
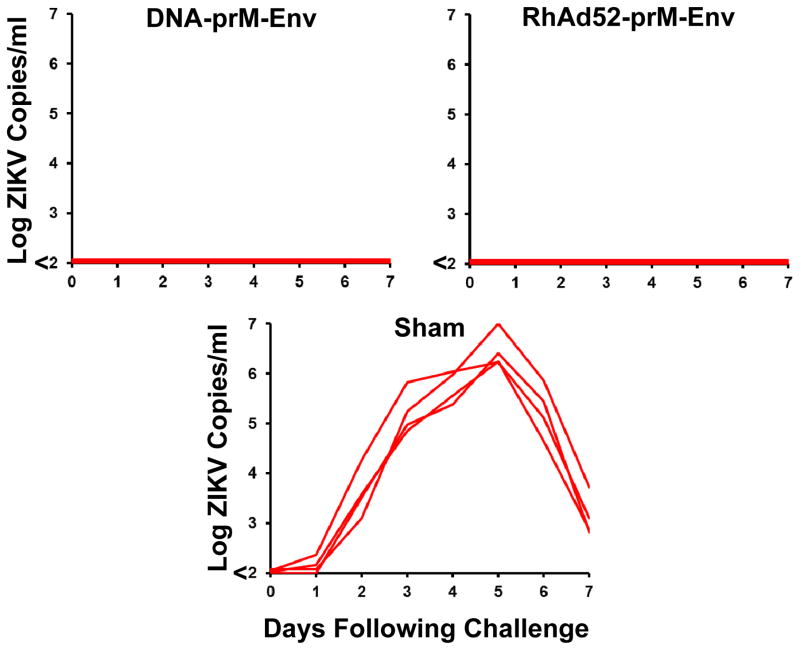
To evaluate the immunogenicity and protective efficacy of gene-based and vector-based ZIKV vaccines, we immunized 12 rhesus monkeys with a plasmid DNA vaccine (15) or a rhesus adenovirus serotype 52 (RhAd52) vector-based vaccine (16) (Fig. S1). Monkeys were immunized by the intramuscular route with 5 mg DNA vaccine expressing prM-Env at weeks 0 and 4 (N=4), a single immunization of 1011 VP RhAd52 vector expressing prM-Env at week 0 (N=4), or sham vaccine (N=4). The DNA-prM-Env vaccine induced ZIKV-specific neutralizing antibody titers in all animals after the week 4 boost immunization, although only minimal MN50 titers were detected after the initial priming immunization (Fig. 5A). In contrast, the RhAd52-prM-Env vaccine induced ZIKV-specific neutralizing antibody responses in all animals at week 2 after the initial priming immunization (Fig. 5A). Moreover, the RhAd52 vector induced substantial breadth of antibody responses against linear ZIKV Env epitopes by peptide microarray assays as compared to the other vaccines tested (17) (Fig. S7). The DNA-prM-Env vaccine also induced detectable Env-specific IFN-γ ELISPOT responses after the week 4 boost immunization, and the RhAd52-prM-Env vaccine induced Env-specific cellular immune responses after the initial week 0 priming immunization (Fig. 5B). Monkeys were challenged 4 weeks after the final vaccination, and both the DNA and RhAd52 vaccines provided complete protection against subcutaneous challenge with 106 VP (103 PFU) of ZIKV-BR as measured by plasma viral loads (Fig. 6).
Figure 5. Immunogenicity of the ZIKV DNA-prM-Env and RhAd52-prM-Env vaccines.
(A) ZIKV-specific microneutralization (MN50) titers following immunization of rhesus monkeys by the i.m. route with 5 mg DNA-prM-Env vaccine at weeks 0 and 4 (red arrows) or a single immunization with 1011 vp RhAd52-prM-Env at week 0. (B) Cellular immune responses by IFN-γ ELISPOT assays to prM, Env, Cap, and NS1 at week 6 for the DNA vaccine or at week 4 for the RhAd52 vaccine. Red bars reflect medians.
Figure 6. Protective efficacy of the ZIKV DNA-prM-Env and RhAd52-prM-Env vaccines.
DNA vaccinated, RhAd52 vaccinated, and sham control rhesus monkeys (N=4/group) were challenged by the s.c route with 106 VP (103 PFU) ZIKV-BR or ZIKV-PR. Plasma viral loads are shown.
Discussion
In this study, we demonstrate that three different vaccine platforms provided complete protection against ZIKV challenge in rhesus monkeys. No specific clinical safety adverse effects related to the vaccines were observed. We recently reported the protective efficacy of the ZIKV PIV vaccine and the DNA-prM-Env vaccine in mice (15). The present data confirm and extend these prior studies by demonstrating robust protection with these vaccines against ZIKV challenge in nonhuman primates, and specifically utilizing the dose, route, and schedule of these vaccines that are typically evaluated in clinical trials. Although the ZIKV PIV vaccine and the DNA-prM-Env vaccine appeared comparably immunogenic in mice (15), the PIV vaccine proved more potent than the DNA vaccine in rhesus monkeys under the conditions tested (Figs. 1, 5). To generalize these observations to a vector-based vaccine, we also evaluated the RhAd52-prM-Env vaccine, which proved highly immunogenic and afforded complete protection after a single immunization in monkeys (Fig. 5). Rhesus adenovirus vectors have the potential advantage of minimal baseline vector-specific neutralizing antibodies in human populations (16).
The adoptive transfer studies demonstrate that vaccine-elicited antibodies are sufficient for protection against ZIKV challenge. Moreover, passive protection in mice and rhesus monkeys was observed at relatively low antibody titers (Figs. 3, 4). Such antibody titers are likely achievable by these vaccine platforms in humans, thus raising optimism for the development of a ZIKV vaccine for humans. Future preclinical and clinical studies will need to address the potential impact of cross-reactive antibodies against dengue virus and other flaviviruses. Secondary infection with a heterologous dengue serotype can be clinically more severe than initial infection, which may or may not reflect antibody-dependent enhancement (18, 19). Cross-reactive antibodies between ZIKV and dengue virus have also been described (20, 21), and dengue-specific antibodies have been reported to increase ZIKV replication in vitro (22). The relevance and implications of these findings for ZIKV vaccine development remain to be determined.
The consistent and robust antibody-based correlates of vaccine protection against ZIKV challenge in both rodents and primates suggest the generalizability of these findings. Similar correlates of protection, and specifically neutralizing antibody titers >10, have been reported for other flavivirus vaccines in humans (23–25). Taken together, these data suggest a path forward for clinical development of ZIKV vaccines in humans. PIV vaccines have been evaluated previously in clinical trials for other flaviviruses, including dengue virus, tick-borne encephalitis virus, and Japanese encephalitis virus (26–30). Phase 1 clinical trials with the ZIKV PIV vaccine, as well as other candidate ZIKV vaccines, are expected to begin later this year.
Supplementary Material
Acknowledgments
We thank J. Mascola, B. Graham, H. Marston, P. Vasconcelos, N. Collins, R. Olson, K. Kabra, C. Springer, G. Ballarini, N. Botero, K. Chandrika, G. Donofrio, M. Robb, D. Weiss, A. Cook, J. Campbell, S. Hetzel, U. Reimer, H. Wenschuh, T. Suscovich, C. Linde, R. Lu, L. Peter, J. Le Suer, P. Gandhi, M. Iampietro, K. Visitsunthorn, A. Badamchi-Zadeh, L. Maxfield, and F. Stephens for generous advice, assistance, and reagents. The data presented in this paper are tabulated in the main paper and in the supplementary materials. P.A., R.A.L., D.H.B., R.G.J., K.H.E., and S.J.T. are co-inventors on pending patent applications related to ZIKV vaccines, antigens, and vectors, and licensure discussions with industry partners are currently ongoing. P.A. and D.H.B. are co-founders and equity holders in AVVI Biotech. ZIKV challenge stocks and vaccine constructs are available with appropriate MTAs. We acknowledge support from the U.S. Military Research and Materiel Command and the U.S. Military HIV Research Program through its cooperative agreement with the Henry M. Jackson Foundation (W81XWH-11-2-0174); the National Institutes of Health (AI095985, AI096040, AI100663, AI124377); the Ragon Institute of MGH, MIT, and Harvard; and the São Paulo Research Foundation (FAPESP 2011/18703-2, 2014/17766-9). The views expressed in this manuscript are those of the authors and do not represent the official views of the Department of the Army or the Department of Defense.
References
- 1.Fauci AS, Morens DM. Zika Virus in the Americas--Yet Another Arbovirus Threat. N Engl J Med. 2016 Feb 18;374:601. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 2.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016 Apr 21;374:1552. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 3.Mlakar J, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016 Mar 10;374:951. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 4.Brasil P, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016 Mar 4; doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016 May 19;374:1981. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 6.Johansson MA, Mier YT-RL, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med. 2016 May 25; doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016 May 9; doi: 10.1016/j.stem.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Miner JJ, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016 May 19;165:1081. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cugola FR, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016 Jun 9;534:267. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brasil P, et al. Guillain-Barre syndrome associated with Zika virus infection. Lancet. 2016 Apr 2;387:1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- 11.Driggers RW, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 2016 Mar 30; doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 12.Dudley DM, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nature communications. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcez PP, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016 May 13;352:816. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 14.Qian X, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016 May 19;165:1238. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larocca RA, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016 Jun 28; doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbink P, et al. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J Virol. 2015 Feb;89:1512. doi: 10.1128/JVI.02950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson KE, et al. Quantification of the epitope diversity of HIV-1-specific binding antibodies by peptide microarrays for global HIV-1 vaccine development. J Immunol Methods. 2015 Jan;416:105. doi: 10.1016/j.jim.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endy TP, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. American journal of epidemiology. 2002 Jul 1;156:40. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 19.Libraty DH, et al. A prospective nested case-control study of Dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009 Oct;6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barba-Spaeth G, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016 Jun 23; doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 21.Stettler K, et al. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science. 2016 Jul 14; doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 22.Dejnirattisai W, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016 Jun 23; doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005 Nov 1;23:5205. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin Exp Immunol. 1997 Dec;110:358. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Applied microbiology. 1973 Apr;25:539. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez LJ, et al. Safety and Immunogenicity of a Dengue Virus Serotype-1 Purified-Inactivated Vaccine: Results of a Phase 1 Clinical Trial. Am J Trop Med Hyg. 2015 Sep;93:454. doi: 10.4269/ajtmh.14-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez S, et al. An adjuvanted, tetravalent dengue virus purified inactivated vaccine candidate induces long-lasting and protective antibody responses against dengue challenge in rhesus macaques. Am J Trop Med Hyg. 2015 Apr;92:698. doi: 10.4269/ajtmh.14-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demicheli V, Debalini MG, Rivetti A. Vaccines for preventing tick-borne encephalitis. The Cochrane database of systematic reviews. 2009:CD000977. doi: 10.1002/14651858.CD000977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demicheli V, Graves P, Pratt M, Jefferson T. Vaccines for preventing tick-borne encephalitis. The Cochrane database of systematic reviews. 2000:CD000977. doi: 10.1002/14651858.CD000977. [DOI] [PubMed] [Google Scholar]
- 30.Erra EO, Kantele A. The Vero cell-derived, inactivated, SA14-14-2 strain-based vaccine (Ixiaro) for prevention of Japanese encephalitis. Expert Rev Vaccines. 2015;14:1167. doi: 10.1586/14760584.2015.1061939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.